Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation
Abstract
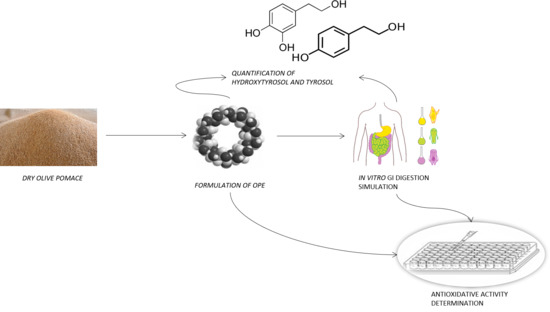
1. Introduction
2. Results and Discussion
2.1. Cell Viability
2.2. Reactive Oxygen Species (ROS) Determination
2.3. GSH Determination
2.4. GPx and SOD Activity Determination
3. Materials and Methods
3.1. Samples
3.2. Reagents
3.3. Quantification of Phenolic Components by HPLC-FLD
3.4. In Vitro Simulated Gastrointestinal Digestion
3.5. Cell Culture
3.6. Preparations of Cells for Cell Viability, ROS and GSH Determination
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silenzi, A.; Giovannini, C.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Santangelo, C.; Masella, R. Extra virgin olive oil polyphenols: Biological properties and antioxidant activity. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–233. [Google Scholar]
- Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 2033. [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macià, A.; Motilva, M.J. Methods for preparing phenolic extracts from olive cake for potential application as food antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Vitali Čepo, D. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Jurmanović, S.; Jug, M.; Safner, T.; Radić, K.; Domijan, A.-M.; Pedisić, S.; Šimić, S.; Jablan, J.; Vitali Čepo, D. Utilization of olive pomace as the source of polyphenols: Optimization of microwave-assisted extraction and characterization of spray-dried extract. J. Food Nutr. Res. 2019, 58, 51–62. [Google Scholar]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Radić, K.; Jurmanović, S.; Jug, M.; Rajković, M.G.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of olive pomace-based nutraceuticals as antioxidants in chemical, food, and biological models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef]
- Veras, K.S.; Silveira Fachel, F.N.; Delagustin, M.G.; Teixeira, H.F.; Barcellos, T.; Henriques, A.T.; Bassani, V.L.; Koester, L.S. Complexation of rosmarinic acid with hydroxypropyl-β-cyclodextrin and methyl-β-cyclodextrin: Formation of 2:1 complexes with improved antioxidant activity. J. Mol. Struct. 2019, 1195, 582–590. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin and cosolvent on the solubility and antioxidant activity of caffeic acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef]
- Radić, K.; Dukovski, B.J.; Čepo, D.V. Influence of pomace matrix and cyclodextrin encapsulation on olive pomace polyphenols’ bioaccessibility and intestinal permeability. Nutrients 2020, 12, 669. [Google Scholar] [CrossRef]
- Thomas-Valdés, S.; Theoduloz, C.; Jiménez-Aspee, F.; Burgos-Edwards, A.; Schmeda-Hirschmann, G. Changes in polyphenol composition and bioactivity of the native Chilean white strawberry (Fragaria chiloensis spp. chiloensis f. chiloensis) after in vitro gastrointestinal digestion. Food Res. Int. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.S.; Gao, N.; Li, T.; Chen, H.; Wang, Y.; Si, X.; Tian, J.; Shu, C.; Luo, S.; Zhang, J.; et al. Effect of In Vitro Digestion on Phytochemical Profiles and Cellular Antioxidant Activity of Whole Grains. J. Agric. Food Chem. 2019, 67, 7016–7024. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.Á.; Ramos, S.; Bravo, L.; Goya, L. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress. Toxicology 2011, 288, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chiesi, C.; Fernandez-Blanco, C.; Cossignani, L.; Font, G.; Ruiz, M.J. Alternariol-induced cytotoxicity in Caco-2 cells. Protective effect of the phenolic fraction from virgin olive oil. Toxicon 2015, 93, 103–111. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; Garrido, A. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits and derived products. J. Agric. Food Chem. 2002, 50, 3835–3839. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Van Camp, J.; Vissenaekens, H.; Raes, K.; Smagghe, G.; Grootaert, C. Review on the Use of Cell Cultures to Study Metabolism, Transport, and Accumulation of Flavonoids: From Mono-Cultures to Co-Culture Systems. Compr. Rev. Food Sci. Food Saf. 2015, 14, 741–754. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar]
