Figure 1.
Schematic representation of the most important orbital interactions between carbene ligands CR2 and carbones CL2 with Lewis acids A.(a) Carbene complex with a monodentate Lewis acid; (b) Carbone with a bidentate Lewis acid; (c) Carbone with a monodentate Lewis acid; (d) Carbone with two monodentate Lewis acids.
Figure 1.
Schematic representation of the most important orbital interactions between carbene ligands CR2 and carbones CL2 with Lewis acids A.(a) Carbene complex with a monodentate Lewis acid; (b) Carbone with a bidentate Lewis acid; (c) Carbone with a monodentate Lewis acid; (d) Carbone with two monodentate Lewis acids.
Figure 2.
Calculated and (in parentheses) experimental bond angles of carbones CL
2 with different ligands L and partial charges Δq of the divalent carbon atom. The data are taken from [
19].
Figure 2.
Calculated and (in parentheses) experimental bond angles of carbones CL
2 with different ligands L and partial charges Δq of the divalent carbon atom. The data are taken from [
19].
Figure 3.
Symmetric carbones 1a–1e as ligands for transition metal complexes.
Figure 3.
Symmetric carbones 1a–1e as ligands for transition metal complexes.
Scheme 1.
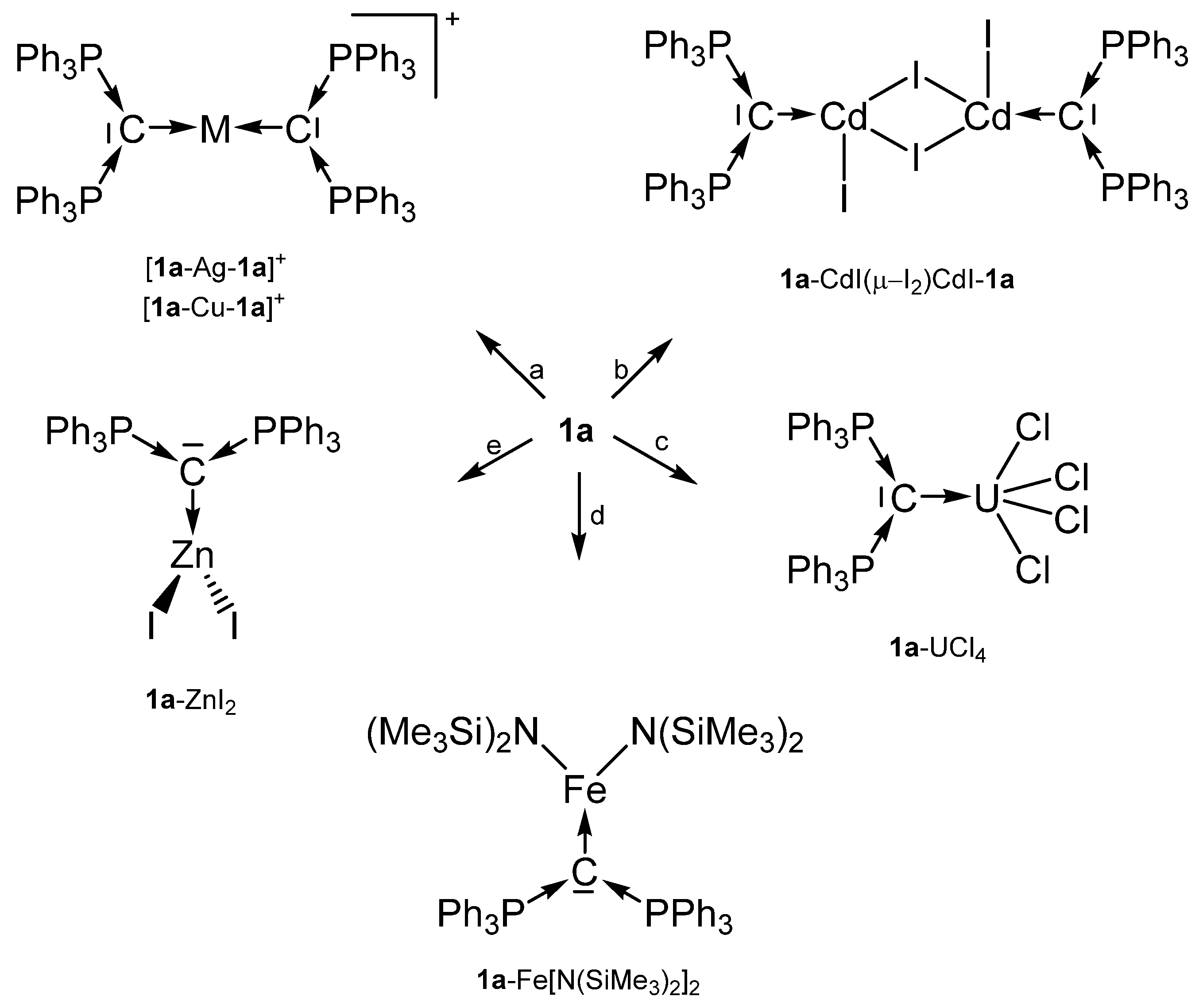
Selected transition metal compounds with the carbone 1a as two electron donor ligand; (a) MI, (b) CdI2, (c) UCl4, (d) Fe(N{SiMe3}2)2, (e) ZnI2.
Scheme 1.
Selected transition metal compounds with the carbone 1a as two electron donor ligand; (a) MI, (b) CdI2, (c) UCl4, (d) Fe(N{SiMe3}2)2, (e) ZnI2.
Scheme 2.
Transition metal complex with the carbone 1c as two and four electron donor ligand. (a) [Tp*(CO)2W≡CPMe3]+/PMe3, (b) 1c/2 MeAuPMe3.
Scheme 2.
Transition metal complex with the carbone 1c as two and four electron donor ligand. (a) [Tp*(CO)2W≡CPMe3]+/PMe3, (b) 1c/2 MeAuPMe3.
Scheme 3.
Selected transition metal compounds with the carbone 1a as four electron donor ligand.
Scheme 3.
Selected transition metal compounds with the carbone 1a as four electron donor ligand.
Scheme 4.
Selected transition metal complexes with the carbone 1b as two and four electron donor ligand. (a) Ni(CO)4, (b) Ni(CO)4 under CO atm, (c) Fe(N{SiMe3}2)2, (d) AuX(tht).
Scheme 4.
Selected transition metal complexes with the carbone 1b as two and four electron donor ligand. (a) Ni(CO)4, (b) Ni(CO)4 under CO atm, (c) Fe(N{SiMe3}2)2, (d) AuX(tht).
Scheme 5.
Selected transition metal complex with the carbone 1e as two electron donor ligand.
Scheme 5.
Selected transition metal complex with the carbone 1e as two electron donor ligand.
Figure 4.
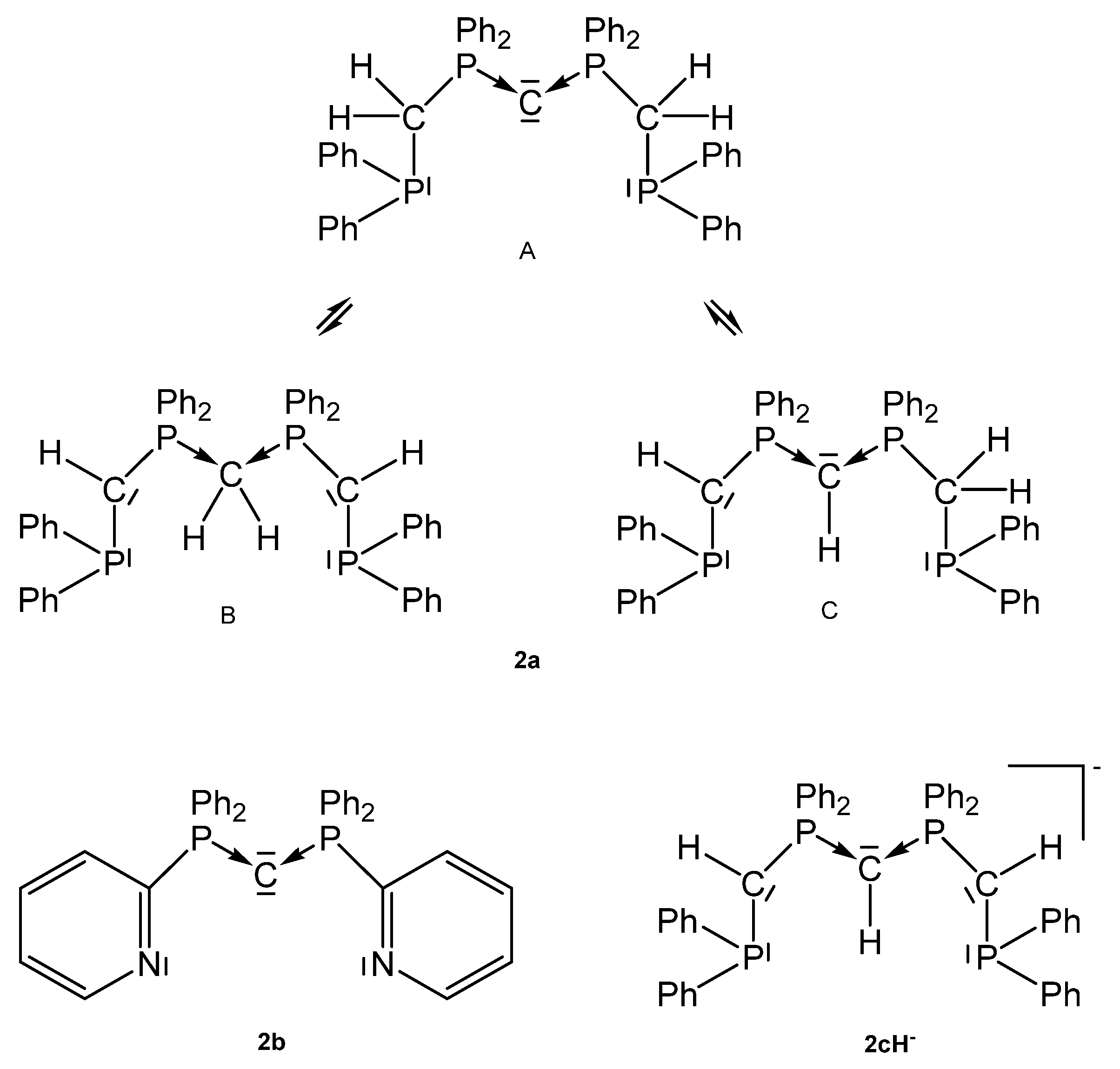
Tripodal basic pincer ligand 2a with its tautomers, the anionic pincer ligand 2cH− and the pyridyl pincer ligand 2b.
