Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages
Abstract
:1. Introduction
2. Results
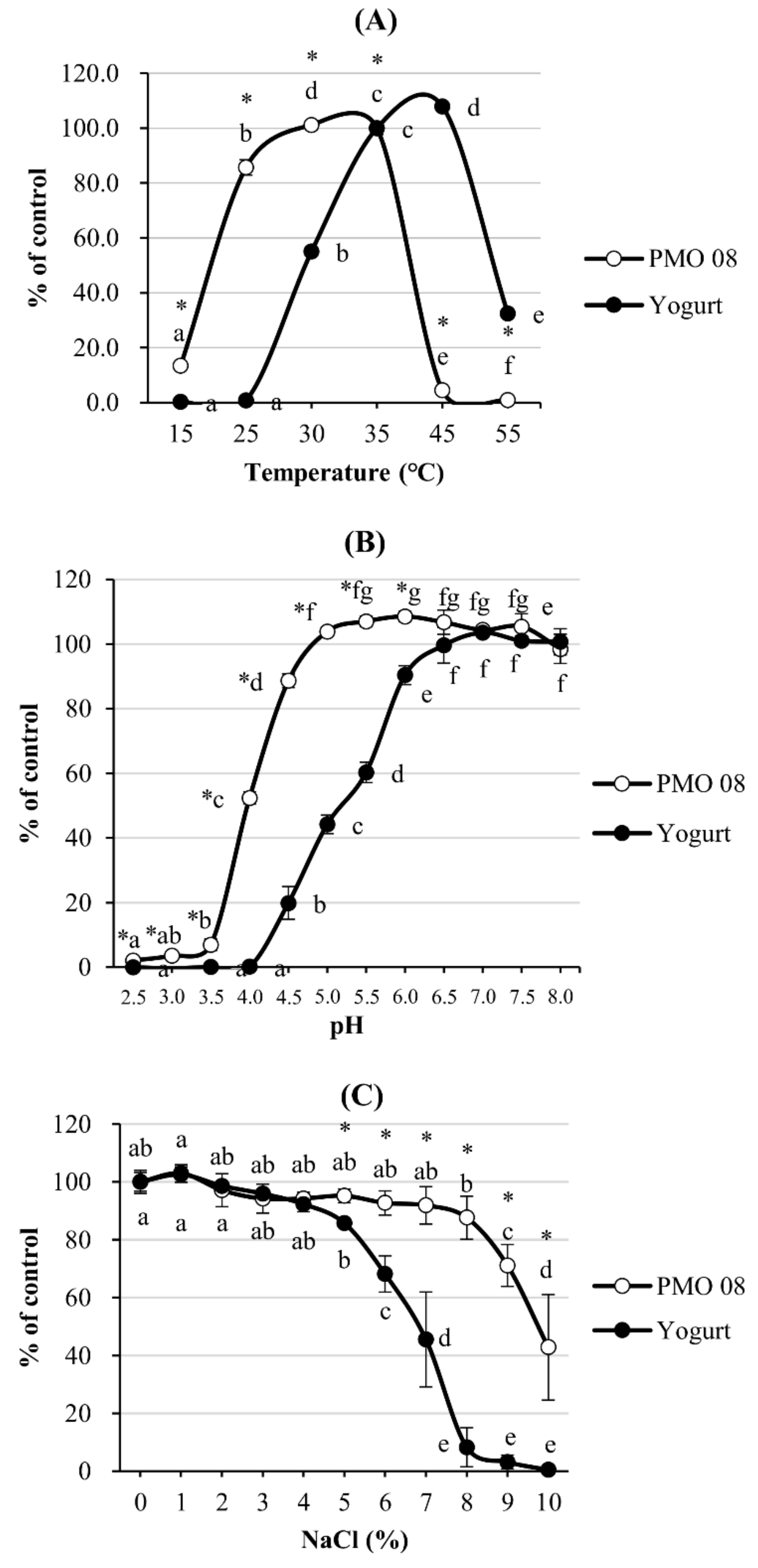
2.1. Effect of Incubation Temperature, pH, and NaCl Concentration on L. plantarum PMO 08 Viability
2.2. Acidification Ability of Plant-Based Substrates
2.3. Physicochemical and Microbial Characterization of the Plant-Based Probiotic Beverages
2.4. Viability in Artificial Gastrointestinal Juice
2.5. Polyphenol Content and Antioxidant Activity
2.6. Free Amino Acid Composition
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Effect of Temperature, pH, and NaCl Concentration on Bacterial Viability
4.3. Preparation of Plant Culture Substrate and Acidification Ability
4.4. Preparation of a Plant-Based Probiotic Beverage
4.5. Analysis of Brix, pH, and Titratable Acidity
4.6. Microbial Analysis
4.7. Tolerance to Artificial Gastrointestinal Juice
4.8. DPPH Radical Scavenging Activity
4.9. Measurement of Total Polyphenol Content
4.10. Measurement of Free Amino Acid Composition
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial ecology of fermented vegetables and non-alcoholic drinks and current knowledge on their impact on human health. Adv. Food. Nutr. Res. 2019, 87, 147–185. [Google Scholar] [PubMed]
- Food and Agriculture Organization; World Health Organization. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional in Food Properties of Probiotics Including Powder Milk with Live LAB, FAO/WHO: Cordoba, Argentina, 2001.
