Macrocyclic Chelates Bridged by a Diaza-Crown Ether: Towards Multinuclear Bimodal Molecular Imaging Probes
Abstract
:1. Introduction
2. Results and Discussion
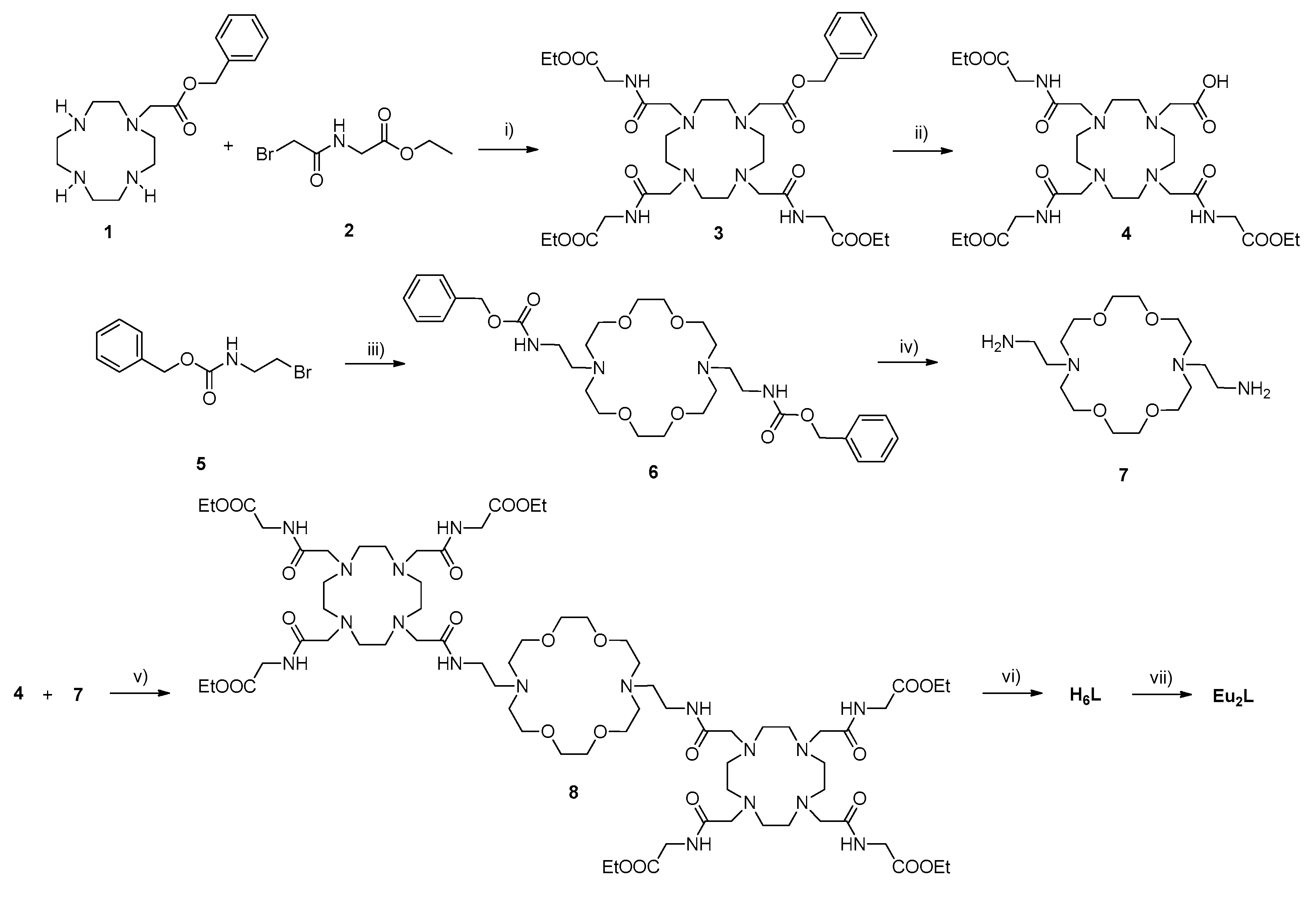
2.1. Synthesis of Complexes Eu2L and Eu2LTb
2.2. CEST Effect Measurements of Eu2L
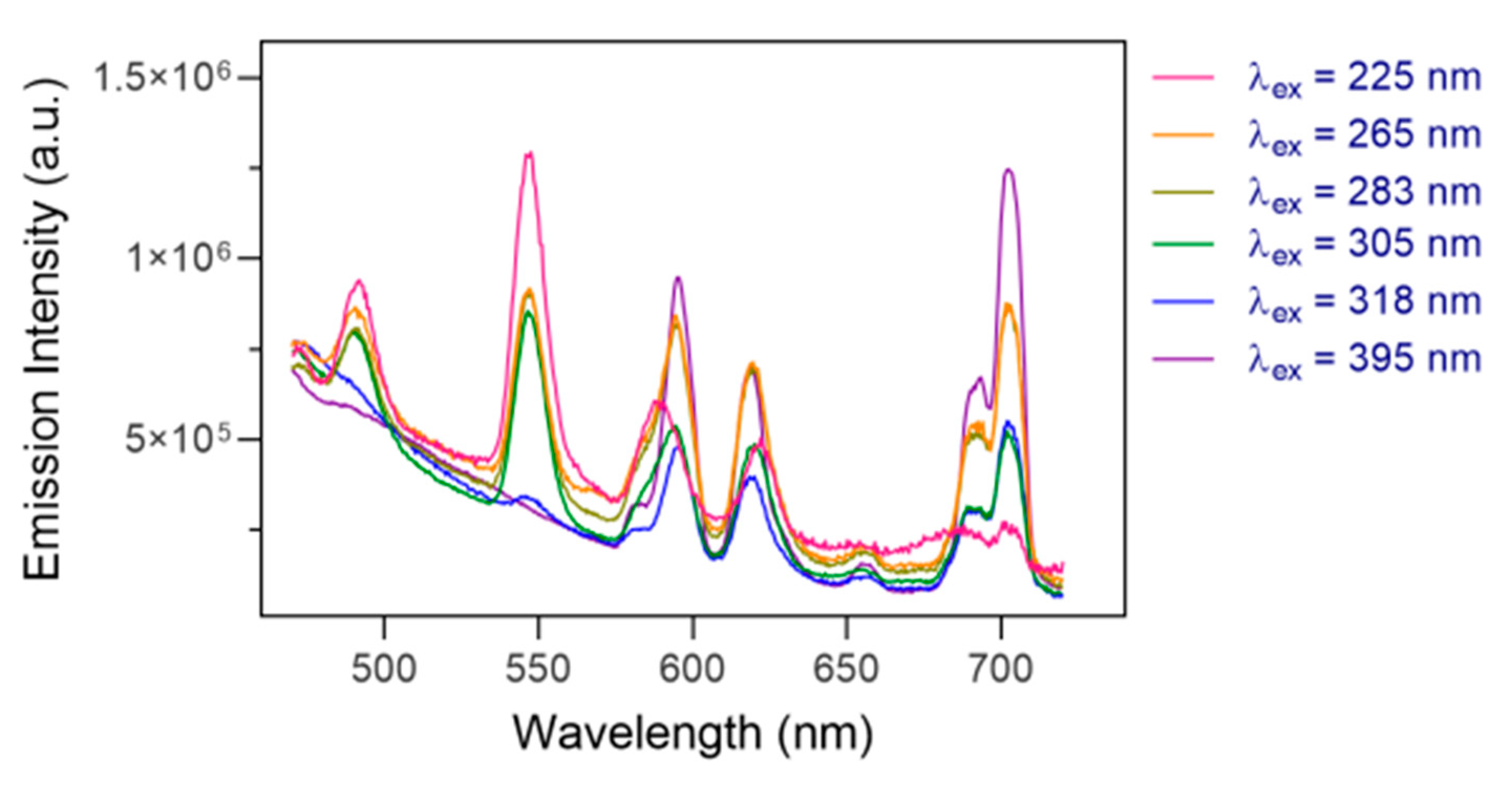
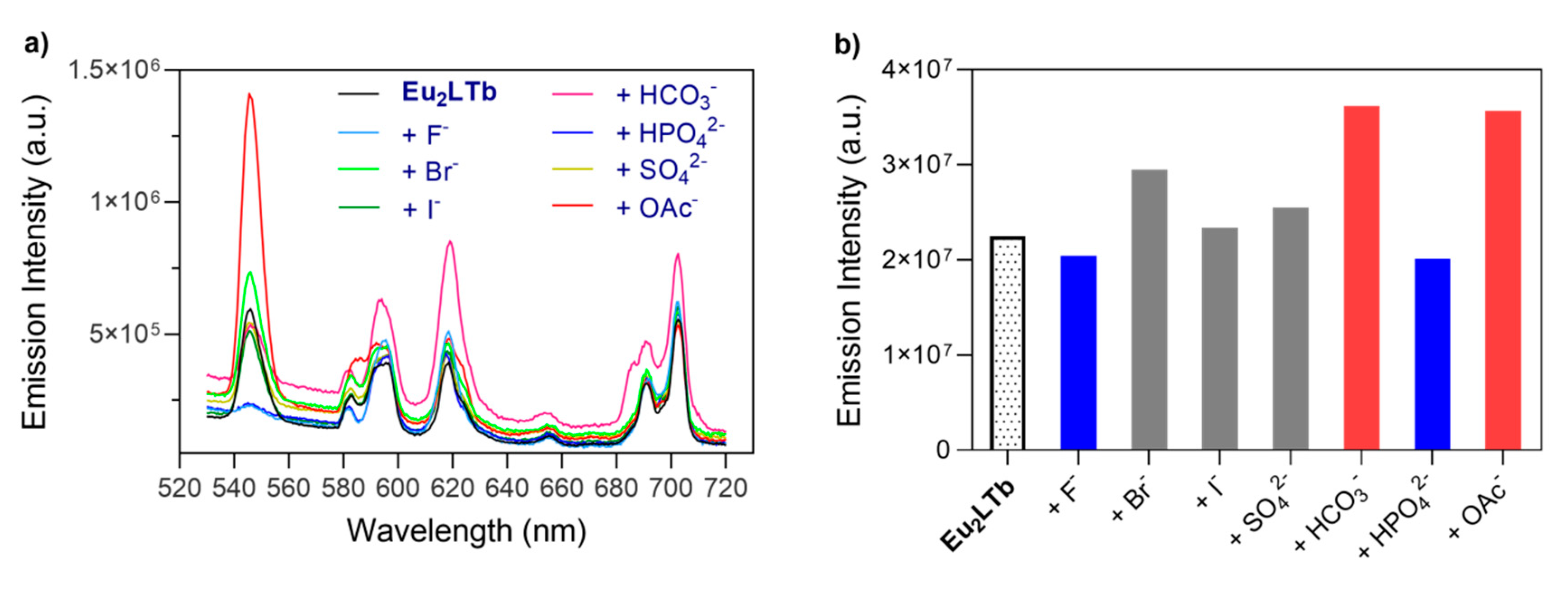
2.3. Photophysical Characterization of Eu2LTb
3. Experimental Section
3.1. Materials
3.2. General Methods
3.3. Synthesis
3.4. NMR Spectroscopy
3.5. Optical Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edelman, R.R.; Hesselink, J.R.; Zlatkin, M.B. MRI: Clinical Magnetic Resonance Imaging, 2nd ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1996; Volume 2. [Google Scholar]
- Zhang, K.; Cheng, Y.; Ren, W.W.; Sun, L.P.; Liu, C.; Wang, D.; Guo, L.H.; Xu, H.X.; Zhao, Y.X. Coordination-Responsive Longitudinal Relaxation Tuning as a Versatile MRI Sensing Protocol for Malignancy Targets. Adv. Sci. 2018, 5, 1800021. [Google Scholar] [CrossRef] [PubMed]
- Helm, L.; Merbach, A.E.; Tóth, E.V. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; p. 496. [Google Scholar]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Evbuomwan, O.M.; Terreno, E.; Aime, S.; Sherry, A.D. CEST and PARACEST Agents for Molecular Imaging. In The Chemistry of Molecular Imaging; Long, N., Wong, W.T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 224–243. [Google Scholar]
- Zhang, S.R.; Merritt, M.; Woessner, D.E.; Lenkinski, R.E.; Sherry, A.D. PARACEST agents: Modulating MRI contrast via water proton exchange. Acc. Chem. Res. 2003, 36, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, A.; Zaiss, M.; Esteban-Gómez, D.; Angelovski, G.; Platas-Iglesias, C. Paramagnetic chemical exchange saturation transfer agents and their perspectives for application inmagnetic resonance imaging. Int. Rev. Phys. Chem. 2020, 40, 51–79. [Google Scholar]