Figure 4.
Tripodal basic pincer ligand 2a with its tautomers, the anionic pincer ligand 2cH− and the pyridyl pincer ligand 2b.
Scheme 6.
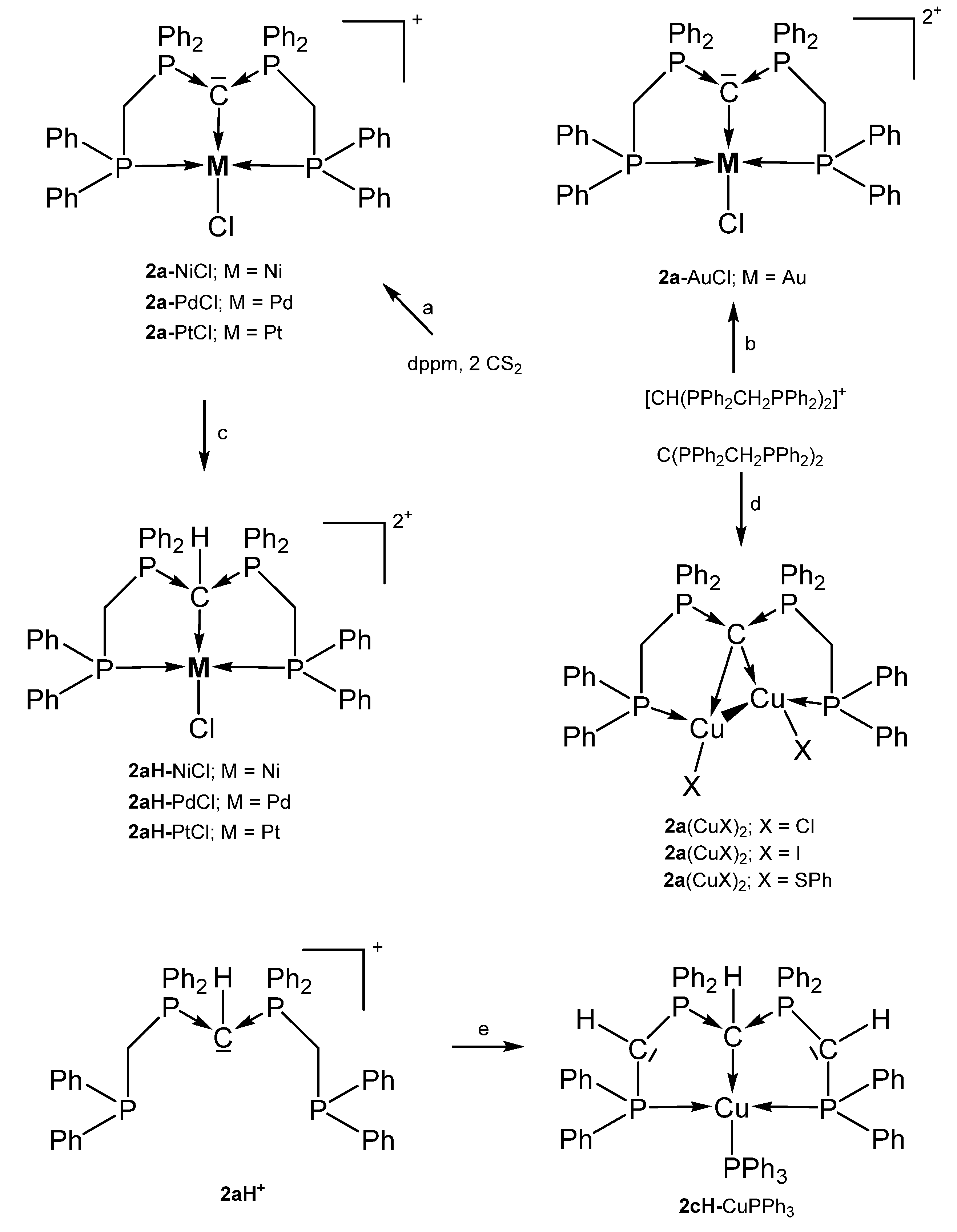
Selected compounds with the pincer ligands 2a and 2aH. (a) MCl2 with a mixture of dppm and 2 eq. of CS2, (b) AuCl(tht)/HNO3, (c) HCl. (d) two eq. of CuX. (e) 2 nBuLi, [Cu(NCMe)4]PF6/PPh3.
Scheme 6.
Selected compounds with the pincer ligands 2a and 2aH. (a) MCl2 with a mixture of dppm and 2 eq. of CS2, (b) AuCl(tht)/HNO3, (c) HCl. (d) two eq. of CuX. (e) 2 nBuLi, [Cu(NCMe)4]PF6/PPh3.
Scheme 7.
Selected compounds with the pincer ligand 2b as two and four electron donor. (a) CeBr3 in THF, (b) UCl4, (c) 2 eq. of Mo(CO)3(NCMe)3, (d) 2 eq. of Ni(CO)4, (e) 2 eq. of CuX, (f) 2 eq. of [Cu]PF6/1 eq. of P-P.
Scheme 7.
Selected compounds with the pincer ligand 2b as two and four electron donor. (a) CeBr3 in THF, (b) UCl4, (c) 2 eq. of Mo(CO)3(NCMe)3, (d) 2 eq. of Ni(CO)4, (e) 2 eq. of CuX, (f) 2 eq. of [Cu]PF6/1 eq. of P-P.
Scheme 8.
Selected addition compounds with the pincer ligand 3a and 3aH and those with the Ag-bridged cations or dication, respectively. (a) from 3aH-PtCl via 3a-Pt(CCl2) and H2O, (b) PMe3, (c) from 5a-Pt(C8H11) (see Scheme 11) and CHCl3, (d) PPh3, (e) 2 AgOTf.
Scheme 8.
Selected addition compounds with the pincer ligand 3a and 3aH and those with the Ag-bridged cations or dication, respectively. (a) from 3aH-PtCl via 3a-Pt(CCl2) and H2O, (b) PMe3, (c) from 5a-Pt(C8H11) (see Scheme 11) and CHCl3, (d) PPh3, (e) 2 AgOTf.
Scheme 9.
Two mesomeric forms of
3b-Pt(CO);
3ba favors a tricarbene coordination at Pt(0) whereas
3bb is consistent Pt(II) forming two C-Pt s-bonds similar to
3a-Pt(CO). The short central C-Pt bond length of 2.002 Å indicates a partial doubly donation of the carbone C atom as shown in
Figure 5. The planar environment at Pt is typical for Pt(II) and supports this view [
72].
Scheme 9.
Two mesomeric forms of
3b-Pt(CO);
3ba favors a tricarbene coordination at Pt(0) whereas
3bb is consistent Pt(II) forming two C-Pt s-bonds similar to
3a-Pt(CO). The short central C-Pt bond length of 2.002 Å indicates a partial doubly donation of the carbone C atom as shown in
Figure 5. The planar environment at Pt is typical for Pt(II) and supports this view [
72].
Figure 5.
Bis-ortho metallated pincer complexes 3a and 3b.
Figure 5.
Bis-ortho metallated pincer complexes 3a and 3b.
Figure 6.
Structure of compound 4.
Figure 6.
Structure of compound 4.
Scheme 10.
Selected complexes with the cyclic carbone 4. R = iPr. a) [{PdCl(allyl)}2], b) [{RhCl(nbd)}2].
Scheme 10.
Selected complexes with the cyclic carbone 4. R = iPr. a) [{PdCl(allyl)}2], b) [{RhCl(nbd)}2].
Figure 7.
Structures of compounds 5a-M, 5b, 5c and 5d-M.
Figure 7.
Structures of compounds 5a-M, 5b, 5c and 5d-M.
Scheme 11.
