Condition-Dependent Coordination and Peroxidase Activity of Hemin-Aβ Complexes
Abstract
:1. Introduction
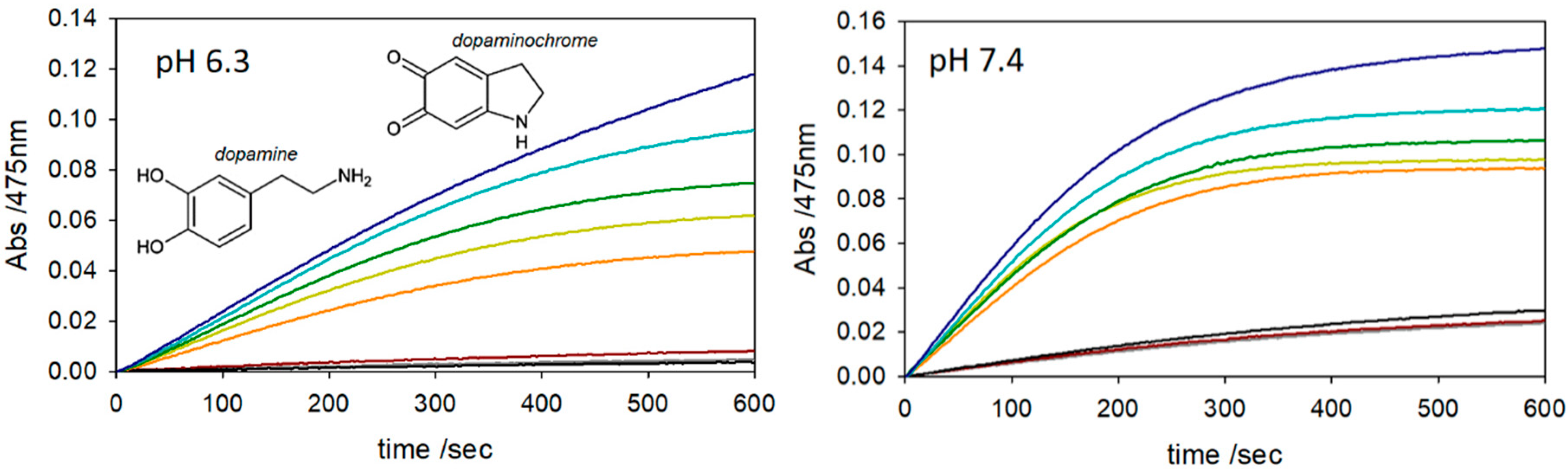
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
| Step 1: [hemin(H2O)2] + Aβ40 ⇄ [hemin(Aβ40)(H2O)] + H2O (K1) |
| Step 2: [hemin(Aβ40)(H2O)] + Aβ40 ⇄ [hemin(Aβ40)2] + H2O (K2) |
| Global: [hemin(H2O)2] + 2 Aβ40 ⇄ [hemin(Aβ40)2] + 2 H2O (β2) |
3.2. Kinetics of ABTS Oxidation
3.3. Kinetics of Dopamine (DA) Oxidation
3.4. Aggregate Formation
3.5. Aβ16 Modification in the presence of Hydrogen Peroxide and Hemin by LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gitler, D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Pievani, M.; Filippini, N.; van den Heuvel, P.M.; Cappa, S.F.; Frisoni, G.B. Brain connectivity in neurodegenerative diseases--from phenotype to proteinopathy. Nat. Rev. Neurol. 2014, 10, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, A.; Hider, R.C. The crucial role of metal ions in neurodegeneration: The basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005, 146, 1041–1059. [Google Scholar] [CrossRef] [Green Version]
- Aizenman, E.; Mastroberardino, P.G. Metals and neurodegeneration. Neurobiol. Dis. 2015, 81, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Cicero, E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Smith, G.; Cappai, R.; Barnahm, K.J. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef] [Green Version]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Khodarahmi, R.; Ashrafi-Kooshk, M.R. Is there correlation between Aβ-heme peroxidase activity and the peptide aggregation state? A literature review combined with hypothesis. Int. J. Biol. Macromol. 2017, 100, 18–36. [Google Scholar] [CrossRef]
- Pramanik, D.; Ghosh, C.; Mukherjee, S.; Dey, S.G. Interaction of amyloid β peptides with redox active heme cofactor: Relevance to Alzheimer’s disease. Coord. Chem. Rev. 2013, 257, 81–92. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Lee, C.-W.; Shih, Y.-H.; Yang, T.; Yu, L.; Kuo, Y.-M. Interactions between amyloid-β and hemoglobin: Implications for amyloid plaque formation in Alzheimer’s disease. PLoS ONE 2012, 7, e33120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Q.; Luo, Y.; Li, W.; Sun, X.; Zhu, C.; Li, P.; Huang, Z.-X.; Tan, X. The mechanism for heme to prevent Aβ1–40 aggregation and its cytotoxicity. J. Biol. Inorg. Chem. 2011, 16, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Thiabaud, G.; Pizzocaro, S.; Garcia-Serres, R.; Latour, J.-M.; Monzani, E.; Casella, L. Heme binding induces dimerization and nitration of truncated β-amyloid peptide Aβ16 under oxidative stress. Angew. Chem. Int. Ed. 2013, 52, 8041–8044. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Boyle, K. Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atamna, H.; Frey, W.H., II. A role for heme in Alzheimer’s disease: Heme binds amyloid β and has altered metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 11153–11158. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Frey, W.H., II; Ko, N. Human and rodent amyloid-beta peptides differentially bind heme: Relevance to the human susceptibility to Alzheimer’s disease. Arc. Biochem. Biophys. 2009, 487, 59–65. [Google Scholar] [CrossRef]
