Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer
Abstract
:1. Introduction
2. Bioconjugation Targets in PDT and PDD of Cancer
2.1. Tissue Factor (CD142)
2.2. Human Epidermal Growth Factor Receptor 2
2.3. Estrogen Receptor
2.4. Integrins
2.5. Folate Receptor
2.6. Epidermal Growth Factor Receptor
2.7. P-glycoprotein
3. Bioconjugation Strategies
3.1. Chemical Linkage Methods
3.2. Recombinant DNA Technology for Making Fluorescent Fusion Protein Photosensitizers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J. Photodynamic therapy (PDT) of malignant tumors. Crit. Rev. Oncol. Hematol. 1984, 2, 83–116. [Google Scholar] [CrossRef]
- Gomer, C.J. Photodynamic therapy in the treatment of malignancies. Semin. Hematol. 1989, 26, 27–34. [Google Scholar] [PubMed]
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch. Ophthalmol. 1999, 117, 1329–1345. [Google Scholar]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [PubMed]
- Biel, M.A. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol. Biol. 2010, 635, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Buzalewicz, I.; Holowacz, I.; Ulatowska-Jarza, A.; Podbielska, H. Towards dosimetry for photodynamic diagnosis with the low-level dose of photosensitizer. J. Photochem. Photobiol. B 2017, 173, 333–343. [Google Scholar] [CrossRef]
- Gold, M.H. Introduction to photodynamic therapy: Early experience. Dermatol. Clin. 2007, 25, 1–4. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Simultaneous Photodiagnosis and Photodynamic Treatment of Metastatic Melanoma. Molecules 2019, 24, 3153. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Matoba, Y.; Banno, K.; Kisu, I.; Aoki, D. Clinical application of photodynamic diagnosis and photodynamic therapy for gynecologic malignant diseases: A review. Photodiagnosis Photodyn. Ther. 2018, 24, 52–57. [Google Scholar] [CrossRef]
- Jocham, D.; Stepp, H.; Waidelich, R. Photodynamic diagnosis in urology: State-of-the-art. Eur. Urol. 2008, 53, 1138–1148. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Antimicrobial photoinactivation with functionalized fullerenes. In Nanobiomaterials in Antimicrobial Therapy; William Andrew Publishing: Oxford, UK; Cambridge, MA, USA, 2016; pp. 1–27. [Google Scholar] [CrossRef]
- Jiayuan Kou, D.D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [Green Version]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody-Drug Conjugates: Past, Present, and Future. Bioconjug. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef]
- Qi, Y.B.; Garren, E.J.; Shu, X.; Tsien, R.Y.; Jin, Y. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc. Natl. Acad. Sci. USA 2012, 109, 7499–7504. [Google Scholar] [CrossRef] [Green Version]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, E.O.E.; Edelweiss, E.F.; Stremovskiy, O.A.; Lukyanov, K.A.; Chudakov, D.M.; Deyev, S.M. Targeting Cancer Cells by Using an Antireceptor Antibody-Photosensitizer Fusion Protein. Proc. Natl. Acad. Sci. USA 2009, 106, 9221–9225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011, 9, e1001041. [Google Scholar] [CrossRef] [Green Version]
- Westberg, M.; Holmegaard, L.; Pimenta, F.M.; Etzerodt, M.; Ogilby, P.R. Rational design of an efficient, genetically encodable, protein-encased singlet oxygen photosensitizer. J. Am. Chem. Soc. 2015, 137, 1632–1642. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Missinato, M.A.; Onuoha, E.; Perkins, L.A.; Watkins, S.C.; St Croix, C.M.; Tsang, M.; Bruchez, M.P. A genetically targetable near-infrared photosensitizer. Nat. Methods 2016, 13, 263–268. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, C.; Schmidt, B.F.; Creeger, Y.; Fisher, G.W.; Zakel, K.L.; Adler, S.; Fitzpatrick, J.A.; Woolford, C.A.; Yan, Q.; Vasilev, K.V.; et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat. Biotechnol. 2008, 26, 235–240. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis and tissue factor. Nat. Med. 1996, 2, 167–168. [Google Scholar] [CrossRef]
- Modzelewski, R.A.; Davies, P.; Watkins, S.C.; Auerbach, R.; Chang, M.J.; Johnson, C.S. Isolation and identification of fresh tumor-derived endothelial cells from a murine RIF-1 fibrosarcoma. Cancer Res. 1994, 54, 336–339. [Google Scholar]
- Hu, Z.; Xu, J.; Cheng, J.; McMichael, E.; Yu, L.; Carson, W.E., 3rd. Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget 2017, 8, 1481–1494. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [Green Version]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrandina, G.; Petrillo, M.; Bonanno, G.; Scambia, G. Targeting CD133 antigen in cancer. Expert. Opin. Ther. Targets 2009, 13, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. How to eliminate MYCN-positive hepatic cancer stem cells to prevent the recurrence? Proc. Natl. Acad. Sci. USA 2018, 115, E6388–E6389. [Google Scholar] [CrossRef] [Green Version]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef] [Green Version]