Selected structures of transition metal complexes with the carbone 5a; (a) ½ [PdCl(allyl); (b) 1/3 [PtI2(cod)]; (c) ¼ [RhCl(cod)]. All complexes are formed upon release of the cation [1aH]+.
Scheme 11.
Selected structures of transition metal complexes with the carbone 5a; (a) ½ [PdCl(allyl); (b) 1/3 [PtI2(cod)]; (c) ¼ [RhCl(cod)]. All complexes are formed upon release of the cation [1aH]+.
Scheme 12.
Selected structures of transition metal complexes with the carbone 5b. (a) [AuCl(tht)], (b) 2 [AuCl(tht), 3 [AuCl(tht)].
Scheme 12.
Selected structures of transition metal complexes with the carbone 5b. (a) [AuCl(tht)], (b) 2 [AuCl(tht), 3 [AuCl(tht)].
Scheme 13.
Selected structures of transition metal complexes with the mono pyridyl substituted carbone 5c.
Scheme 13.
Selected structures of transition metal complexes with the mono pyridyl substituted carbone 5c.
Figure 8.
Structures of compounds 6a and 6b.
Figure 8.
Structures of compounds 6a and 6b.
Scheme 14.
Selected structures of complexes with the cyclic carbones 6a and 6b. Preparation see text.
Scheme 14.
Selected structures of complexes with the cyclic carbones 6a and 6b. Preparation see text.
Figure 9.
Possible description of the bonding in the carbone 7.
Figure 9.
Possible description of the bonding in the carbone 7.
Scheme 15.
Selected structures of complexes with the cyclo propylidene stabilized carbone 7. (a) KHMDS/(NHC)AuOTf.
Scheme 15.
Selected structures of complexes with the cyclo propylidene stabilized carbone 7. (a) KHMDS/(NHC)AuOTf.
Figure 10.
Symmetrical CDCs from which transition metal complexes are known.
Figure 10.
Symmetrical CDCs from which transition metal complexes are known.
Figure 11.
Unsymmetrical CDCs from which transition metal complexes are reported.
Figure 11.
Unsymmetrical CDCs from which transition metal complexes are reported.
Scheme 16.
Selected structures of transition metal complexes with symmetric CDCs 8a and 8b; (a) Fe(OTf)2(MeCN)2.
Scheme 16.
Selected structures of transition metal complexes with symmetric CDCs 8a and 8b; (a) Fe(OTf)2(MeCN)2.
Scheme 17.
Selected structural representation of 8e-PdCl2P(OiPr)3 (a) PdCl2P(OiPr)3.
Scheme 17.
Selected structural representation of 8e-PdCl2P(OiPr)3 (a) PdCl2P(OiPr)3.
Figure 12.
Hypothetical free carbones 9a and 9b.
Figure 12.
Hypothetical free carbones 9a and 9b.
Scheme 18.
Selected structures of transition metal complexes with the carbones 9a and 9b. (a) [Rh(cod)Cl]2/NaOMe, (b) 9a-RhCl/styrene/NaBF4.
Scheme 18.
Selected structures of transition metal complexes with the carbones 9a and 9b. (a) [Rh(cod)Cl]2/NaOMe, (b) 9a-RhCl/styrene/NaBF4.
Scheme 19.
Preparation of [10-AuPPh3]SbF6; a) AuClPPh3/NaSbF6.
Scheme 19.
Preparation of [10-AuPPh3]SbF6; a) AuClPPh3/NaSbF6.
Figure 13.
Bonding description of tetraaminoallene (TAA) (
10). TAA’s may have a bent geometry with hidden or masked pairs of electrons, which are delocalized but serve as double donor orbitals in complexes with CO
2 and CS
2 [
96].
Figure 13.
Bonding description of tetraaminoallene (TAA) (
10). TAA’s may have a bent geometry with hidden or masked pairs of electrons, which are delocalized but serve as double donor orbitals in complexes with CO
2 and CS
2 [
96].
Figure 14.
In compounds 11 the C(0) atoms are stabilized by a phophine or a carbene ligand.
Figure 14.
In compounds 11 the C(0) atoms are stabilized by a phophine or a carbene ligand.
Scheme 20.
Selected structural representation of transition metal complexes of 11a. (a) one equiv. of AuCl(SMe2), (b) two equiv. of AuCl(SMe2).
Scheme 20.
Selected structural representation of transition metal complexes of 11a. (a) one equiv. of AuCl(SMe2), (b) two equiv. of AuCl(SMe2).
Figure 15.
Carbone complex reported by Kato et al. [
99].
Figure 15.
Carbone complex reported by Kato et al. [
99].
Figure 16.
Carbone complexes reported by Fujii et al. [
100].
Figure 16.
Carbone complexes reported by Fujii et al. [
100].
Scheme 21.
Selected structures with the carbones
13a und
13b: (
a) 0.5 eq. of AgOTf, (
b) 2 eq. of AuCl(PPh
3)/2 eq. of AgSbF
6, (
c) 1 eq. of AuCl(PPh
3)/1 eq. of AgSbF
6, (
d) ion exchange (OH
− form), 1 eq. of AuClPPh
3/1 eq. of AgOTf [
100].
Scheme 21.
Selected structures with the carbones
13a und
13b: (
a) 0.5 eq. of AgOTf, (
b) 2 eq. of AuCl(PPh
3)/2 eq. of AgSbF
6, (
c) 1 eq. of AuCl(PPh
3)/1 eq. of AgSbF
6, (
d) ion exchange (OH
− form), 1 eq. of AuClPPh
3/1 eq. of AgOTf [
100].
Figure 17.
Mixed P and S stabilized carbone 14.
Figure 17.
Mixed P and S stabilized carbone 14.
Figure 18.
Sulfur based carbones 15 as ligands for transition metal complexes.
Figure 18.
Sulfur based carbones 15 as ligands for transition metal complexes.
Scheme 22.
Selected of complexes with the carbone 15a. (a) 2 eq AuCl(PPh3), (b) AuCl(PPh3)2.
Scheme 22.
Selected of complexes with the carbone 15a. (a) 2 eq AuCl(PPh3), (b) AuCl(PPh3)2.
Scheme 23.
Selected of complexes with the carbone 15b. (a) 0.5 eq AgOTf, (b) 1.0 eq AgOTf, (c) 2.0 eq AgOTf, (d) 2 eq AuCl(PPh3), (e) AuCl(PPh3).
Scheme 23.
Selected of complexes with the carbone 15b. (a) 0.5 eq AgOTf, (b) 1.0 eq AgOTf, (c) 2.0 eq AgOTf, (d) 2 eq AuCl(PPh3), (e) AuCl(PPh3).
Scheme 24.
Selected complexes with the carbone ligand 15c; (a) AgOTf, (b) 0.5 eq AgOTf, (c) 1.0 eq AgOTf, (d) 2.0 eq AgOTf. {[15c-(AuPPh3)2AgOTf](OTf)4}2 is dimeric linked by two OTf anions.
Scheme 24.
Selected complexes with the carbone ligand 15c; (a) AgOTf, (b) 0.5 eq AgOTf, (c) 1.0 eq AgOTf, (d) 2.0 eq AgOTf. {[15c-(AuPPh3)2AgOTf](OTf)4}2 is dimeric linked by two OTf anions.
Figure 19.
Carbone with Se and S based ligands L.
Figure 19.
Carbone with Se and S based ligands L.
Scheme 25.
Transition metal complexes with the carbone 16 as two and four electron donor. (a) 0.5 eq AgOTf, (b) 1 eq AgOTf, (c) 2.0 eq AgOTf, (d) AgBF4/CH2Cl2.
Scheme 25.
Transition metal complexes with the carbone 16 as two and four electron donor. (a) 0.5 eq AgOTf, (b) 1 eq AgOTf, (c) 2.0 eq AgOTf, (d) AgBF4/CH2Cl2.
Figure 20.