- Baranyai, Z.; Brucher, E.; Ivanyi, T.; Kiraly, R.; Lazar, I.; Zekany, L. Complexation Properties of N,N′,N″,N‴-[1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayltetrakis(1-oxoethane-2,1-diyl)]tetrakis[glycine] (H4dotagl). Equilibrium, Kinetic, and Relaxation Behavior of the Lanthanide(III) Complexes. Helv. Chim. Acta 2005, 88, 604–617. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Tircso, G.; Benyo, E.T.; Garda, Z.; Singh, J.; Trokowski, R.; Brucher, E.; Sherry, A.D.; Toth, E.; Kovacs, Z. Comparison of the equilibrium, kinetic and water exchange properties of some metal ion-DOTA and DOTA-bis(amide) complexes. J. Inorg. Biochem. 2020, 206, 111042–111054. [Google Scholar] [CrossRef]
- Bunzli, J.C. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Mamedov, I.; Parac-Vogt, T.N.; Logothetis, N.K.; Angelovski, G. Synthesis and characterization of dinuclear heterometallic lanthanide complexes exhibiting MRI and luminescence response. Dalton Trans. 2010, 39, 5721–5727. [Google Scholar] [CrossRef]
- Jones, J.E.; Amoroso, A.J.; Dorin, I.M.; Parigi, G.; Ward, B.D.; Buurma, N.J.; Pope, S.J.A. Bimodal, dimetallic lanthanide complexes that bind to DNA: The nature of binding and its influence on water relaxivity. Chem. Commun. 2011, 47, 3374–3376. [Google Scholar] [CrossRef] [PubMed]
- Terreno, E.; Boffa, C.; Menchise, V.; Fedeli, F.; Carrera, C.; Delli Castelli, D.; Digilio, G.; Aime, S. Gadolinium-doped LipoCEST agents: A potential novel class of dual 1H-MRI probes. Chem. Commun. 2011, 47, 4667–4669. [Google Scholar] [CrossRef]
- Debroye, E.; Parac-Vogt, T.N. Towards polymetallic lanthanide complexes as dual contrast agents for magnetic resonance and optical imaging. Chem. Soc. Rev. 2014, 43, 8178–8192. [Google Scholar] [CrossRef] [Green Version]
- Placidi, M.P.; Villaraza, A.J.; Natrajan, L.S.; Sykes, D.; Kenwright, A.M.; Faulkner, S. Synthesis and spectroscopic studies on azo-dye derivatives of polymetallic lanthanide complexes: Using diazotization to link metal complexes together. J. Am. Chem. Soc. 2009, 131, 9916–9917. [Google Scholar] [CrossRef]