- Adorno-Cruz, V.; Kibria, G.; Liu, X.; Doherty, M.; Junk, D.J.; Guan, D.; Hubert, C.; Venere, M.; Mulkearns-Hubert, E.; Sinyuk, M.; et al. Cancer stem cells: Targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015, 75, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, J.; Wesolowski, R.; Papenfuss, T.; Brooks, T.R.; Carson, W.E., 3rd. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res. Treat. 2013, 140, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Mitchell, R.A.; Yaddanapudi, K. Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. J. Leukoc. Biol. 2017, 102, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Montero, C.M.; Finke, J.; Montero, A.J. Myeloid-derived suppressor cells in cancer: Therapeutic, predictive, and prognostic implications. Semin. Oncol. 2014, 41, 174–184. [Google Scholar] [CrossRef] [Green Version]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 2016, 539, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Du, W.; Yan, F.; Wang, Y.; Li, H.; Cao, S.; Yu, W.; Shen, C.; Liu, J.; Ren, X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 2013, 190, 3783–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, D.; Trad, M.; Hanke, N.T.; Larmonier, C.B.; Janikashvili, N.; Bonnotte, B.; Katsanis, E.; Larmonier, N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014, 74, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Keeley, T.; Costanzo-Garvey, D.L.; Cook, L.M. Unmasking the Many Faces of Tumor-Associated Neutrophils and Macrophages: Considerations for Targeting Innate Immune Cells in Cancer. Trends Cancer 2019, 5, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Schlossman, S.F.; Boumsell, L.; Kishimoto, T.; Knapp, W.; Mason, D.; McMichael, A.; Shaw, S.; Springer, T.A.; Tedder, T.; Todd, R.; et al. CD antigens 1996: Updated nomenclature for clusters of differentiation on human cells. B World Health Organ. 1997, 75, 385–387. [Google Scholar]
- Engel, P.; Boumsell, L.; Balderas, R.; Bensussan, A.; Gattei, V.; Horejsi, V.; Jin, B.Q.; Malavasi, F.; Mortari, F.; Schwartz-Albiez, R.; et al. CD Nomenclature 2015: Human Leukocyte Differentiation Antigen Workshops as a Driving Force in Immunology. J. Immunol. 2015, 195, 4555–4563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrissey, J.H.; Fakhrai, H.; Edgington, T.S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell 1987, 50, 129–135. [Google Scholar] [CrossRef]
- Spicer, E.K.; Horton, R.; Bloem, L.; Bach, R.; Williams, K.R.; Guha, A.; Kraus, J.; Lin, T.C.; Nemerson, Y.; Konigsberg, W.H. Isolation of cDNA clones coding for human tissue factor: Primary structure of the protein and cDNA. Proc. Natl. Acad. Sci. USA 1987, 84, 5148–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konigsberg, W.H.; Nemerson, Y. Molecular cloning of the cDNA for human tissue factor. Cell 1988, 52, 639–640. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [Green Version]
- Osterud, B.; Bjorklid, E. The production and availability of tissue thromboplastin in cellular populations of whole blood exposed to various concentrations of endotoxin. An assay for detection of endotoxin. Scand. J. Haematol. 1982, 29, 175–184. [Google Scholar] [CrossRef]
- Lewis, J.C.; Jones, N.L.; Hermanns, M.I.; Rohrig, O.; Klein, C.L.; Kirkpatrick, C.J. Tissue factor expression during coculture of endothelial cells and monocytes. Exp. Mol. Pathol. 1995, 62, 207–218. [Google Scholar] [CrossRef]
- Mechiche, H.; Cornillet-Lefebvre, P.; Nguyen, P. A subpopulation of human B lymphocytes can express a functional Tissue Factor in response to phorbol myristate acetate. Thromb. Haemost. 2005, 94, 146–154. [Google Scholar] [CrossRef]
- Williams, J.C.; Mackman, N. Tissue factor in health and disease. Front. Biosci. (Elite Ed.) 2012, 4, 358–372. [Google Scholar] [CrossRef]
- Semeraro, N.; Colucci, M. Tissue factor in health and disease. Thromb. Haemost. 1997, 78, 759–764. [Google Scholar] [CrossRef]