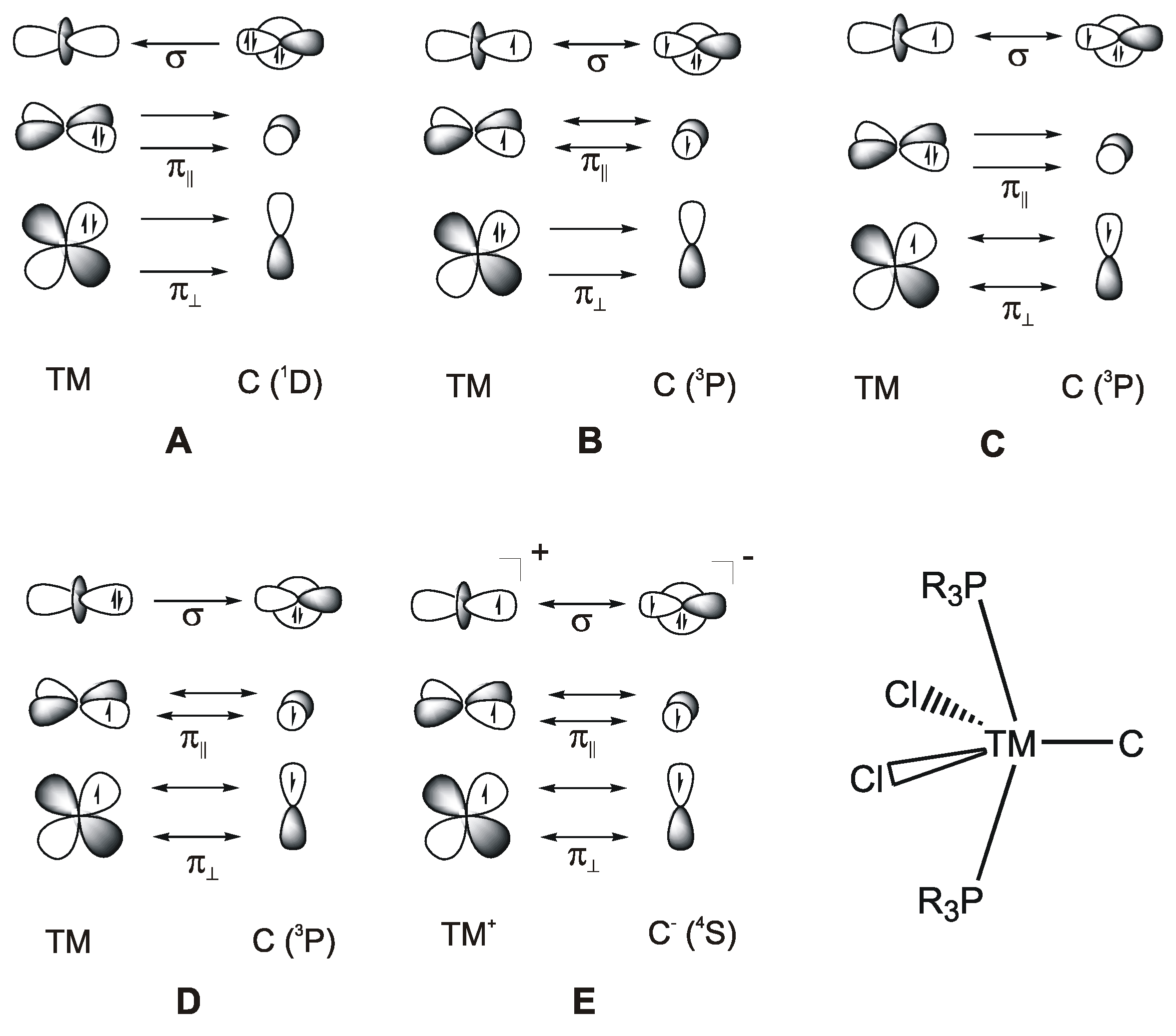
Bonding models (A–E) for the bonding between a transition metal (TM) and a naked carbon atom in the compound [(R3P)2Cl2Ru-C].
Figure 20.
Bonding models (A–E) for the bonding between a transition metal (TM) and a naked carbon atom in the compound [(R3P)2Cl2Ru-C].
Figure 21.
The [Ru]C core.
Figure 21.
The [Ru]C core.
Figure 22.
Spezification of ligands of
Table 14.
Figure 22.
Spezification of ligands of
Table 14.
Scheme 26.
Selected [Ru]C→M carbido complexes and synthesis of {[Ru]C→PtCl}2(μ-Cl)pz.
Scheme 26.
Selected [Ru]C→M carbido complexes and synthesis of {[Ru]C→PtCl}2(μ-Cl)pz.
Figure 23.
The NHC[Ru]C core.
Figure 23.
The NHC[Ru]C core.
Figure 24.
The NHC[RuCl3]−C core.
Figure 24.
The NHC[RuCl3]−C core.
Figure 25.
Structural representation of the Ru carbido complex Ru2(NHC)2(≡C)Cl3H.
Figure 25.
Structural representation of the Ru carbido complex Ru2(NHC)2(≡C)Cl3H.
Figure 26.
Carbido compounds of [Ru]XC with various X.
Figure 26.
Carbido compounds of [Ru]XC with various X.
Figure 27.
The [Os]C core.
Figure 27.
The [Os]C core.
Figure 28.
The [Mo]−C core. Tp* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]− or [HB(pz)3]−.
Figure 28.
The [Mo]−C core. Tp* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]− or [HB(pz)3]−.
Figure 29.
Selected structure of compounds with the [Mo]−C moiety.
Figure 29.
Selected structure of compounds with the [Mo]−C moiety.
Figure 30.
The carbido complex with the P2(CO)Mo≡C core.
Figure 30.
The carbido complex with the P2(CO)Mo≡C core.
Figure 31.
The [W]−C core. T* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]−.
Figure 31.
The [W]−C core. T* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]−.
Figure 32.
Tetrameric unit from [W]C→AuAsPh
3 and [W]C→AuPPh
3 [
122].
Figure 32.
Tetrameric unit from [W]C→AuAsPh
3 and [W]C→AuPPh
3 [
122].
Figure 33.
Structural representation of [W]C→Pt complexes [
125].
Figure 33.
Structural representation of [W]C→Pt complexes [
125].
Figure 34.
The [NMo]−C and [OW]−C core.
Figure 34.
The [NMo]−C and [OW]−C core.
Figure 35.
Selected structures of Rh=C=Rh complexes.
Figure 35.
Selected structures of Rh=C=Rh complexes.
Figure 36.
Structural representation of the Ru carbido complex [Ru(PEt3)Cl(μ-Cl3)RuAr]2C.
Figure 36.
Structural representation of the Ru carbido complex [Ru(PEt3)Cl(μ-Cl3)RuAr]2C.
Figure 37.
Structural representation of the Re carbido complex [Re(CO)2Cp]2C.
Figure 37.
Structural representation of the Re carbido complex [Re(CO)2Cp]2C.
Figure 38.
Structural representation of the W carbido complexes (tBu3SiO)2(NR)W=C=WCl2(OSitBu3)2 and (tBu3SiO)2(O)W=C=WCl2(OSitBu3)2.
Figure 38.
Structural representation of the W carbido complexes (tBu3SiO)2(NR)W=C=WCl2(OSitBu3)2 and (tBu3SiO)2(O)W=C=WCl2(OSitBu3)2.
Figure 39.
Structural representation of the Fe=C=Re carbido complex (TPP)Fe=C=Re2(CO)9.
Figure 39.
Structural representation of the Fe=C=Re carbido complex (TPP)Fe=C=Re2(CO)9.
Figure 40.
Structural representation of the Fe=C=Mn carbido complex (TCNP)Fe=C=Mn2(CO)9.
Figure 40.
Structural representation of the Fe=C=Mn carbido complex (TCNP)Fe=C=Mn2(CO)9.
Figure 41.
Structural representation of the Fe=C=Cr carbido complexes (TPP)Fe=C=Cr(CO)5 and (TAP)Fe=C=Cr(CO)5.
Figure 41.
Structural representation of the Fe=C=Cr carbido complexes (TPP)Fe=C=Cr(CO)5 and (TAP)Fe=C=Cr(CO)5.
Table 1.
Transition metal complexes with the carbones 1a to 1e including C-M and P-C bond lengths and P-C-P angles and 31PNMR shifts in ppm.
Table 1.
Transition metal complexes with the carbones 1a to 1e including C-M and P-C bond lengths and P-C-P angles and 31PNMR shifts in ppm.