- Yuan, C.; Yu, L.; Yang, Z.; Deng, Q.; Huang, Y.; Li, H.; Gao, Z. Amyloid beta-heme peroxidase promoted protein nitrotyrosination: Relevance to widespread protein nitration in Alzheimer’s disease. J. Biol. Inorg. Chem. 2012, 17, 197–207. [Google Scholar] [CrossRef]
- Ghosh, C.; Mukherjee, S.; Seal, M.; Dey, S.G. Peroxidase to cytochrome b type transition in the active site of heme-bound amyloid β peptides relevant to Alzheimer’s Disease. Inorg. Chem. 2016, 55, 1748–1757. [Google Scholar] [CrossRef]
- Seal, M.; Ghosh, C.; Basu, O.; Dey, S.G. Cytochrome c peroxidase activity of heme bound amyloid β peptides. J. Biol. Inorg. Chem. 2016, 21, 683–690. [Google Scholar] [CrossRef]
- Pramanik, D.; Dey, S.G. Active site environment of heme-bound amyloid β peptide associated with Alzheimer’s Disease. J. Am. Chem. Soc. 2011, 133, 81–87. [Google Scholar] [CrossRef]
- Chiziane, E.; Telemann, H.; Krueger, M.; Adler, J.; Arnhold, J.; Alia, A.; Flemmig, J. Free heme and Amyloid-β: A fatal liaison in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 963–984. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Li, J.; Tian, R.; Peng, Y.-Y. Key roles of Arg(5), Tyr(10) and His residues in Aβ-heme peroxidase: Relevance to Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2014, 452, 676–681. [Google Scholar] [CrossRef]
- Wißbrock, A.; Kuhl, T.; Silbermann, K.; Becker, A.J.; Ohlenschlager, O.; Imhof, D. Synthesis and evaluation of amyloid β derived and amyloid β independent enhancers of the peroxidase-like activity of heme. J. Med. Chem. 2017, 60, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, T.; Imhof, D. Regulatory FeII/III Heme: The reconstruction of a molecule’s biography. ChemBioChem 2014, 15, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.; Halliday, G.M. What is the dominant Abeta species in human brain tissue? A review. Neuro. Res. 2005, 7, 29–41. [Google Scholar] [CrossRef]
- Chesler, M. Regulation and Modulation of pH in the Brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Steen, K.H.; Steen, A.E.; Reeh, P.W. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J. Neurosci. 1995, 15, 3982–3989. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010, 11, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective β-Amyloid clearance pathways in aging Alzheimer’s Disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- De Villiers, K.A.; Kaschula, C.H.; Egan, T.J.; Marques, H.M. Speciation and structure of ferriprotoporphyrin IX in aqueous solution: Spectroscopic and diffusion measurements demonstrate dimerization, but not mu-oxo dimer formation. J. Biol. Inorg. Chem. 2007, 12, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Asher, C.; De Villiers, K.A.; Egan, T.J. Speciation of ferriprotoporphyrin IX in aqueous and mixed aqueous solution is controlled by solvent identity, pH, and salt concentration. Inorg. Chem. 2009, 48, 7994–8003. [Google Scholar] [CrossRef] [PubMed]
- Huffman, D.L.; Rosenblatt, M.M.; Suslick, K.S. Synthetic heme-peptide complexes. J. Am. Chem. Soc. 1998, 120, 6183–6184. [Google Scholar] [CrossRef]