- Wada, H.; Wakita, Y.; Shiku, H. Tissue factor expression in endothelial cells in health and disease. Blood Coagul. Fibrinol. 1995, 6 (Suppl. 1), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z. Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases. Antibodies 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Shen, R.; Campbell, A.; McMichael, E.; Yu, L.; Ramaswamy, B.; London, C.A.; Xu, T.; Carson, W.E., 3rd. Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON. Cancer Immunol. Res. 2018, 6, 671–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contrino, J.; Hair, G.; Kreutzer, D.L.; Rickles, F.R. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nat. Med. 1996, 2, 209–215. [Google Scholar] [CrossRef]
- Contrino, J.; Hair, G.A.; Schmeizl, M.A.; Rickles, F.R.; Kreutzer, D.L. In situ characterization of antigenic and functional tissue factor expression in human tumors utilizing monoclonal antibodies and recombinant factor VIIa as probes. Am. J. Pathol. 1994, 145, 1315–1322. [Google Scholar]
- Callander, N.S.; Varki, N.; Rao, L.V. Immunohistochemical identification of tissue factor in solid tumors. Cancer 1992, 70, 1194–1201. [Google Scholar] [CrossRef]
- Hu, Z. Factor VII-Targeted Photodynamic Therapy for Breast Cancer and Its Therapeutic Potential for Other Solid Cancers and Leukemia, Breast Cancer—Current and Alternative Therapeutic Modalities; Esra, G., Mehmet, G., Eds.; InTech: London, UK, 2011; pp. 175–196. ISBN 978-953-307-776-5. [Google Scholar]
- Cesarman-Maus, G.; Braggio, E.; Lome-Maldonado, C.; Morales-Leyte, A.L.; Fonseca, R. Absence of tissue factor is characteristic of lymphoid malignancies of both T- and B-cell origin. Thromb. Res. 2014, 133, 606–609. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Hayes, T.R.; Liu, Y.C.; Hurford, M.T.; Solomides, C.; Bromberg, M. Tissue Factor Is Frequently Expressed in Multiple Myeloma Cells. Blood 2009, 114, 2132. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16 (Suppl. 1), 1–11. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9 (Suppl. 2), S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, R.S.; Taubman, M.B.; Mackman, N. Role of tissue factor in cancer. J. Clin. Oncol. 2009, 27, 4834–4838. [Google Scholar] [CrossRef] [Green Version]
- Versteeg, H.H.; Spek, C.A.; Peppelenbosch, M.P.; Richel, D.J. Tissue factor and cancer metastasis: The role of intracellular and extracellular signaling pathways. Mol. Med. 2004, 10, 6–11. [Google Scholar] [CrossRef]
- Lykke, J.; Nielsen, H.J. The role of tissue factor in colorectal cancer. Eur. J. Surg. Oncol. 2003, 29, 417–422. [Google Scholar] [CrossRef]
- Rickles, F.R.; Shoji, M.; Abe, K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: Tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int. J. Hematol. 2001, 73, 145–150. [Google Scholar] [CrossRef]
- Ruf, W.; Disse, J.; Carneiro-Lobo, T.C.; Yokota, N.; Schaffner, F. Tissue factor and cell signalling in cancer progression and thrombosis. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 306–315. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, M.E.; Konigsberg, W.H.; Madison, J.F.; Pawashe, A.; Garen, A. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc. Natl. Acad. Sci. USA 1995, 92, 8205–8209. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Toi, M.; Koike, M.; Nakamura, S.; Tominaga, T. Tissue factor expression in breast cancer tissues: Its correlation with prognosis and plasma concentration. Br. J. Cancer 2000, 83, 164–170. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, J.; Xu, J.; Ruf, W.; Lockwood, C.J. Tissue factor is an angiogenic-specific receptor for factor VII-targeted immunotherapy and photodynamic therapy. Angiogenesis 2017, 20, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Garen, A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2000, 97, 9221–9225. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Sun, Y.; Garen, A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc. Natl. Acad. Sci. USA 1999, 96, 8161–8166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Xu, J.; Duanmu, J.; Zhou, H.; Booth, C.J.; Hu, Z. Effective treatment of human lung cancer by targeting tissue factor with a factor VII-targeted photodynamic therapy. Curr. Cancer Drug Targets 2011, 11, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, J.; Cheng, J.; Xu, J.; Booth, C.J.; Hu, Z. Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy. Br. J. Cancer 2011, 104, 1401–1409. [Google Scholar] [CrossRef]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC Cancer 2010, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Pedrera, C.; Barbarroja, N.; Dorado, G.; Siendones, E.; Velasco, F. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia 2006, 20, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Rak, J.; Milsom, C.; May, L.; Klement, P.; Yu, J. Tissue factor in cancer and angiogenesis: The molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin. Thromb. Hemost. 