| 1-M | 31P NMR | C-M | P-C | P-C-P | Ref |
|---|
| Transition metal complexes with the carbone 1a |
| 1a-Ni(CO)2 | 19.20 | 1.990(3) | 1.677(3) 1.676(3) | 132.13(16) | [39] |
| 1a-Ni(CO)3 | 9.92 | 2.110(3) | 1.681(3) 1.674(3) | 124.58(19) | [39] |
| 1a-ZnI2 | 17.8 | 2.000(9) | 1.691(9) 1.703(8) | 128.3(6) | [40] |
| 1a-CdI(μ I2)CdI-1a | 18.5 | 2.25(1) | 1.700(9) 1.68(1) | 124.8(7) | [40] |
| [1a-Hg-1a][Hg2Cl6] | 21.2 | 2.057(6) 2.082(7) | 1.731(6) 1.706(6) 1.737(6) 1.702(7) | 124.2(4) 125.7(3) | [41] |
| [1a-Ag-1a]I | 13.6 | 2.115(8) 2.134(7) | 1.656(7) 1.690(7) 1.667(7) 1.663(7) | 128.5(5) 129.1(5) | [42] |
| [1a-Cu-1a]I | 15.8 | 1.944(5) 1.951(5) | 1.683(6) 1.688(6) 1.673(6) 1.694(5) | 125.6(3) 128.3(3) | [41] |
| [1a-ReO3][ReO4] | 29.5 | 1.997(7) | 1.771(8) | 123.1(4) | [43] |
| 1a-CuCl | 16.5 | 1.906(2) | nr | 123.8(1) | [44] |
| 1a-Cu-C5H5 | 8.5 | nr | nr | nr | [45] |
| 1a-Cu-C5Me5 | 7.5 | 1.922(6) | 1.668(5) 1.660(6) | 136.0(4) | [45] |
| 1a-CuPPh3 | 3.7 | nr | nr | nr | [45] |
| 1a-AgCl | 16.5 | nr | nr | nr | [44] |
| 1a-AgCp* | 6.5 | nr | nr | nr | [45] |
1a-Au-C≡C-R
R = C6H4NO2-p | nr | 2.082(2) | 1.688(2) 1.682(2) | 133.64(13) | [46] |
| 1a-Au-CH(COMe)2 | nr | nr | nr | nr | [46] |
| 1a-AuCl | 13.7 14.4 | nr | nr | nr | [44] |
| [1a-Ir(COD)]PF6 | nr | nr | nr | nr | [47] |
| 1a-VCl3 | 21.13 | 2.050(3) | 1.712(2) 1.722(2) | 123.6(2) | [48] |
| 1a-FeCl (μ Cl2)FeCl-1a | par | 2.043(7) | 1.689(7) 1.712(7) | 121.3(4) | [49] |
| 1a-Fe[N(SiMe3)2]2 | par | 2.147(2) | 1.702(2) 1.720(2) | 120.0(1) | [50] |
| 1a-FeCl2 | par | 2.055(8) | 1.709(7) 1.702(7) | 122.7(5) | [49] |
| 1a-Fe(CH2Ph)2 | par | 2.097(5) | 1.694(5) 1.671(5) | 124.5(3) | [49] |
| 1a-FeCl[N(TMS)2] | par | nr | nr | nr | [49] |
| 1a-FeOTf[N(TMS)2] | par | 2.040(3) | 1.701(3) 1.704(3) | 122.1(2) | [49] |
| 1a-UCl4 | nr | 2.411(3) | 1.705(3) 1.719(3) | 125.05(16) | [51] |
| 1a-(AuCl)2 | 21.2 | 2.078(3) 2.074(3) | 1.776(3) 1.776(3) | 117.30(15) | [46] |
| [1aH-Ag-1aH](BF4)3 | 23.6 | 2.221(5) | 1.770(7) 1.779(7) | 119.9(4) | [52] |
| [1aH-Au-1aH](OTf)3 | 26.1 | nr | nr | nr | [46] |
| [1aH-AuCl](OTf) | 22.1 | nr | nr | nr | [46] |
| Transition metal complexes with the carbone 1b |
| 1b-Fe[N(SiMe3)2]2 | par | 2.100(2) | 1.694(2) 1.696(1) | 120.8(9) | [50] |
| 1b-Ni(CO)3 | 2.6 | 2.091(2) | 1.683(2) 1.673(2) | 122.3(1) | [53] |
| 1b-Ni2(CO)5 | 12.1 | 2.080(5)
2.070(5) | 1.742(5) 1.743(5) | 117.1(3) | [53] |
| [1bH-AuC6F5](CF3SO3) | 22.7 | 2.029(6) | 1.781(2) 1.792(2) | 119.1 | [54] |
| [1bH-AuCl](CF3SO3) | 22.1 | nr | nr | nr | [54] |
| Transition metal complexes with the carbone 1c |
| [1c-W(CO)2(Tp*)]PF6 | 36 | 2.11(1) | 1.75(2) 1.77(1) | 114.5(8) | [55] |
| 1c-(AuMe)2 | nr | nr | nr | nr | [56] |
| Transition metal complexes with the carbone 1d |
| 1d-Ni(CO)3 | 3.5 | 2.0661(9) | 1.712(2) 1.722(2) | 117.19(9) | [48] |
| Transition metal complexes with the carbone 1e |
| 1e-ZnCl2 | 28.9 | 1.994(2) | 1.686(2) | 125.3(1) | [32] |
| 1e-Rh(CO)(acac) | 32.9 | 2.092(3) | 1.685(3) | 128.56(17) | [32] |
Table 2.
Transition metal complexes with the phosphine based pincer ligands 2a and the pyridyl based pincer ligand 2b; C-M and P-C distances are included and 31P NMR shifts in ppm.
Table 2.
Transition metal complexes with the phosphine based pincer ligands 2a and the pyridyl based pincer ligand 2b; C-M and P-C distances are included and 31P NMR shifts in ppm.
| | 31P NMR | C-M | P-C | P-C-P | Ref |
|---|
| Transition metal complexes with the tripodal carbone 2a |
| [2a-(PdCl)]Cl | 34.5 | 2.062(2) | 1.694(3) | 124.9(2) | [62,65] |
| [2a-(NiCl)]Cl | 36.4 | 1.942(4) | 1.6925(18) | 125.1(2) | [65] |
| [2a-(NiCl)]2NiCl4 | nr | 1.930(7) | 1.696(7) 1.701(7) | 126.3(4) | [65] |
| [2a-(PtCl)]Cl | 35.7 | 2.060(4) | 1.692(5) | 124.86(15) | [65] |
| [2a-(NiMe)][AlCl2Me2] | 31.8 | 1.959 | 1.697 | 120.9 | [66] |
| [2a-(AuCl)]TfO2 | nr | 2.080(8) | 1.723(8) | 124.5(5) | [67] |
| [2a-(AuCl)](NO3)2 | 40.8 | 2.060(3) | 1.721(3) | 125.1(2) | [67] |
| [2a-(AuI)](TfO)2 | 41.1 | 2.082(8) | 1.723(8) | 124.9(5) | [67] |
| [2aH-PdCl]Cl2 | 42.4 | 2.102(3) | 1.803(3) | 121.9(2) | [62] |
| [2aH-PtCl]Cl2 | 44.4 | 2.106(4) | 1.811(4) 1.823(4) | 120.4(2) | [62] |
| [2aH-NiCl]Cl2 | 32.7 | 1.990 | 1.801–1.834 | 121.1 | [65,66] |
| [2aH-(CuCl)]PF6 | nr | 2.304(2) | 1.745(2) | 125.26(14) | [63] |
| 2a-(CuCl)2 | 20.4 | 2.2041 | 1.718 | 122.86(14) | [63] |
| 2a-(CuI)2 | 22.5 | 2.4936 | 1.717 | 126.3(4) | [63] |
| 2a-(CuSPh)2 | 19.8–19.0 | 2.195 | 1.712 | 126.9(7) | [63] |
| Transition metal complexes with the tripodal carbone 2b |
| 2b-(CeBr3THF) | −10.2 | 2.597(6) | 1.672(6) | 122.5(4) | [68] |
| 2b-(CeBr)-2b | nr | 2.573(6) 2.597(6) | 1.684(7) | 120.5(4) | [68] |
| 2b-(UCl4) | nr | 2.471(7) | 1.696(7) | 121.3(4) | [41] |
| 2b-(TiCl3) [57] | 18.24 | 2.144(6) | 1.670(3)
1.670(3) | 129.9(4) | [64] |
| 2b-(Cr(CO)3) | 6.97 | 2.212(2) | 1.651(3) 1.650(3) | 133.6(2) | [64] |
| 2b-(MnCl2) | par | 2.1843(14) | 1.6671(17)
1.6636(17) | 127.70(9) | [64] |
| 2b-(CoCl2) | par | 2.015(6) | 1.680(7)
1.661(7) | 127.5(3) | [64] |
| 2b-[Mo2(CO)7] | 9.49 | 2.355(4) | 1.722(4)
1.724(4) | 120.4(2) | [64] |
| [2b-(PdCl)]Cl | 31.6 | 2.004(4) | 1.689(4)
1.676(4) | 132.4 | [64] |
| 2b-[Ni2(CO)4] | 34.20 | 2.0635(18) | 1.7142(18) | nr | [64] |
| | | 2.0912(18) | 1.7146(18) | | |
| 2b-(Cu2Cl2) | 21.4 | nr | 1.714(3) | 121.51(14) | [63] |
| | | | 1.718(2) | | |
| 2b-(Cu2I2) | 21.5 | nr | 1.679(5) | 128.5(3) | [63] |
| | | | 1.702(5) | | |
| [2b-Cu2(PPh3)2](PF6)2 | 32.9 | nr | 1.709(10) | 126.8(6) | [63] |
| | | | 1.693(9) | | |
| 2b-Cu2(PC6H4OMe)2](PF6)2 | 32.8 | nr | 1.707(3) | 123.64(18) | [63] |
| | | | 1.710(3) | | |
| [2b-Cu2(DPEPhos)](PF6)2 | 29.7 | nr | 1.710(4) | 124.0(2) | [63] |
| [2b-Cu2(XantPhos)](PF6)2 | 34.9 | nr | 1.712(4) | | |
| | | | 1.7064(19) | 122.10(11) | [63] |
| [2b-Cu2(dppf)](PF6)2 | 36.5 | nr | 1.7211(18) | | |
| | | | 1.730(6) | 121.8(4) | [63] |
| 2b-Cu2(SC6F5)2 | 23.1 | nr | 1.717(6) | | |
| | | | 1.710(3) | 123.70(17) | [63] |
| 2b-Cu2(Carb)2 | 22.8 | nr | 1.710(3) | | |
| 1.726(2) | 120.45(15) | [63] |
| 1.728(2) | | |
| Transition metal complex with 2cH |
| 2cH-(CuPPh3) | 23.6 | 2.196(3) | 1.761(3) | 124.26(17) | [63] |
| | | 1.777(3) | | |
Table 3.
Transition metal complexes with ortho metallated tripodal pincer ligand 3a derived from 1a and the related pincer ligand 3b and 31P NMR shifts.
Table 3.