- Sorensen, T.J.; Tropiano, M.; Blackburn, O.A.; Tilney, J.A.; Kenwright, A.M.; Faulkner, S. Preparation and study of an f,f,f′,f″ covalently linked tetranuclear hetero-trimetallic complex—A europium, terbium, dysprosium triad. Chem. Commun. 2013, 49, 783–785. [Google Scholar] [CrossRef]
- Faulkner, S.; Pope, S.J. Lanthanide-sensitized lanthanide luminescence: Terbium-sensitized ytterbium luminescence in a trinuclear complex. J. Am. Chem. Soc. 2003, 125, 10526–10527. [Google Scholar] [CrossRef]
- Tseng, M.C.; Chu, Y.H. Chemoselective gas sensing ionic liquids. Chem. Commun. 2010, 46, 2983–2985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Winter, P.; Wu, K.; Sherry, A.D. A novel europium(III)-based MRI contrast agent. J. Am. Chem. Soc. 2001, 123, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Evbuomwan, O.M.; Kiefer, G.; Sherry, A.D. Amphiphilic EuDOTA-tetraamide complexes form micelles with enhanced CEST sensitivity. Eur. J. Inorg. Chem. 2012, 2012, 2126–2134. [Google Scholar] [CrossRef] [Green Version]
- Cakic, N.; Verbic, T.Z.; Jelic, R.M.; Platas-Iglesias, C.; Angelovski, G. Synthesis and characterisation of bismacrocyclic DO3A-amide derivatives—An approach towards metal-responsive PARACEST agents. Dalton Trans. 2016, 45, 6555–6565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zijl, P.C.M.; Yadav, N.N. Chemical Exchange Saturation Transfer (CEST): What is in a Name and What Isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Malloy, C.R.; Sherry, A.D. MRI thermometry based on PARACEST agents. J. Am. Chem. Soc. 2005, 127, 17572–17573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaiss, M.; Angelovski, G.; Demetriou, E.; McMahon, M.T.; Golay, X.; Scheffler, K. QUESP and QUEST revisited—Fast and accurate quantitative CEST experiments. Magn. Reson. Med. 2018, 79, 1708–1721. [Google Scholar] [CrossRef]