2006, 32, 54–70. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zhao, X.; Wang, J.; Di, C.; Zhao, Y.; Ji, T.; Cheng, K.; Wang, Y.; Chen, L.; et al. Tumor-Specific Silencing of Tissue Factor Suppresses Metastasis and Prevents Cancer-Associated Hypercoagulability. Nano Lett. 2019, 19, 4721–4730. [Google Scholar] [CrossRef]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of Tissue Factor in Tumor Progression and Cancer-Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Hu, Z.; Osteen, K.; Bruner-Tran, K.L.; Schatz, F.; Taylor, H.S.; Toti, P.; Arcuri, F.; Konigsberg, W.; Garen, A.; et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am. J. Pathol. 2010, 176, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.; Hu, Z.; Tezel, T.H.; Sohn, J.H.; Kang, S.G.; Cruz, J.M.; Bora, N.S.; Garen, A.; Kaplan, H.J. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 2679–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Garen, A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 12180–12185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezel, T.H.; Bodek, E.; Sonmez, K.; Kaliappan, S.; Kaplan, H.J.; Hu, Z.; Garen, A. Targeting tissue factor for immunotherapy of choroidal neovascularization by intravitreal delivery of factor VII-Fc chimeric antibody. Ocul. Immunol. Inflamm. 2007, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.; Goetz, T.G.; Hu, Z.; Nyachieo, A.; D’Hooghe, T.; Fazleabas, A.; Duleba, A.; Krikun, G.; Taylor, H.S.; Lockwood, C.J. Icon immunoconjugate treatment results in regression of red lesions in a non-human primate (Papio anubis) model of endometriosis. Reprod. Biol. 2018, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pecen, P.E.; Kaiser, P.K. Current phase 1/2 research for neovascular age-related macular degeneration. Curr. Opin. Ophthalmol. 2015, 26, 188–193. [Google Scholar] [CrossRef]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.P.; Arkenau, H.T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, J.W.; Cai, A.G.; Bhatti, M.M.; Cooper, A.B.; Avery, A.D.; Dorfman, R.; Guelman, S.; Levashova, Z.; Migone, T.S. Treating Tissue Factor-Positive Cancers with Antibody-Drug Conjugates That Do Not Affect Blood Clotting. Mol. Cancer Ther. 2018, 17, 2412–2426. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci. Rep. 2020, 10, 2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer. Breast Cancer Res. Treat. 2011, 126, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Hu, Z.; Sinard, J.; Garen, A.; Adelman, R.A. Factor VII-verteporfin for targeted photodynamic therapy in a rat model of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3890–3896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, C.D.; Kelly, C.R.; Ruf, W. Identification of surface residues mediating tissue factor binding and catalytic function of the serine protease factor VIIa. Proc. Natl. Acad. Sci. USA 1996, 93, 14379–14384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Li, J. Natural killer cells are crucial for the efficacy of Icon (factor VII/human IgG1 Fc) immunotherapy in human tongue cancer. BMC Immunol. 2010, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurti, U.; Silverman, J.F. HER2 in breast cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Wilson, F.R.; Coombes, M.E.; Wylie, Q.; Yurchenko, M.; Brezden-Masley, C.; Hutton, B.; Skidmore, B.; Cameron, C. Herceptin(R) (trastuzumab) in HER2-positive early breast cancer: Protocol for a systematic review and cumulative network meta-analysis. Syst. Rev. 2017, 6, 196. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Nelson, E.L. HER2/Neu: An Increasingly Important Therapeutic Target. Part 2: Distribution of HER2/Neu Overexpression and Gene Amplification by Organ, Tumor Site and Histology. Clin. Investig. (Lond.) 2014, 4, 705–728. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Mitsunaga, M.; Arihiro, S.; Saruta, M.; Matsuoka, M.; Kobayashi, H.; Tajiri, H. Molecular targeted photoimmunotherapy for HER2-positive human gastric cancer in combination with chemotherapy results in improved treatment outcomes through different cytotoxic mechanisms. BMC Cancer 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korsak, B.; Almeida, G.M.; Rocha, S.; Pereira, C.; Mendes, N.; Osorio, H.; Pereira, P.M.R.; Rodrigues, J.M.M.; Schneider, R.J.; Sarmento, B.; et al. Porphyrin modified trastuzumab improves efficacy of HER2 targeted photodynamic therapy of gastric cancer. Int. J. Cancer 2017, 141, 1478–1489. [Google Scholar] [CrossRef]