- Pirota, V.; Monzani, E.; Dell’Acqua, S.; Casella, L. Interactions between heme and tau-derived R1 peptides: Binding and oxidative reactivity. Dalton Trans. 2016, 45, 14343–14351. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Liu, L.; Wang, R.; La, X.; Xu, M. Interaction between amyloid-β peptide and heme probed by electrochemistry and atomic force microscopy. ACS Chem. Neurosci. 2013, 4, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, P.A. The peroxidasic activity of the haem octapeptide microperoxidase-8 (MP-8): The kinetic mechanism of the catalytic reduction of H2O2 by MP-8 using 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate)(ABTS) as reducing substrate. J. Chem. Soc. Perkin Trans 2 1990, 1407–1414. [Google Scholar] [CrossRef]
- Sengupta, K.; Chatterjee, S.; Pramanik, D.; Dey, S.G.; Dey, A. Self-assembly of stable oligomeric and fibrillar aggregates of Aβ peptides relevant to Alzheimer’s disease: Morphology dependent Cu/heme toxicity and inhibition of PROS generation. Dalton Trans. 2014, 43, 13377–13383. [Google Scholar] [CrossRef]
- Resende, R.; Ferreiro, E.; Pereira, C.; Resende de Oliveira, C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 2008, 155, 725–737. [Google Scholar] [CrossRef]
- Syme, C.D.; Nadal, R.C.; Rigby, S.E.J.; Viles, J.H. Copper binding to the amyloid-β (Aβ) peptide associated with Alzheimer’s Disease. Folding, coordination geometry, pH dependence, stoichiometry, and affinity of Aβ-(1–28): Insights from range of complementary spectroscopic techniques. J. Biol. Chem. 2004, 279, 18169–18177. [Google Scholar] [CrossRef] [Green Version]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Cappucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef] [PubMed]
- Herlinger, E.; Jameson, R.F.; Linert, W. Spontaneous autoxidation of dopamine. J. Chem. Soc. Perkin Trans. 2 1995, 259–263. [Google Scholar] [CrossRef]
- Ros, J.R.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Tyrosinase: Kinetic analysis of the transient phase and the steady state. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1994, 1204, 33–42. [Google Scholar] [CrossRef]
- Mohammad-Shiri, H.; Ghaemi, M.; Riahi, S.; Akbari-Sehat, A. Computational and electrochemical studies on the redox reaction of dopamine in aqueous solution. Int. J. Electrochem. Sci. 2011, 6, 317–336. [Google Scholar]
- Dunford, B. Peroxidases in Chemistry and Biology; Everse, K.E., Grisham, M.B., Eds.; CRC: Boca Raton, FL, USA, 1991; Volume 2, p. 1. [Google Scholar]
- Pal, I.; Nath, A.K.; Roy, M.; Seal, M.; Ghosh, C.; Dey, A.; Dey, S.G. Formation of compound I in heme bound Aβ-peptides relevant to Alzheimer’s disease. Chem. Sci. 2019, 10, 8405–8410. [Google Scholar] [CrossRef] [PubMed]
- Casella, L.; Monzani, E.; Fantucci, P.; Gullotti, M.; De Gioia, L.; Strini, A.; Chillemi, F. Axial imidazole distortion effects on the catalytic and binding properties of chelated deuterohemin complexes. Inorg. Chem. 1996, 35, 439–444. [Google Scholar] [CrossRef]
- Pirota, V.; Dell’Acqua, S.; Monzani, E.; Nicolis, S.; Casella, L. Copper-Aβ peptides and oxidation of catecholic substrates: Reactivity and endogenous peptide damage. Chem. Eur. J. 2016, 22, 16964. [Google Scholar] [CrossRef]