- Visioli, F.; Wolfram, R.; Richard, D.; Abdullah, M.I.C.B.; Crea, R. Olive Phenolics increase glutathione levels in healthy volunteers. J. Agric. Food Chem. 2009, 57, 1793–1796. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Mateos, R.; Sarria, B.; Cert, R.; Goya, L.; Bravo, L. Hydroxytyrosyl acetate contributes to the protective effects against oxidative stress of virgin olive oil. Food Chem. 2012, 131, 869–878. [Google Scholar] [CrossRef]
- Lubos, E.; Kelly, N.J.; Oldebeken, S.R.; Leopold, J.A.; Zhang, Y.Y.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. J. Biol. Chem. 2011, 286, 35407–35417. [Google Scholar] [CrossRef] [PubMed]
- Orena, S.; Owen, J.; Jin, F.; Fabian, M.; Gillitt, N.D.; Zeisel, S.H. Extracts of fruits and vegetables activate the antioxidant response element in IMR-32 cells. J. Nutr. 2015, 145, 2006–2011. [Google Scholar] [CrossRef][Green Version]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [PubMed]
- Marinić, J.; Broznić, D.; Milin, Č. Preexposure to Olive Oil Polyphenols Extract Increases Oxidative Load and Improves Liver Mass Restoration after Hepatectomy in Mice via Stress-Sensitive Genes. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Leskovec, J.; Rezar, V.; Svete, A.N. Antioxidative effects of olive polyphenols compared to vitamin e in piglets fed a diet rich in n-3 pufa. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
- Tsarbopoulos, A.; Gikas, E.; Papadopoulos, N.; Aligiannis, N.; Kafatos, A. Simultaneous determination of oleuropein and its metabolites in plasma by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 157–164. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Alía, M.; Ramos, S.; Mateos, R.; Bravo, L.; Goya, L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J. Biochem. Mol. Toxicol. 2005, 19, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Benink, H.A.; Worzlla, T.J.; Minor, L. Cell Viability Assays. Assay Guid. Man. 2004, 1, 1–23. [Google Scholar]
- Held, P. An Introduction to Reactive Oxygen Species Measurement of ROS in Cells. BioTek Instrum. Inc. 2012, 1, 1–14. [Google Scholar]
- Čapek, J.; Hauschke, M.; Brůčková, L.; Roušar, T. Comparison of glutathione levels measured using optimized monochlorobimane assay with those from ortho-phthalaldehyde assay in intact cells. J. Pharmacol. Toxicol. Methods 2017, 88, 40–45. [Google Scholar] [CrossRef]
- Forstrom, J.W.; Zakowski, J.J.; Tappel, A.L. Identification of the Catalytic Site of Rat Liver Glutathione Peroxidase as Selenocysteine†. Biochemistry 1978, 17, 2639–2644. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| γOPE (mg/mL) | γHTS (µM) | γTS (µM) | |
|---|---|---|---|
| nat | 0.8 | - | - |
| nat_bf | 0.8 | - | - |
| hpbCD | 2.5 | - | - |
| hpbCD_bf | 2.5 | - | - |
| mix | - | 3.7 | 1.2 |
| TS | - | - | 20 |
| HTS | - | 20 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, K.; Vinković Vrček, I.; Pavičić, I.; Čepo, D.V. Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation. Molecules 2020, 25, 5027. https://doi.org/10.3390/molecules25215027
Radić K, Vinković Vrček I, Pavičić I, Čepo DV. Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation. Molecules. 2020; 25(21):5027. https://doi.org/10.3390/molecules25215027
Chicago/Turabian StyleRadić, Kristina, Ivana Vinković Vrček, Ivan Pavičić, and Dubravka Vitali Čepo. 2020. "Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation" Molecules 25, no. 21: 5027. https://doi.org/10.3390/molecules25215027
APA StyleRadić, K., Vinković Vrček, I., Pavičić, I., & Čepo, D. V. (2020). Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation. Molecules, 25(21), 5027. https://doi.org/10.3390/molecules25215027