- Li, S.; Jin, Y.; Su, Y.; Li, W.; Xing, Y.; Wang, F.; Hong, Z. Anti-HER2 Affibody-Conjugated Photosensitizer for Tumor Targeting Photodynamic Therapy. Mol. Pharm. 2020, 17, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Ma, Y.T. Synthesis, characterization, and biological verification of anti-HER2 indocyanine green-doxorubicin-loaded polyethyleneimine-coated perfluorocarbon double nanoemulsions for targeted photochemotherapy of breast cancer cells. J. Nanobiotechnol. 2017, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Pye, H.; Butt, M.A.; Reinert, H.W.; Maruani, A.; Nunes, J.P.; Marklew, J.S.; Qurashi, M.; Funnell, L.; May, A.; Stamati, I.; et al. A HER2 selective theranostic agent for surgical resection guidance and photodynamic therapy. Photochem. Photobiol. Sci. 2016, 15, 1227–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Garcia, G.; Panikar, S.S.; Lopez-Luke, T.; Piazza, V.; Honorato-Colin, M.A.; Camacho-Villegas, T.; Hernandez-Gutierrez, R.; De la Rosa, E. An immunoconjugated up-conversion nanocomplex for selective imaging and photodynamic therapy against HER2-positive breast cancer. Nanoscale 2018, 10, 10154–10165. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.H.; Man, H.T.; Zhao, X.D.; Dong, N.; Ma, S.L. Estrogen receptor-positive breast cancer molecular signatures and therapeutic potentials (Review). Biomed. Rep. 2014, 2, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef]
- Zhang, F.L.; Song, M.R.; Yuan, G.K.; Ye, H.N.; Tian, Y.; Huang, M.D.; Xue, J.P.; Zhang, Z.H.; Liu, J.Y. A Molecular Combination of Zinc(II) Phthalocyanine and Tamoxifen Derivative for Dual Targeting Photodynamic Therapy and Hormone Therapy. J. Med. Chem. 2017, 60, 6693–6703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Sun, W.; Fan, J.; Du, J.; Peng, X. An Estrogen Receptor Targeted Ruthenium Complex as a TwoPhoton Photodynamic Therapy Agent for Breast Cancer Cells. Chem. Commun. 2018, 54, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yuan, H.; Zhu, C.; Yang, Q.; Lv, F.; Liu, L.; Wang, S. Polymer-drug conjugates for intracellar molecule-targeted photoinduced inactivation of protein and growth inhibition of cancer cells. Sci. Rep. 2012, 2, 766. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Gacio, A.; Fernandez-Marcos, C.; Swamy, N.; Dunn, D.; Ray, R. Photodynamic cell-kill analysis of breast tumor cells with a tamoxifen-pyropheophorbide conjugate. J. Cell Biochem. 2006, 99, 665–670. [Google Scholar] [CrossRef]
- Swamy, N.; Purohit, A.; Fernandez-Gacio, A.; Jones, G.B.; Ray, R. Nuclear estrogen receptor targeted photodynamic therapy: Selective uptake and killing of MCF-7 breast cancer cells by a C17alpha-alkynylestradiol-porphyrin conjugate. J. Cell Biochem. 2006, 99, 966–977. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Li, Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.Y.; Yang, X.Q.; Zhao, Y.; Zheng, Q.F.; Ke, M.R.; Lin, T.; Chen, R.X.; Ho, K.K.K.; Kumar, N.; Huang, J.D. Synthesis and photodynamic activities of integrin-targeting silicon(IV) phthalocyanine-cRGD conjugates. Eur. J. Med. Chem. 2018, 155, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chatzisideri, T.; Thysiadis, S.; Katsamakas, S.; Dalezis, P.; Sigala, I.; Lazarides, T.; Nikolakaki, E.; Trafalis, D.; Gederaas, O.A.; Lindgren, M.; et al. Synthesis and biological evaluation of a Platinum(II)-c(RGDyK) conjugate for integrin-targeted photodynamic therapy. Eur. J. Med. Chem. 2017, 141, 221–231. [Google Scholar] [CrossRef]
- Klein, O.J.; Yuan, H.; Nowell, N.H.; Kaittanis, C.; Josephson, L.; Evans, C.L. An Integrin-Targeted, Highly Diffusive Construct for Photodynamic Therapy. Sci. Rep. 2017, 7, 13375. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.Y.Y.; Wong, R.C.H.; Wong, C.T.T.; Ng, D.K.P. An integrin-targeting glutathione-activated zinc(II) phthalocyanine for dual targeted photodynamic therapy. Eur. J. Med. Chem. 2019, 174, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; He, R.; Liu, Q.; Dong, Y.; Lin, M.; Li, W.; Xu, F. Theranostics of Triple-Negative Breast Cancer Based on Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 10634–10646. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.A.; Reader, J.; Roque, D.M. Review of Immune Therapies Targeting Ovarian Cancer. Curr. Treat. Op. Oncol. 2018, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Cancer Res. 2019, 38, 125. [Google Scholar] [CrossRef] [Green Version]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Azais, H.; Schmitt, C.; Tardivel, M.; Kerdraon, O.; Stallivieri, A.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Assessment of the specificity of a new folate-targeted photosensitizer for peritoneal metastasis of epithelial ovarian cancer to enable intraperitoneal photodynamic therapy. A preclinical study. Photodiagnosis Photodyn. Ther. 2016, 13, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carron, P.M.; Crowley, A.; O’Shea, D.; McCann, M.; Howe, O.; Hunt, M.; Devereux, M. Targeting the Folate Receptor: Improving Efficacy in Inorganic Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 2675–2708. [Google Scholar] [CrossRef]