Transition metal complexes with ortho metallated tripodal pincer ligand 3a derived from 1a and the related pincer ligand 3b and 31P NMR shifts.
| 3-M | 31P NMR | C-M | P-C | P-C-P | Ref |
|---|
| Transition metal complexes with the tripodal ligand 3a |
| 3a-Rh(PMe3)2H | 8.56 | 2.203(3) | 1.674(3) | 138.32(18) | [69] |
| 3a-PtSMe2 | 30.42 | nr | nr | nr | [69] |
| 3a-PtCO | 41.5 | 2.037(5) | 1.706(3) | 128.4(3) | [71] |
| 3a-PtPEt3 | 28.5 | 2.067(2) | 1.697(2) | 124.88(14) | [70] |
| 3a-PtP(OPh)3 | nr | nr | nr | nr | [70] |
| [3a-PtPEt3(μ-AgPPh3)3](OTf) | 32.5 | 2.130(4) | 1.737 | 126.0(2) | [70] |
| [3a-PtP(OPh)3(μ-AgPEt3](OTf) | 36.0 | 2.105(3) | 1.743 | 122.9(2) | [70] |
| [3a-PtPEt3(μ-Ag2)Et3PPt-3a](OTf)2 | 33.4 | 2.128(3) | 1.749 | 125.29(18) | [70] |
| 3aH-PtCl | 27.9 | 2.077(6) | 1.796(6) | 123.4(4) | [71] |
| Transition metal complexes with the tripodal ligand 3b |
| 3b-Pt(CO) | 46.9 | 2.002(5) | nr | 133.3(3) | [72] |
Table 4.
Transition metal complexes with the cyclic carbone 4, containing 31P NMR shifts and relevant structural parameters.
Table 4.
Transition metal complexes with the cyclic carbone 4, containing 31P NMR shifts and relevant structural parameters.
| 4-M | 31P NMR | M-C | P1-C P2-C | P-C-P | Ref |
|---|
| 4-PdCl(π-C3H5) | 61.2 71.9 (225) | 2.120(2) | 1.673(2) 1.694(2) | 106.66(13) | [73] |
| 4-RhCl(nbd) | 64.6 75.7 (230) | 2.115(18) | 1.676(18) 1.702(18) | 106.86(10) | [73] |
| 4-Rh(CO)2Cl | 68.2 75.6 (224) | nr | nr | nr | [73] |
| 4-AuOBut | 64.1 60.4 (225) | 2.018(6) | 1.674(7) 1.687(7) | 108.5(4) | [74] |
| 4-CuOBut | 69.8 62.6 (195) | 1.8923(15) | 1.6763(15) 1.6887(15) | 106.90(8) | [74] |
| 4-CuCl | 63.2 70.6 (186) | 1.8914(19) | 1.6700(19) 1.6869(19) | 107.20(11) | [74] |
Table 5.
Transition metal complexes with the unsymmetrical carbones 5a–5d; 31P NMR shifts in ppm.
Table 5.
Transition metal complexes with the unsymmetrical carbones 5a–5d; 31P NMR shifts in ppm.
| 5-M | 31P NMR (2JPP) | M-C | P1-C P2-C | P-C-P | Ref. |
|---|
| Transition metal complexes of 5a-M |
| 5a-Ptcod(C8H11) | 14.9 5.7 (59.8) | 2.072(3) | 1.694(4) 1.716(4) | 114.8(2) | [77] |
| 5a-Rhcod(p) | 10.15 12.40 (50.9) | 2.165(2) | 1.693(2) 1.692(2) | 124.50(13) | [69] |
| 5a-PdC3H5 | 39.8 9.9 (54) | nr | nr | nr | [73] |
| Transition metal complexes with the carbone 5b |
| 5b-AuCl (X = PPh2) | 8.6 18.7 (52) | 2.043 | 1.701(4) 1.696(2) | 126.0(2) | [75] |
| 5b-AuCl (X = PPh2-AuCl) | 25.6 20.2 (47) | 2.037(3) | 1.690(3) 1.689(3) | 131.4(2) | [75] |
| 5b-(AuCl)2 (X = PPh2-AuCl) | 25.4 26.9 | 2.089 2.064 | 1.774(5) 1.763(5) | 123.6(3) | [75] |
| 5b-PtMe2 (X = Me) | 19.3 | nr | nr | nr | [78] |
| Transition metal complexes with the carbone 5c |
| 5c-UCl4 | par | 2.461(5) | 1.699(5) 1.711(5) | 120.6(3) | [41] |
| [5cAuPPh3]+ | 19.70 15.03 (30.7) | 2.067(9) | 1.688(9) 1.707(9) | 124.3(5) | [76] |
| [5c(CuCl)(AuPPh3)]+ | 39.7 26.2 (m) | 2.111(4) Au 1.981(5) Cu | 1.732(5) 1.750(5) | 120.2(3) | [76] |
| [5c(AuCl)(AuPPh3)]+ | 35.4 27.5 (m) | 2.080(9) Au2 2.127(8) Au1 | 1.756(9) | 119.3(5) | [76] |
| Transition metal complexes with the carbone 5d-M |
| 5d-Pt-5d | 19.3 | nr | nr | nr | [78] |
Table 6.
Transition metal complexes with the all carbon ligand 6; 13C NMR shifts (in ppm) of the donating carbon atom. Distances in Å, angles in deg.
Table 6.
Transition metal complexes with the all carbon ligand 6; 13C NMR shifts (in ppm) of the donating carbon atom. Distances in Å, angles in deg.
| | 13C NMR | C-M | C-C | C-C-C | Ref. |
|---|
| Transition metal complexes with the carbone 6a |
| 6a-RhCl(cod) | 136.6 | 2.038(5) | 1.405(6) | 88.4(3) | [79] |
| 6a-IrCl(cod) | 138.6 | nr | nr | nr | [79] |
| 6a-RhCl(CO)2 | 124.7 | nr | nr | nr | [79] |
| 6a-IrCl(CO)2 | 129.2 | nr | nr | nr | [79] |
| Transition metal complexes with the carbone 6b |
| 6b-W(CO)5 | 130.1 | 2.319(3) | 1.419(4) | 88.0(2) | [80] |
| 6b-AuCl | 123.6 | 2.001(4) | 1.409(5) | 90.5(3) | [80] |
| 6b-RhCl(CO)2 | 131.2 | 2.0602(14) | 1.4102(19) | 89.73(11) | [80] |
Table 7.
Complexes with the carbone 7. 13C NMR shifts (in ppm) of the donating carbon atom.
Table 7.
Complexes with the carbone 7. 13C NMR shifts (in ppm) of the donating carbon atom.
| 7-M | 13C NMR | M-C | C-C | C-C-C | Ref. |
|---|
| [7-AuNHC-Ad](OTf) | 92.7 | 2.071(6) 2.047(6) | nr | nr | [81] |
| [7-AuNHC-Dipp](OTf) | 98.0 | nr | nr | nr | [81] |
Table 8.
Collection of transition metal complexes with the CDCs 8a–8h. 13C NMR shifts of the central carbon atom (in ppm).
Table 8.
Collection of transition metal complexes with the CDCs 8a–8h. 13C NMR shifts of the central carbon atom (in ppm).
| | 13C NMR | M-C | C-C | C-C-C | Ref. |
|---|
| Transition metal complexes with the CDC 8a |
| 8a-RhCl(CO)2 | 64.1 | 2.089(7) | 1.398(10) | 121.2(7) | [83] |
| 8a-RuCl2(=CHPh)NHC | 73.01 mes | 2.2069(18) | 1.352(3) 1.429(3) | 119.84(17) | [86] |
| 8a-RuCl2(=CHPh)NHC | 73.4 iPr | 2.210(7) | 1.345(11) 1.439(9) | 116.9(6) | [86] |
| Transition metal complexes with the CDC 8b |
| [8b-PdCl]+ | nr | 1.973(3) | 1.369(5) 1.398(5) | 126.5(3) | [84] |
| [8b-Fe0.5]2+ | | 2.018(3) | 1.374(3) | 128.4(3) | [87] |
| [8b-Fe0.5]3+ | | 1.968(4) | 1.387(6) | 125.2(4) | [87] |
| [8b-Fe0.5]4+ | | 1.928(3) | 1.407(4) | 125.4(2) | [87] |
| Transition metal complexes with the CDC 8c |
| 8c-PdClC3H5 | nr | 2.207(4) | 1.404(5) 1.377(5) | 119.7(4) | [85] |
| 8c-RhCl(CO)2 | 63.7 | 2.109(2) | 1.411(3) 1.385(3) | 117.4(2) | [85] |
| Transition metal complexes with the CDC 8d |
| 8d--RhCl(CO)2 | | 2.123(2) | 1.416(3) 1.368(3) | 116.8(2) | [85] |
| Transition metal complexes with the asymmetric CDC 8e |
| 8e-PdCl2(POR)3 | nr | 2.0398(18) | 1.395(3) 1.328(3) | 119.20(16) | [88] |
| 8e-PdCl2PPh3 | nr | 2.063(2) | 1.383(3) 1.409(3) tP | 115.63(19) | [89] |
| 8e-PdCl2PTol3 | nr | 2.049(4) | 1.374(7) 1.412(8) tP | 117.7(4) | [89] |
| 8e-PdCl2PCy3 | nr | 2.111(2) | 1.343(3) 1.415(4) tP | 123.6(2) | [89] |
| Transition metal complexes with the asymmetric CDC 8f |
| 8f-RhCl(CO)2 | 67.1 | 2.117(2) | 1.369(3) 1.424(3) | 117.8(2) | [90] |
| Transition metal complexes with the asymmetric CDC 8g |
| 8g-RhCl(CO)2 | 63.2 | 2.1164(17) | 1.374(2)NHC 1.420(3) | 118.77(16) | [90] |
| Transition metal complexes with the asymmetric CDC 8h |
| 8h-IrCl(CO)2 | nr | nr | nr | nr | [91] |
| 8h-IrCl(cod) | 166.4 | nr | nr | nr | [91] |
Table 9.