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Front. Microbiol. 2019, 9, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit beverage: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijaya Kumar, B.; Vijayendra, S.V.; Reddy, O.V. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant polyphenol-based second-generation synbiotic agents: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic product. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res. Int. 2018, 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, I.K.; Kim, T.S.; Tae, S.G. Microorganism, Lactobacillus Plantarum PMO 08 (KFCC-11028), Decreasing Blood Cholesterol Concentration. KR. Patent 0264361, 30 May 2000. [Google Scholar]
- OH, Y.J.; Kim, H.J.; Kim, T.S.; Yeo, I.H.; Ji, G.E. Effects of Lactobacillus plantarum PMO 08 alone and combined with chia seeds on metabolic syndrome and parameters related to gut health in high-fat diet-induced obese mice. J. Med. Food. 2019, 22, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Hyun, Y.J.; Oh, Y.J.; Choi, K.B.; Kim, T.S.; Yeo, I.H.; Han, M.J.; Kim, D.H. Adhesion activity of Lactobacillus plantarum PMO 08 isolated from Kimchi on the intestine of mice. J. Bacteriol. Virol. 2011, 41, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.E.; Hyun, Y.J.; Trinh, H.T.; Han, M.J.; Kim, D.H. Anti-scratching behavioral effect of Lactobacillus plantarum PMO 08 isolated from kimchi in mice. Immunopharmacol. Immunotoxicol. 2011, 33, 539–544. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandr, G. Phytochemical composition and antioxidant activity of high lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. (Amsterdam) 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. 2004, 42, 315–318. [Google Scholar]
- Mzidi, M.; Katsiki, N.; George, E.S.; Banach, M. Tomato and lycopene consumption is inversely associated with total and cause-specific mortality: A population-based cohort study, on behalf of the international lipid expert panel (ILEP). Br. J. Nutr. 2019, 22, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization; World Health Organization. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. FAO/WHO: London, ON, Canada, 30 April–1 May 2002.
- Stroehle, L.; Zweytick, G.; Berghofer, E. Sauerkraut fermentation with L(+)-lactic acid producing-bacteria. Ernaehrung 2006, 30, 293–303. [Google Scholar]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Tsen, J.H.; Lin, Y.P.; Huang, H.Y.; King, V.A. Studies on the fermentation of tomato juice by using k-carrageenan immobilized Lactobacillus acidophilus. J. Food Process. Preserv. 2008, 32, 178–189. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; da Costa, W.C.; de Sousa, O.K.; Franco, O.L. Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Front. Microbiol. 2016, 7, 1371–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mater, D.D.; Bretigny, L.; Firmesse, O.; Flores, M.J.; Mogenet, A.; Bresson, J.L. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 2010, 250, 185–187. [Google Scholar]
- Rudolph, A.S.; Crowe, J.H. Membrane stabilization during freezing: The role of two natural cryoprotectants, trehalose and proline. Cryobiology 1985, 22, 367–377. [Google Scholar] [CrossRef]
- Samuel, D.; Kumar, T.K.; Ganesh, G.; Jayaraman, G.; Yang, P.W.; Chang, M.M.; Trivedi, V.D.; Wang, S.L.; Hwang, K.C.; Chang, D.K.; et al. Proline inhibits aggregation during protein refolding. Protein Sci. 2000, 9, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Wang, Y.; Chu, J.; Zhuang, Y.; Zhang, S. Enhanced L-lactic acid production in Lactobacillus paracasei by exogenous proline addition based on comparative metabolite profiling analysis. Appl. Microbiol. Biotechnol. 2016, 100, 2301–2310. [Google Scholar] [CrossRef]
- Parlindungan, E.; May, B.K.; Jones, O. Metabolic insights into the effects of nutrient stress on Lactobacillus plantarum B21. Front. Mol. Biosci. 2019, 6, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.C.; Chu, C.C.; Ko, T.L.; Yeh, C.L.; Yeh, S.L. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur. J. Nutr. 2013, 52, 1089–1098. [Google Scholar] [CrossRef]