- Dixon, W.T.; Ren, J.; Lubag, A.J.; Ratnakar, J.; Vinogradov, E.; Hancu, I.; Lenkinski, R.E.; Sherry, A.D. A concentration-independent method to measure exchange rates in PARACEST agents. Magn. Reson. Med. 2010, 63, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.; Mamula, O.; Scopelliti, R.; Donnio, B.; Guillon, D.; Terazzi, E.; Piguet, C.; Bunzli, J.C.G. Lanthanide luminescent mesomorphic complexes with macrocycles derived from diaza-18-crown-6. New J. Chem. 2005, 29, 1323–1334. [Google Scholar] [CrossRef]
- Sazonov, P.K.; Stolyarenko, V.Y.; Shtern, M.M.; Beletskaya, I.P. Unexpected lanthanide cation selectivity of bis-β-ketovinylated diaza-18-crown-6 and open-chain diamines: Cooperative effect of the second keto group. J. Inclusion Phenom. Macrocyclic Chem. 2014, 79, 193–203. [Google Scholar] [CrossRef]
- Gao, C.J.; Kirillov, A.M.; Dou, W.; Tang, X.L.; Liu, L.L.; Yan, X.H.; Xie, Y.J.; Zang, P.X.; Liu, W.S.; Tang, Y. Self-Assembly Synthesis, Structural Features, and Photophysical Properties of Dilanthanide Complexes Derived from a Novel Amide Type Ligand: Energy Transfer from Tb(III) to Eu(III) in a Heterodinuclear Derivative. Inorg. Chem. 2014, 53, 935–942. [Google Scholar] [CrossRef]
- Zhang, H.X.; Chen, Z.H.; Liu, X.; Zhang, F. A mini-review on recent progress of new sensitizers for luminescence of lanthanide doped nanomaterials. Nano Res. 2020, 13, 1795–1809. [Google Scholar] [CrossRef]
- Kaczmarek, M. Lanthanide-sensitized luminescence and chemiluminescence in the systems containing most often used medicines; a review. J. Lumin. 2020, 222, 117174. [Google Scholar] [CrossRef]
- Parker, D.; Dickins, R.S.; Puschmann, H.; Crossland, C.; Howard, J.A. Being excited by lanthanide coordination complexes: Aqua species, chirality, excited-state chemistry, and exchange dynamics. Chem. Rev. 2002, 102, 1977–2010. [Google Scholar] [CrossRef] [PubMed]