Transition metal complexes with the carbones 9a and 9b; 13C NMR signal of the central donating carbon atom.
Table 9.
Transition metal complexes with the carbones 9a and 9b; 13C NMR signal of the central donating carbon atom.
| 9-M | 13C NMR | M-C | C-C | C-C-C | Ref. |
|---|
| Transition metal complexes with the carbone 9a |
| 9a-RhCl | 73.0 | nr | nr | nr | [93] |
| [9a-RhNCMe]+ | nr | 2.043 | 1.398 1.387 | nr | [93] |
| [9a-Rh(CO)]BF4 | nr | nr | nr | nr | [93] |
| [9a-Rh(styrene)]BF4 | nr | 2.075(2) | 1.404(3) 1.391(3) | 121.7(2) | [94] |
| [9aH-Rh(CO)](BF4)2 | nr | nr | nr | nr | [94] |
| Transition metal complexes with the carbone 9b |
| 9b-RhCl | 73.4 | nr | nr | nr | [93] |
| [9b-RhNCMe]BF4 | nr | nr | nr | nr | [93] |
| [9b-Rh(CO)]BF4 | nr | nr | nr | nr | [93] |
Table 10.
Transition metal complexes with the mixed carbones 11a and 11b. 31P NMR shifts in ppm.
Table 10.
Transition metal complexes with the mixed carbones 11a and 11b. 31P NMR shifts in ppm.
| 11-M | 31P NMR | M-C | P-C C-C | P-C-C | Ref. |
|---|
| Transition metal complexes with the carbone 11a |
| 11a-RhCl(CO)2 | 25.1 | nr | nr | nr | [98] |
| 11a-AuCl | 26.7 | 2.014(16) | 1.7449(16) 1.362(2) | 114.30(12) | [98] |
| 11a-(AuCl)2 | 28.1 | 2.081(4) 2.103(4) | 1.785(4) 1.425(6) | 114.2(3) | [98] |
| Transition metal complexes with the carbone 11b |
| 11b-AuCl | 22.2 | nr | nr | nr | [98] |
Table 11.
Collection of transition metal complexes with the carbones 13a and 13b. 31P NMR signals (in ppm) are given.
Table 11.
Collection of transition metal complexes with the carbones 13a and 13b. 31P NMR signals (in ppm) are given.
| 13-M | 31P NMR | M-C | P-C S-C | P-C-S | Ref |
|---|
| Transition metal complexes with the carbone 13a based on a P-C-S core |
| 13a-AgCl | 10.8 | 2.131 | 1.711 1.648 | 121.9 | [100] |
| [13a-AuPPh3](OTf) | 15.2 | nr | nr | nr | [100] |
| [13a-(AuPPh3)2](OTf)2 | 29.7 | nr | nr | nr | [100] |
| Transition metal complexes with the carbone 13b based on a P-C-S core |
| 13b-AgCl | 9.13 | 2.098 | 1.728 1.636 | 119.1 | [100] |
| [13b-AuPh3](SbF6) | 12.88 | nr | nr | nr | [100] |
| [13b-(AuPPh3)2](SbF6)2 | 27.45 | 2.127 2.118 | 1.788 1.737 | 115.6 | [100] |
| [13b-Ag-13b][OTf) | 8.43 | 2.160 | 1.707 1.635 | 121.8 127.0 | [100] |
| [13bH-AuPPh3](OTf)2 | 17.1 | 2.106 | 1.817 1.782 | 116.3 | [100] |
Table 12.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone ligands 15a to 15c. 13C NMR signal (in ppm) of the central carbon atom.
Table 12.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone ligands 15a to 15c. 13C NMR signal (in ppm) of the central carbon atom.
| 15-M | 13C NMR | C-M | SII-C | SII-M-SII | Ref. |
|---|
| 15a-AgCl | not obs | 2.058(8) | 1.707(8) 1.698(8) | 107.3(5) | [102] |
| [15a-AuPPh3]OTf | 65.4 | nr | nr | nr | [102] |
| [15a-(AuPPh3)2]2+ | not obs | 2.116(6) 2.084(5) | 1.782(6) 1.767(6) | 115.4(3) | [102] |
| [15aH-AuPPh3]2+ | 66.0 | 2.090(7) | 1.837(7) 1.805(7) | 104.4 | [102] |
| Transition metal complexes with the CDS 15b |
| | | C-M | SII-C SIV-C | SII-M-SIV | |
| [15b-AuPPh3]OTf | 67.4 | nr | nr | nr | [102] |
| [15b-Ag-15b]OTf | not obs | 2.111(7) 2.097(7) | 1.718(6) 1.664(7) | 106.3(6) | [102,105] |
| [15b-(AuPPh3)2](OTf)2 | not obs | 2.130(3) 2.103(3) | 1.792(3) 1.746(3) | 106.27(18) | [102] |
| [15b-Ag2-15b](OTf)2 | not obs | nr | nr | nr | [105] |
| [15b-Ag4-15b](OTf)4 | not obs | 2.192 2.187 | nr | nr | [105] |
| [15bH-AuPPh3](OTf)2 | 72.1 | 2.098(3) | 1.796(3) 1.789(3) | 106.83(17) | [102] |
| Transition metal complexes with the CDS 15c |
| | | C-M | SIV-C | SIV-M-SIV | |
| [15c-AuPPh3]OTf | 65.1 | nr | nr | nr | [102] |
| 15c-AgCl | not obs | 2.134(3) | 1.690(3) 1.678(3) | 112.16(14) | [102] |
| [15c-(AuPPh3)2](OTf)2 | not obs | 2.126(4) 2.125(4) | 1.789(4) 1.735(5) | 112.5(2) | [102] |
| [15c-Ag-15c]OTf | 40.0 | 2.116 2.127 | 1.671–1.696 | 114.6 115.6 | [105] |
| [15c-Ag2-15c](OTf)2 | 43.1 | 2.147 | 1.666 1.696 | 114.7 | [105] |
| [15c-Ag4-15c](OTf)4 | nr | 2.228 2.193 | nr | nr | [105] |
| {[15c-(AuPPh3)2AgOTf](OTf)4}2 | nr | 2.139 2.108 | 1.757 1.747 | 116.8 | [102] |
Table 13.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone 16. 13C NMR signal (in ppm) of the central carbon atom.
Table 13.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone 16. 13C NMR signal (in ppm) of the central carbon atom.
| 16-M | 13C NMR | C-M | C-S C-Se | S-C-Se | Ref. |
|---|
| [16-Ag-16](OTf) | not obs. | nr | nr | nr | [105] |
| [16-Ag2-16](OTf)2 | 52.7 | nr | nr | nr | [105] |
| [16-Ag4-16](OTf)4 | not obs | 2.174(5) | 1.714(5) 1.923(6) | 106.4(3) | [105] |
| [16H-Ag-16H](BF4)3 | not obs | 2.164(4) 2.177(4) | 1.772(5) 1.771(5)
1.936(4) 1.948(5) | 103.8(2) | [103] |
Table 14.
Selected structural (in Å and deg) and spectroscopic (13C NMR in ppm) details of [Ru]C addition compounds.
Table 14.
Selected structural (in Å and deg) and spectroscopic (13C NMR in ppm) details of [Ru]C addition compounds.