- Andrade, M.E.; Araújo, R.S.; de Barros, P.A.; Soares, A.D.; Abrantes, F.A.; Generoso Sde, V.; Fernandes, S.O.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Roy, N.C.; Frutos, M.J.; Anderson, R.C. Influence of the fruit juice carriers on the ability of Lactobacillus plantarum DSM 20205 to improve in vitro intestinal barrier integrity and its probiotic properties. J. Agric. Food Chem. 2017, 65, 5632–5638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial effects of tomato juice fermented by Lactobacillus plantarum and Lactobacillus casei: Antioxidation, antimicrobial effect, and volatile profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.H.; Kim, Y.S.; Oh, J.H. Chemical characterization of tomato juice fermented with Bifidobacteria. J. Food Sci. 2010, 75, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Hsu, C.K.; Chou, S.T.; Chen, Y.C.; Huang, F.S.; Chung, Y.C. Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int. J. Food Sci. Technol. 2009, 44, 799–806. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.; Ghaedian, R.; Shetty, K. Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol. 2007, 21, 217–236. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Rio, D.D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of dairy and plant-derived lactobacilli as starters for cherry juice fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Sulhvir, K.; Harjot, P.K.; Jyotsana, G. Fermentation of tomato juice by probiotic lactic acid bacteria. Int. J. Pharm. Biol. Sci. 2016, 5, 212–219. [Google Scholar]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chen, H.; Jin, L.; Chang, Q.; Peng, T.; Hu, X.; Fan, C.; Pang, G.; Lu, M.; Wang, W. Discrimination of botanical origins for Chinese honey according to free amino acids content by high-performance liquid chromatography with fluorescence detection with chemometric approaches J. Sci. Food Agric. 2017, 97, 2042–2049. [Google Scholar] [CrossRef]



| Non-Fermented Beverage | Fermented Beverage | ||
|---|---|---|---|
| Control | PMO 08 | Yogurt | |
| °Brix (%) | 4.66 ± 0.02 NS | 4.63 ± 0.03 | 4.36 ± 0.01 |
| Titratable acidity (%) | 0.312 ± 0.016 a | 0.958 ± 0.002 c | 0.867 ± 0.003 b |
| pH | 4.47 ± 0.01 a | 3.39 ± 0.03 c | 3.60 ± 0.03 b |
| Escherichia coli/coliforms(Log10CFU/mL) | ND | ND | ND |
| Yeast and molds(Log10CFU/mL) | ND | ND | ND |
| Total aerobic bacteria(Log10CFU/mL) | ND | - | - |
| Lactic acid bacteria (Log10CFU/mL) | - | 9.78 ± 0.14 * | 7.64 ± 0.27 |
| Amino Acid (mg/100 g) | Non-Fermented Beverage | Fermented Beverage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | PMO 08 | Yogurt | |||||||
| Threonine | 6.22 | ± | 0.005 a | 4.30 | ± | 0.065 c | 5.50 | ± | 0.050 b |
| Tyrosine | 2.66 | ± | 0.001 a | 0.94 | ± | 0.025 c | 2.21 | ± | 0.015 b |
| Arginine | 2.44 | ± | 0.010 a | 1.16 | ± | 0.025 c | 1.76 | ± | 0.010 b |
| Alanine | 14.63 | ± | 0.005 a | 8.58 | ± | 0.110 c | 10.91 | ± | 0.080 b |
| Proline | 4.36 | ± | 0.040 c | 4.82 | ± | 0.210 a | 4.71 | ± | 0.285 ab |
| Lysine | 2.58 | ± | 0.030 a | 1.52 | ± | 0.190 b | 1.26 | ± | 0.155 b |
| Histidine | 3.87 | ± | 0.045 a | 3.26 | ± | 0.055 c | 3.55 | ± | 0.015 b |
| Isoleucine | 2.12 | ± | 0.010 a | 0.00 | ± | 0.000 c | 0.67 | ± | 0.045 b |
| Leucine | 2.37 | ± | 0.135 a | 0.25 | ± | 0.000 c | 1.16 | ± | 0.035 b |
| Methionine | 0.74 | ± | 0.050 a | 0.00 | ± | 0.000 c | 0.00 | ± | 0.000 b |
| Phenylalanine | 7.33 | ± | 0.005 a | 4.91 | ± | 0.045 c | 6.02 | ± | 0.005 b |
| Valine | 1.66 | ± | 0.026 a | 0.00 | ± | 0.00 c | 0.72 | ± | 0.06 b |
| Glutamic acid | 126.05 | ± | 0.020 a | 109.78 | ± | 1.97 c | 107.86 | ± | 1.61 b |
| Aspartic acid | 70.00 | ± | 1.780 a | 65.05 | ± | 0.27 b | 59.55 | ± | 2.14 c |
| Serine | 6.48 | ± | 0.010 a | 2.86 | ± | 0.06 c | 5.81 | ± | 0.02 b |
| Glycine | 1.35 | ± | 0.005 a | 0.75 | ± | 0.02 c | 0.77 | ± | 0.07 b |
| Glutamine | 0.21 | ± | 0.005 c | 0.83 | ± | 0.00 b | 3.79 | ± | 0.39 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules 2020, 25, 5056. https://doi.org/10.3390/molecules25215056
Oh YJ, Kim TS, Moon HW, Lee SY, Lee SY, Ji GE, Hwang KT. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules. 2020; 25(21):5056. https://doi.org/10.3390/molecules25215056
Chicago/Turabian StyleOh, Young Joo, Tae Seok Kim, Hwang Woo Moon, So Young Lee, Sang Yun Lee, Geun Eog Ji, and Keum Taek Hwang. 2020. "Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages" Molecules 25, no. 21: 5056. https://doi.org/10.3390/molecules25215056