- Kato, T.; Jin, C.S.; Ujiie, H.; Lee, D.; Fujino, K.; Wada, H.; Hu, H.P.; Weersink, R.A.; Chen, J.; Kaji, M.; et al. Nanoparticle targeted folate receptor 1-enhanced photodynamic therapy for lung cancer. Lung. Cancer 2017, 113, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Jin, C.S.; Lee, D.; Ujiie, H.; Fujino, K.; Hu, H.P.; Wada, H.; Wu, L.; Chen, J.; Weersink, R.A.; et al. Preclinical investigation of folate receptor-targeted nanoparticles for photodynamic therapy of malignant pleural mesothelioma. Int. J. Oncol. 2018, 53, 2034–2046. [Google Scholar] [CrossRef]
- Girma, W.M.; Dehvari, K.; Ling, Y.C.; Chang, J.Y. Albumin-functionalized CuFeS2/photosensitizer nanohybrid for single-laser-induced folate receptor-targeted photothermal and photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 179–189. [Google Scholar] [CrossRef]
- Yan, S.; Huang, Q.; Chen, J.; Song, X.; Chen, Z.; Huang, M.; Xu, P.; Zhang, J. Tumor-targeting photodynamic therapy based on folate-modified polydopamine nanoparticles. Int. J. Nanomed. 2019, 14, 6799–6812. [Google Scholar] [CrossRef] [Green Version]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Jeong, Y.; Bae, S.Y.; You, D.; Jung, S.P.; Choi, H.J.; Kim, I.; Lee, S.K.; Yu, J.; Kim, S.W.; Lee, J.E.; et al. EGFR is a Therapeutic Target in Hormone Receptor-Positive Breast Cancer. Cell Physiol. Biochem. 2019, 53, 805–819. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Mitchell, R.A.; Luwor, R.B.; Burgess, A.W. Epidermal growth factor receptor: Structure-function informing the design of anticancer therapeutics. Exp. Cell Res. 2018, 371, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Janne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef] [Green Version]
- Driehuis, E.; Spelier, S.; Beltran Hernandez, I.; de Bree, R.; Stefan, M.W.; Clevers, H.; Oliveira, S. Patient-Derived Head and Neck Cancer Organoids Recapitulate EGFR Expression Levels of Respective Tissues and Are Responsive to EGFR-Targeted Photodynamic Therapy. J. Clin. Med. 2019, 8, 1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Driel, P.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; van Bergen En Henegouwen, P.M.P.; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.F.; Kampmeier, F.; von Felbert, V.; Merk, H.F.; Tur, M.K.; Barth, S. SNAP-tag technology mediates site specific conjugation of antibody fragments with a photosensitizer and improves target specific phototoxicity in tumor cells. Bioconjug. Chem. 2011, 22, 2487–2495. [Google Scholar] [CrossRef]
- Amoury, M.; Bauerschlag, D.; Zeppernick, F.; von Felbert, V.; Berges, N.; Di Fiore, S.; Mintert, I.; Bleilevens, A.; Maass, N.; Bräutigam, K.; et al. Photoimmunotheranostic agents for triple-negative breast cancer diagnosis and therapy that can be activated on demand. Oncotarget 2016, 7, 54925–54936. [Google Scholar] [CrossRef] [Green Version]
- Akeba, Y.T.; Sekine, S.; Umai, T.K.; Atsumoto, N.M.; Akaya, S.N.; Suzuki, Y.T.; Anagida, Y.Y.; Akano, H.N.; Sakura, T.A. Irinotecan-Induced Apoptosis Is Inhibited by Increased P-Glycoprotein. Biol. Pharm. Bull. 2007, 30, 1400–1406. [Google Scholar]
- Katayama, R.; Sakashita, T.; Yanagitani, N.; Ninomiya, H.; Horiike, A.; Friboulet, L.; Gainor, J.F.; Motoi, N.; Dobashi, A.; Sakata, S.; et al. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine 2016, 3, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Yano, K.; Tomono, T.; Ogihara, T. Advances in Studies of P-Glycoprotein and Its Expression Regulators. Biol. Pharm. Bull. 2018, 41, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhao, Y.; Li, F.; Li, Z.; Tian, S.; Debinski, W.; Ming, X. P-glycoprotein targeted and near-infrared light-guided depletion of chemoresistant tumors. J. Control. Release 2018, 286, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Qu, P.; Miley, M.J.; Zhao, Y.; Li, Z.; Ming, X. P-glycoprotein targeted photodynamic therapy of chemoresistant tumors using recombinant Fab fragment conjugates. Biomater Sci. 2018, 6, 3063–3074. [Google Scholar] [CrossRef]