| | 13C NMR | Ru-C | M-C | Ru-C-M | Ref |
|---|
| [Ru]C→PdCl2(SMe2) | 381.23 | 1.662(2) | 1.946(2) | 175.1(1) | [109] |
| {[Ru]C→PdCl3}− | 380.9 | nr | nr | nr | [112] |
| [Ru]C→Mo(CO)5 | 446.31 | nr | nr | nr | [109] |
| [Ru]C→PtCl2Py | 350.34 | nr | nr | nr | [29,111] |
| [Ru]C→PtCl2NCr(dbm)2 | nr | 1.676(2) | 1.899(2) | 174.5(1) | [29] |
| {[Ru]C→PtCl3}− | 344.7 | nr | nr | nr | [29,112] |
| {[Ru]C→PtCl2}2 | 326.23 | 1.676(8) | 1.871(8) | 1796(4) | [29,111] |
| [Ru]C→PtCl2PPh3 | 388.81 | 1672(2) | 1.983(2) | 173.7(1) | [111] |
| [Ru]C→PtCl2P(OPh)3 | 387.54 | 1.659(2) | 2.001(2) | 179.3(2) | [111] |
| [Ru]C→PtCl2AsPh3 | 374.68 | 1.670(2) | 1.949(2) | 171.9(2) | [111] |
| [Ru]C→PtCl2CNtBu | 376.26 | 1.661(2) | 1.967(6) | 176.5(3) | [111] |
| [Ru]C→PtCl2CNCy | 376.04 | nr | nr | nr | [111] |
| [Ru]C→PtCl2PCy3 | 396.77 | 1.666(3) | 1.971(2) | 174.5(2) | [111] |
| [Ru]C→PtCl2(dmso) | 349.0 | | | | [112] |
| {[Ru]C→PtCl2}2bipy | 348.27 | 1.679(3) | 1.891(4) | 171.4(2) | [111] |
| {[Ru]C→PtCl2}2pyz | 342.48 | 1.668(6) | 1.895(6) | 176.3(3) | [111] |
| {[Ru]C→PtCl2}2pym | 341.36 | 1.678(3) | 1.893(3) | 176.0(2) | [111] |
| {[Ru]C→PtCl}2(μ-Cl)pz | 355.09 | 1.678(4) | 1.909(4) | 169.9(2) | [111] |
| [Ru]C→AuCl | 395.3 | nr | nr | nr | [112] |
| {[Ru]C→Au←C[Ru]}+ | 395.3 | nr | nr | nr | [112] |
| {[Ru]C→IrCl(CO)←C[Ru]} | 397.4 | nr | nr | nr | [112] |
| {[Ru]C→Rh(CO)}2(μ-Cl)2 | 396.4 | nr | nr | nr | [112] |
| [Ru]C→RhCl(cod) | 411.7 | nr | nr | nr | [112] |
| [Ru]C→IrCl(cod) | 387.6 | nr | nr | nr | [112] |
| {[Ru]C→Ag(4′-H-terpy)} | 433.5 | nr | nr | nr | [112] |
| {[Ru]C→Ag(4′-Ph-terpy)} | 433.1 | nr | nr | nr | [112] |
| [Ru]C→Ag(ttcn) | nr | 1.653(4) | 1.876(4) | 177.3(2) | [112] |
| [Ru]C→Cu(ttcn) | nr | 1.622(7) | 2.098(7) | 176.9(5) | [112] |
| [Ru]C→Pd-S4(MoCp*)3 | nr | 1.672(3) | 1.971(3 | 178.3(2) | [112] |
| [Ru]C→Pt-S4(MoCp*)3 | nr | 1.689(7) | 1.896(7) | 178.2(5) | [112] |
| [Ru]C→Pd-S4(WCp*)3 | nr | 1.668(5) | 1.959(5) | 178.1(3) | [112] |
| [Ru]C→Pt-S4(WCp*)3 | nr | 1.699(9) | 1.874(9) | 178.8(6) | [112] |
Table 15.
Carbido complexes with the [Ru]XC core.
Table 15.
Carbido complexes with the [Ru]XC core.
| | 13C NMR | Ru-C | M-C | Ru-C-M | Ref |
|---|
| {[Ru](MeCN)C}OTf | 464.75 | nr | nr | nr | [115] |
| [Ru](CN)2C | 464.70 | nr | nr | nr | [115] |
| [Ru](F)C | 474.58 | nr | nr | nr | [115] |
| [Ru](Br)C | 471.38 | nr | nr | nr | [115] |
| [Ru](I)C | 469.74 | nr | nr | nr | [115] |
| [Ru](CN)C | 474.91 | nr | nr | nr | [115] |
| [Ru](NCO)C | 473.51 | nr | nr | nr | [115] |
| [Ru](NCS)C | 477.50 | nr | nr | nr | [115] |
Table 16.
Compounds with [Mo]−C core with Tp* = [HB(pzMe2)3]− or [HB(pz)3]−.
Table 16.
Compounds with [Mo]−C core with Tp* = [HB(pzMe2)3]− or [HB(pz)3]−.
| | Mo-C | M-C | Mo-C-M | 13C NMR | Ref |
|---|
| Tp* is [HB(pzMe2)3]− |
| [Mo]C→FeCp(CO)2 | 1.819(6) | 1.911(8) | 172.2(5) | 381 | [117] |
| (μ-Se2)[Ir2-{[Mo]C}2(CO)2(PPh3)2] | 1.843(5) | 1.974(5) | 171.3(3) 168.2(3) | 286.1 | [119] |
| [Mo]C→Hg←C[Mo] | nr | nr | nr | 373 | [120] |
| [Mo]C→AuPPh3 | nr | nr | nr | nr | [122] |
| Tp* is [HB(pz)3]− |
| [Mo]C→Pt(PPh3)2Br | nr | nr | nr | 339.0 | [121] |
Table 17.
Compounds with [W]−C core. Tp* = [HB(pzMe2)3]−.
Table 17.
Compounds with [W]−C core. Tp* = [HB(pzMe2)3]−.
| | W-C | M-C | W-C-M | 13C NMR | Ref |
|---|
| [W]C→NiCl(PEt3)2 | nr | nr | nr | nr | [124] |
| [W]C→Fe(CO)2Cp | nr | nr | nr | nr | [122] |
| [W]C→Hg←C[W] | nr | nr | nr | nr | [122] |
| [W]C→AuAsPh3 | nr | nr | nr | nr | [122] |
| [W]C→AuPPh3 | nr | nr | nr | nr | [122] |
| [W]C→AuPEt3 | nr | nr | nr | 397.7 | [122] |
| {[W]C→Pt(terpy)}PF6 | 1.835(5) | 1.938(5) | 176.3(3) | 368 | [125] |
| [W]C→PtCl(terpyC[W]) | 1.853(14) | 1.890(14) | 173.4(9) | 331.3 | [125] |
Table 18.
Fe-C distances (in Å) and Fe-C-Fe angles (in deg). 13C NMR of the bridging carbon atom in ppm.
Table 18.
Fe-C distances (in Å) and Fe-C-Fe angles (in deg). 13C NMR of the bridging carbon atom in ppm.
| | 13C NMR | Fe-C | Fe-C | Fe-C-Fe | Ref |
|---|
| [Fe(TPP)]2C | nr | 1.683(1) | 1.675 | 180 | [130,131] |
| [Fe(TTP)]2C | nr | nr | nr | nr | [129] |
| [Fe(oep)]2C | nr | 1.6638(9) | 1.6638(9) | 179.5(3) | [132] |
| (TPP)Fe=C=Fe(CO)4 | nr | nr | nr | nr | [121] |
| (TCNP)Fe=C=Fe(CO)4 | nr | nr | nr | nr | [121] |
| [Fe(pc)]2C | nr | nr | nr | nr | [95] |
| {[Fe(pc)]2C}(I3)0.66 | nr | nr | nr | nr | [95] |
| [(py)Fe(pc)]2C | nr | 1.69(2) | 1.69(2) | 177.5(8) | [133] |
| [(1-meim)Fe(pc)]2C | nr | 1.70(1) | 1.70(1) | 178(1) | [134] |
| [(4-Mepy)Fe(pc)]2C | nr | nr | nr | nr | [133] |
| [(pip)Fe(pc)]2C | nr | nr | nr | nr | [133] |
| [(thf)Fe(pc)]2C | nr | 1.71(2) | 1.64(2) | 180(1) | [130] |
| [(thf)(TPP)Fe=C=Fe(pc)(thf)] | nr | 1.71(1) | 1.65(1) | 179(1) | [130] |
| (Bu4N)2{[(F)Fe(pc)]2C} | nr | 1.687(4) | 1.687(4) | 179.5(3) | [135] |
| (Bu4N)2{[(Cl)Fe(pc)]2C} | nr | nr | nr | nr | [135] |
| (Bu4N)2{[(Br)Fe(pc)]2C} | nr | nr | nr | nr | [135] |
Table 19.
Rh-C distances (in Å) and Rh-C-Rh angles (in deg). 13C NMR of the bridging carbon atom in ppm.
Table 19.
Rh-C distances (in Å) and Rh-C-Rh angles (in deg). 13C NMR of the bridging carbon atom in ppm.
| | 13C NMR | Rh-C | Rh-C | Rh-C-Rh | Ref |
|---|
| [Rh(PEt3)2(SGePh3)]2C | 425.8, 1JRhC = 47 | 1.788(4) | 1.798(4) | 175.6(2) | [137] |
| [Rh(PEt3)2(SBpin)]2C | nr | 1.790(7) | 1.766(7) | 176.1(4) | [137,138] |
| [Rh(PEt3)2(SH)]2C | nr | nr | nr | nr | [137] |
| [Rh(Cl)(PPh3)2]2C | 424.4, 1JRhC = 47 | 1.7828(19) | 1.7828(19) | nr | [139] |
| [Rh(H2B(pz)2)(PPh3)]2C | nr | 1.7644(11) | 1.7644(11) | 169.1(7) | [139] |
| [Rh(H2B(pzMe2)2)(PPh3)]2C | nr | 1.7794(9) | 1.7794(9) | 168.8(6) | [139] |
| [Rh(HB(pz)3)(PPh3)]2C | nr | 1.7761(7) | 1.7761(7) | 163.7(4) | [139] |
| [Rh2H(μ-C)(μ-C6H4PPh2-2){HB(pzMe2)3}2] | 447.2 1JRhC = 40, 50 | 1.740(6) | 1.818(6) | 165.9(3) | [139] |
 CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11].
CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11]. C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.
C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.