- Aletti, A.B.; Gillen, D.M.; Gunnlaugsson, T. Luminescent/colorimetric probes and (chemo-) sensors for detecting anions based on transition and lanthanide ion receptor/binding complexes. Coord. Chem. Rev. 2018, 354, 98–120. [Google Scholar] [CrossRef]
- Kropp, J.L.; Windsor, M.W. Luminescence and Energy Transfer in Solutions of Rare Earth Complexes. II. Studies of Solvation Shell in Europium(III) and Terbium(III) as a Function of Acetate Concentration. J. Phys. Chem. 1967, 71, 477–482. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide Ion Probes of Structure in Biology—Laser-Induced Luminescence Decay Constants Provide a Direct Measure of the Number of Metal-Coordinated Water-Molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Corsi, D.M.; Platas-Iglesias, C.; van Bekkum, H.; Peters, J.A. Determination of paramagnetic lanthanide(III) concentrations from bulk magnetic susceptibility shifts in NMR spectra. Magn. Reson. Chem. 2001, 39, 723–726. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]



| Temp./°C | 10 | 15 | 20 | 25 | 30 | 35 | 37 | 40 |
|---|---|---|---|---|---|---|---|---|
| kex/kHz | 3.8 ± 0.4 | 4.7 ± 0.3 | 6.3 ± 0.3 | 8.9 ± 0.3 | 12.9 ± 0.4 | 18.6 ± 0.5 | 21.5 ± 0.5 | 26.7 ± 0.6 |
Sample Availability: Samples of the compounds are not available from the authors. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Angelovski, G. Macrocyclic Chelates Bridged by a Diaza-Crown Ether: Towards Multinuclear Bimodal Molecular Imaging Probes. Molecules 2020, 25, 5019. https://doi.org/10.3390/molecules25215019
Wang G, Angelovski G. Macrocyclic Chelates Bridged by a Diaza-Crown Ether: Towards Multinuclear Bimodal Molecular Imaging Probes. Molecules. 2020; 25(21):5019. https://doi.org/10.3390/molecules25215019
Chicago/Turabian StyleWang, Gaoji, and Goran Angelovski. 2020. "Macrocyclic Chelates Bridged by a Diaza-Crown Ether: Towards Multinuclear Bimodal Molecular Imaging Probes" Molecules 25, no. 21: 5019. https://doi.org/10.3390/molecules25215019