- Mao, C.; Li, F.; Zhao, Y.; Debinski, W.; Ming, X. P-glycoprotein-targeted photodynamic therapy boosts cancer nanomedicine by priming tumor microenvironment. Theranostics 2018, 8, 6274–6290. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Eldridge, B.N.; Zhang, H.; Mao, C.; Min, Y.; Sun, Y.; Singh, R.; Ming, X. P-Glycoprotein-Targeted Photothermal Therapy of Drug-Resistant Cancer Cells Using Antibody-Conjugated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 33464–33473. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [Green Version]
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Jothar, L.; Doulkeridou, S.; Schiffelers, R.M.; Sastre Torano, J.; Oliveira, S.; van Nostrum, C.F.; Hennink, W.E. Insights into maleimide-thiol conjugation chemistry: Conditions for efficient surface functionalization of nanoparticles for receptor targeting. J. Control. Release 2018, 282, 101–109. [Google Scholar] [CrossRef]
- Acherar, S.; Colombeau, L.; Frochot, C.; Vanderesse, R. Synthesis of Porphyrin, Chlorin and Phthalocyanine Derivatives by Azide-Alkyne Click Chemistry. Curr. Med. Chem. 2015, 22, 3217–3254. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B. Site-specific protein labeling with SNAP-tags. Curr. Protoc. Protein Sci. 2013, 73, 30.1.1–30.1.16. [Google Scholar] [CrossRef]
- Hussain, A.F.; Heppenstall, P.A.; Kampmeier, F.; Meinhold-Heerlein, I.; Barth, S. One-step site-specific antibody fragment auto-conjugation using SNAP-tag technology. Nat. Protoc. 2019, 14, 3101–3125. [Google Scholar] [CrossRef]
- Meldal, M.; Schoffelen, S. Recent advances in covalent, site-specific protein immobilization. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Yu, Y.; Brooks, J.C.; Godwin, L.A.; Somasundaram, S.; Torabinejad, F.; Kim, J.; Shannon, C.; Easley, C.J. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J. Am. Chem. Soc. 2014, 136, 8467–8474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, D.; Hackenberger, C.P.; Leonhardt, H.; Helma, J. Current Status: Site-Specific Antibody Drug Conjugates. J. Clin. Immunol. 2016, 36 (Suppl. 1), 100–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhao, J.; Ren, J.L.; Zhang, L.; Wen, W.H.; Zhang, R.; Qin, W.W.; Jia, L.T.; Yao, L.B.; Zhang, Y.Q.; et al. Recombinant immunoproapoptotic proteins with furin site can translocate and kill HER2-positive cancer cells. Cancer Res. 2007, 67, 11830–11839. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Photodynamic Physiology-Photonanomanipulations in Cellular Physiology with Protein Photosensitizers. Front. Physiol. 2017, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Liu, C.; Li, J.; Ma, W.; Yu, X.; Zhang, P.; Ji, Y. The effects of photodynamic therapy on leukemia cells mediated by KillerRed, a genetically encoded fluorescent protein photosensitizer. BMC Cancer 2019, 19, 934. [Google Scholar] [CrossRef] [Green Version]
- Shirmanova, M.V.; Serebrovskaya, E.O.; Lukyanov, K.A.; Snopova, L.B.; Sirotkina, M.A.; Prodanetz, N.N.; Bugrova, M.L.; Minakova, E.A.; Turchin, I.V.; Kamensky, V.A.; et al. Phototoxic effects of fluorescent protein KillerRed on tumor cells in mice. J. Biophotonics 2013, 6, 283–290. [Google Scholar] [CrossRef]
- Shirmanova, M.; Yuzhakova, D.; Snopova, L.; Perelman, G.; Serebrovskaya, E.; Lukyanov, K.; Turchin, I.; Subochev, P.; Lukyanov, S.; Kamensky, V.; et al. Towards PDT with Genetically Encoded Photosensitizer KillerRed: A Comparison of Continuous and Pulsed Laser Regimens in an Animal Tumor Model. PLoS ONE 2015, 10, e0144617. [Google Scholar] [CrossRef]
- Liao, Z.X.; Li, Y.C.; Lu, H.M.; Sung, H.W. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials 2014, 35, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Berry, B.J.; Wei, A.Y.; Bahr, L.L.; Foster, T.H.; Wojtovich, A.P. Light-induced oxidant production by fluorescent proteins. Free Radic. Biol. Med. 2018, 128, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Souslova, E.A.; Mironova, K.E.; Deyev, S.M. Applications of genetically encoded photosensitizer miniSOG: From correlative light electron microscopy to immunophotosensitizing. J. Biophotonics 2017, 10, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Mironova, K.E.; Proshkina, G.M.; Ryabova, A.V.; Stremovskiy, O.A.; Lukyanov, S.A.; Petrov, R.V.; Deyev, S.M. Genetically encoded immunophotosensitizer 4D5scFv-miniSOG is a highly selective agent for targeted photokilling of tumor cells in vitro. Theranostics 2013, 3, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Proshkina, G.M.; Shilova, O.N.; Ryabova, A.V.; Stremovskiy, O.A.; Deyev, S.M. A new anticancer toxin based on HER2/neu-specific DARPin and photoactive flavoprotein miniSOG. Biochimie 2015, 118, 116–122. [Google Scholar] [CrossRef]