- Cassagnes, L.-E.; Herve, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The catalytically active copper-amyloid-Beta state: Coordination site responsible for reactive oxygen species production. Angew. Chem. Int. Ed. 2013, 52, 11110–11113. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, H.Y.; Jeon, M.J.; Kim, D.J.; Kim, Y.S. Efficient access to highly pure β-amyloid peptide by optimized solid-phase synthesis. Amyloid 2012, 19, 133–137. [Google Scholar] [CrossRef]
- Mach, H.; Middaugh, C.R.; Lewis, R.V. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 1992, 200, 74–80. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Smith, K.M. Laboratory Methods in Porphyrins and Metalloporphyrins Research; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Esparza, T.J.; LeDuc, R.D.; Fellers, R.T.; Thomas, P.M.; Cairns, N.J.; Kelleher, N.L.; Bateman, R.J.; Brody, D.L. Diversity of amyloid-beta proteoforms in the Alzheimer’s Disease brain. Sci. Rep. 2017, 7, 9520. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Complex a | log K1 | log K2 b | log β2 |
|---|---|---|---|
| [hemin(Aβ40)2] | 6.08 ± 0.02 | 4.77 | 10.84 ± 0.02 |
| [hemin(Aβ40)2] c | 5.60 ± 0.01 | 4.20 | 9.80 ± 0.01 |
| [hemin(Aβ40)2] d | 4.76 ± 0.03 | 4.30 | 9.05 ± 0.01 |
| [hemin(Aβ40)2] e | 5.30 ± 0.02 | 4.47 | 9.77 ± 0.04 |
| [hemin(Aβ40)2] f | 7.30 ± 0.02 | 4.02 | 11.32 ± 0.16 |
| [hemin(Aβ16)2] g | 4.80 ± 0.02 | 4.03 | 8.82 ± 0.02 |
| Species | pH 7.4−Aβ16 kobs (s−1) | pH 7.4−Aβ40 kobs (s−1) | pH 6.3−Aβ16 kobs (s−1) | pH 6.3−Aβ40 kobs (s−1) |
|---|---|---|---|---|
| Hemin | 0.013 | 0.013 | 0.016 | 0.016 |
| Aβ (1 equiv.) | 0.022 | 0.018 | 0.034 | 0.027 |
| Aβ (5 equiv.) | 0.022 | 0.021 | 0.042 | 0.033 |
| Aβ (20 equiv.) | 0.032 | 0.027 | 0.053 | 0.038 |
| Aβ (100 equiv.) | 0.054 | 0.032 | 0.092 | 0.049 |
| Species | pH 7.4−Aβ16 kobs (s−1) | pH 7.4−Aβ40 kobs (s−1) | pH 6.3−Aβ16 kobs (s−1) | pH 6.3−Aβ40 kobs (s−1) |
|---|---|---|---|---|
| Hemin | 0.053 | 0.052 | 0.018 | 0.018 |
| Aβ (1 equiv.) | 0.049 | 0.058 | 0.024 | 0.023 |
| Aβ (5 equiv.) | 0.056 | 0.060 | 0.023 | 0.027 |
| Aβ (20 equiv.) | 0.077 | 0.068 | 0.037 | 0.031 |
| Aβ (100 equiv.) | 0.171 | 0.076 | 0.076 | 0.034 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacchella, C.; Brewster, J.T., II; Bähring, S.; Dell’Acqua, S.; Root, H.D.; Thiabaud, G.D.; Reuther, J.F.; Monzani, E.; Sessler, J.L.; Casella, L. Condition-Dependent Coordination and Peroxidase Activity of Hemin-Aβ Complexes. Molecules 2020, 25, 5044. https://doi.org/10.3390/molecules25215044
Bacchella C, Brewster JT II, Bähring S, Dell’Acqua S, Root HD, Thiabaud GD, Reuther JF, Monzani E, Sessler JL, Casella L. Condition-Dependent Coordination and Peroxidase Activity of Hemin-Aβ Complexes. Molecules. 2020; 25(21):5044. https://doi.org/10.3390/molecules25215044
Chicago/Turabian StyleBacchella, Chiara, James T. Brewster, II, Steffen Bähring, Simone Dell’Acqua, Harrison D. Root, Gregory D. Thiabaud, James F. Reuther, Enrico Monzani, Jonathan L. Sessler, and Luigi Casella. 2020. "Condition-Dependent Coordination and Peroxidase Activity of Hemin-Aβ Complexes" Molecules 25, no. 21: 5044. https://doi.org/10.3390/molecules25215044