- Li, S.; Yuan, C.; Chen, J.; Chen, D.; Chen, Z.; Chen, W.; Yan, S.; Hu, P.; Xue, J.; Li, R.; et al. Nanoparticle Binding to Urokinase Receptor on Cancer Cell Surface Triggers Nanoparticle Disintegration and Cargo Release. Theranostics 2019, 9, 884–899. [Google Scholar] [CrossRef] [PubMed]

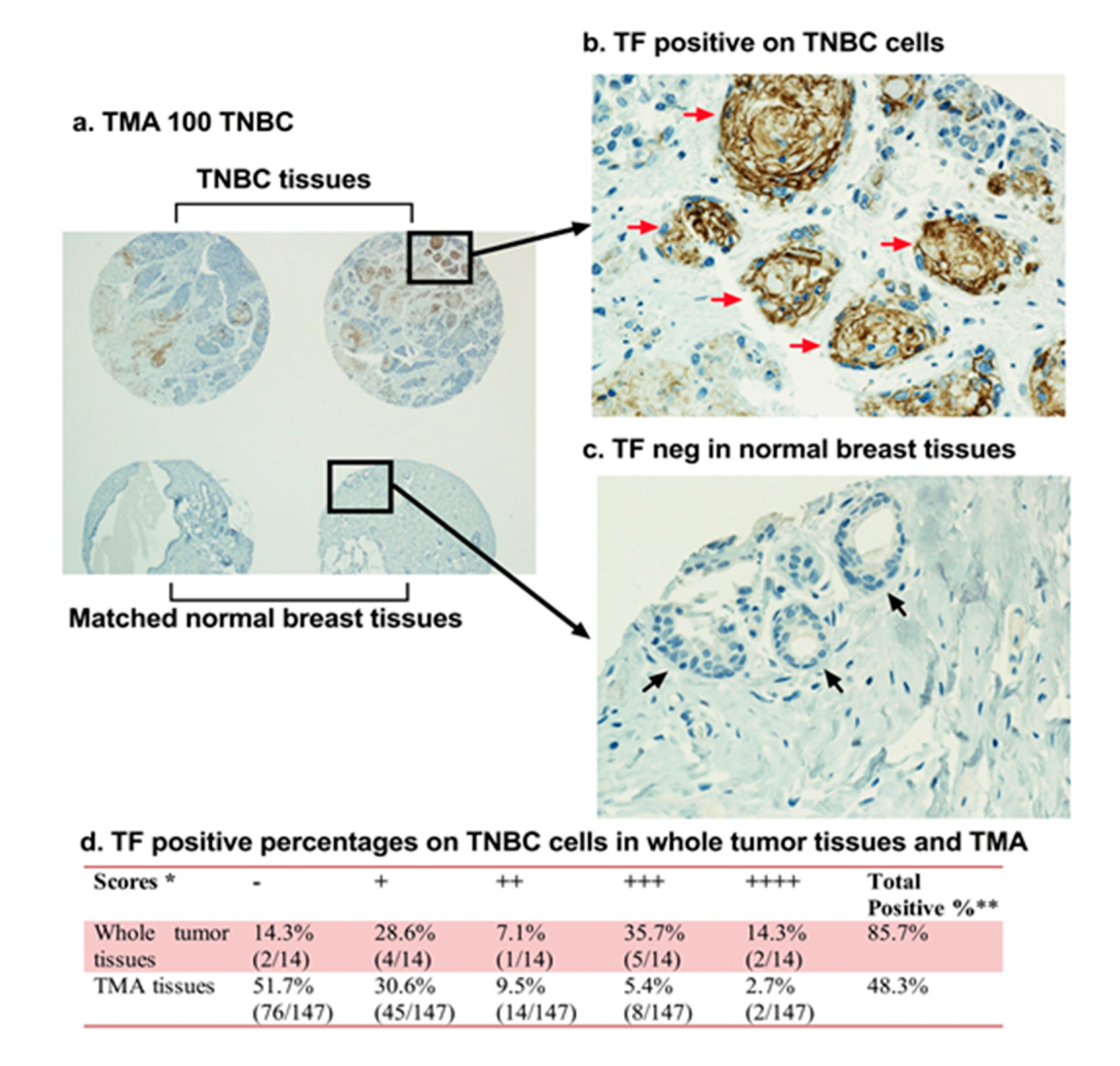

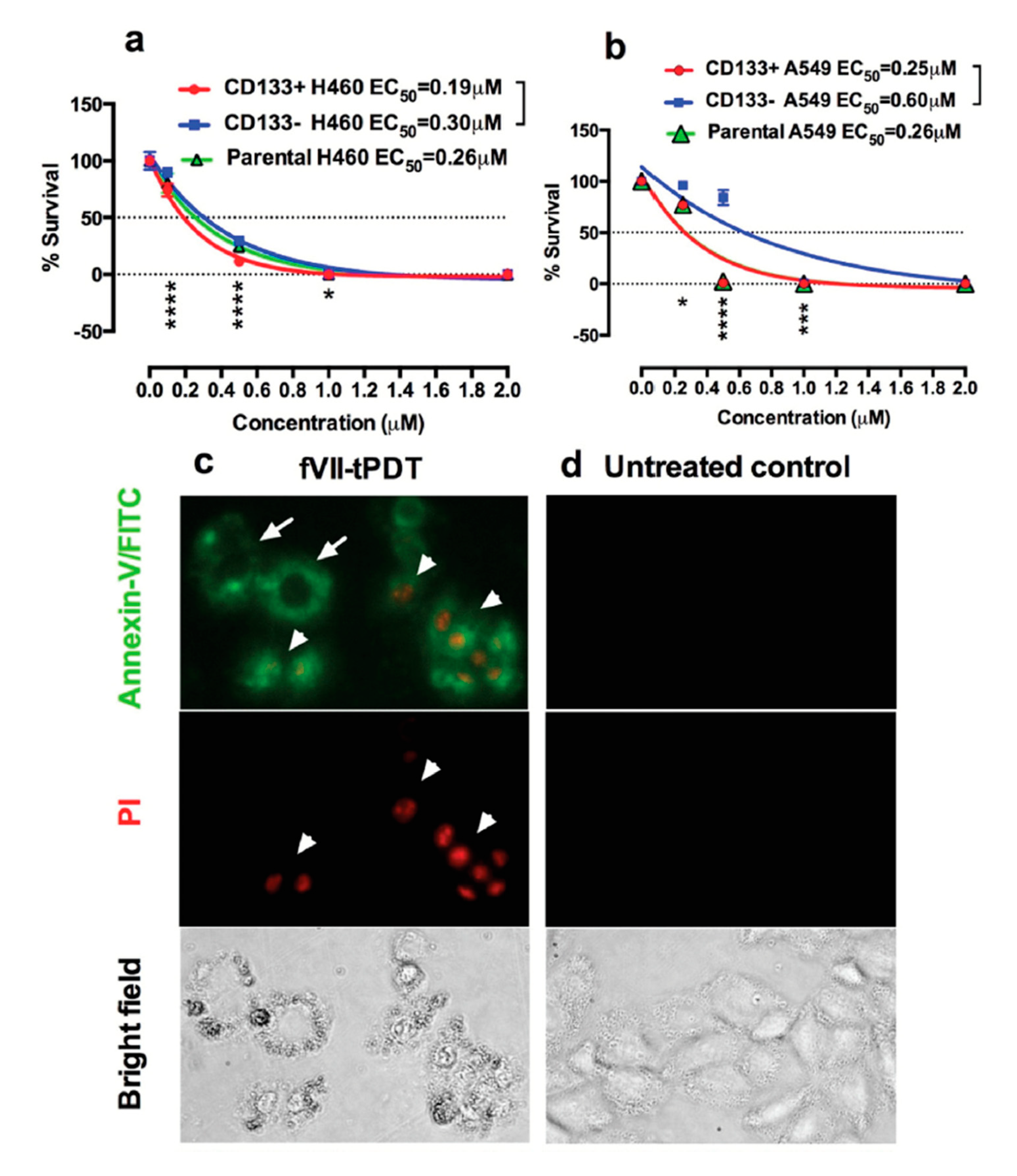
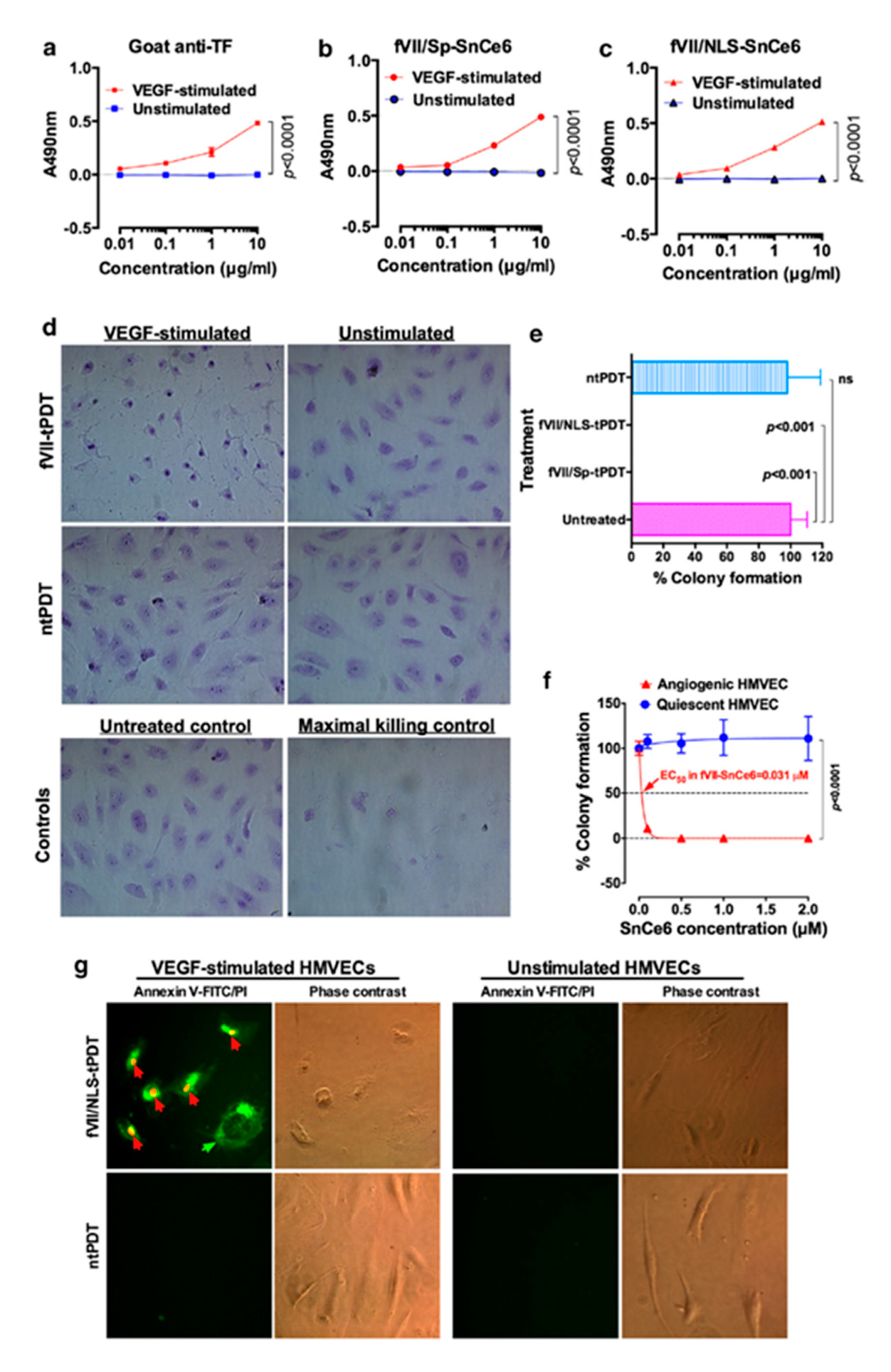
| Target | Targeting Molecule | Photosensitizer | Conjugation Method | Cell Lines/Tumor Xenografts in Mice | Reference |
|---|---|---|---|---|---|
| TF (CD142) | Active-site mutated fVII | SnCe6 | EDC linker | A549, H460 | [86] |
| TF (CD142) | Active-site mutated fVII | Verteporfin | EDC linker | MDA-MB-231 (human TNBC), VEGF-stimulated HUVEC vs. unstimulated HUVEC, murine breast cancer EMT6 xenografts | [88] |
| TF (CD142) | Active-site mutated fVII | SnCe6 | EDC linker | Chemosensitive (MCF-7) and chemoresistant (MCF-7/MDR) breast cancer | [87] |
| TF (CD142) | Active-site mutated fVII | SnCe6 | EDC linker | MDA-MB-231, VEGF-stimulated HUVEC vs. unstimulated HUVEC, murine breast cancer EMT6 xenografts | [104] |
| HER2 | Trastuzumab | IR700 | NHS ester reaction | NCI-N87 | [115] |
| HER2 | Trastuzumab | Porphyrin | NHS ester reaction | NCI-N87 | [116] |
| HER2 | Anti-HER2 affibody | Pyropheophorbide-a | PEG linker | NCI-N87, BT-474 | [117] |
| HER2 | Anti-HER2 antibody | ICG-DOX | PEI linker | MDA-MB-453 | [118] |
| HER2 | HER2 Fab | IRDye800 CW | Maleimide conjugation | OE19 | [119] |
| HER2 | Trastuzumab | Zinc tetracarboxyphenoxy phthalocyanine | NHS ester reaction | SKBR3 | [120] |
| ER | Tamoxifen | Zinc (II) phthalocyanine | Triethylene glycol chain | MCF-7 | [126] |
| ER | Tamoxifen | Ruthenium (II) polypyridyl | “Click” conjugation | MCF-7 | [127] |
| ER | Tamoxifen | Porphyrin | Oligo-ethyleneglycol linker | MCF-7 | [128] |
| ER | Tamoxifen | Pyropheophorbide | Nucleophilic substitution | MCF-7 | [129] |
| ER | C17-α-alkynylestradiol | Ce6-dimethyl ester | Nucleophilic substitution | MCF-7 | [130] |
| Integrin | RGD | Phthalocyanine | “Click” reaction | HT-29, H22 | [135] |
| Integrin | RGD | Platinum (II) complexes | NHS ester reaction | AY27 | [136] |
| Integrin | RGD | EtNBS | “Click” reaction | OVCAR-5 | [137] |
| Integrin | RGD | Zinc (II) phthalocyanine | “Click” reaction | A549, MDA-MB-231 | [138] |
| Integrin | RGD | MEH-PPV | Maleimide conjugation | MDA-MB-231 | [139] |
| FRα | FA | Porphyrin | NHS ester reaction | NuTu-19 | [144] |
| FOLR1 | FA | Porphyrin | PEG linker | A549, H647, H460, SBC5 | [146] |
| FOLR1 | FA | Porphyrin | PEG linker | AE17, AE17-sOVA, AK7, AB12, RN5, H28, H226, H2052, H2452 | [147] |
| FR | FA | Ce6 | NHS ester reaction | HeLa | [148] |
| FR | FA | Phthalocyanine | Polydopamine nanomedicine | HeLa, MCF-7 | [149] |
| FR | FA | Verteporfin | NHS ester reaction | HCT116 | [150] |
| EGFR | NB 7D12 and NB 7D12-9G8 nanobodies | IR700 | NHS ester reaction | 19-luc2-cGFP | [156] |
| EGFR | NB 7D12 and NB 7D12-9G8 nanobodies, cetuximab | IR700 | NHS ester reaction | UM-SCC-14C | [157] |
| EGFR | EGF-conjugated chitosan nanoparticles | Curcumin | NHS ester reaction | MKN45 | [158] |
| EGFR | scFv-425 Fab | Ce6 | SNAP-tag | A431, MDA-MBDA-231, SiHa | [159] |
| EGFR | Anti-EGFR antibody | IR700 | SNAP-tag | Hs758T, MDAMB-231, MDA-MB-453, MDA-MB-468 | [160] |
| Pgp | Pab | IR700 | NHS ester reaction | 3T3-MDR1, NCI/ADRRes, KB-8–5-11 | [166] |
| Pgp | Pgp Fab | IR700 | Maleimide conjugation | 3T3-MDR1, KB-8-5-11 | [167] |
| Pgp | Pab | IR700 | NHS ester reaction | 3T3-MDR1, NCI/ADRRes, KB-8–5-11 | [168] |
| Pgp | Pab | MWCNT nanomedicine | Maleimide conjugation | 3T3-MDR1 | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, S.; Tsung, A.; Hu, Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules 2020, 25, 4964. https://doi.org/10.3390/molecules25214964
Gomez S, Tsung A, Hu Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules. 2020; 25(21):4964. https://doi.org/10.3390/molecules25214964
Chicago/Turabian StyleGomez, Salvador, Allan Tsung, and Zhiwei Hu. 2020. "Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer" Molecules 25, no. 21: 4964. https://doi.org/10.3390/molecules25214964
APA StyleGomez, S., Tsung, A., & Hu, Z. (2020). Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules, 25(21), 4964. https://doi.org/10.3390/molecules25214964








