Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. General Methodology for 13C-NMR Dereplication of Metabolite Mixtures Diluted in Glycerin
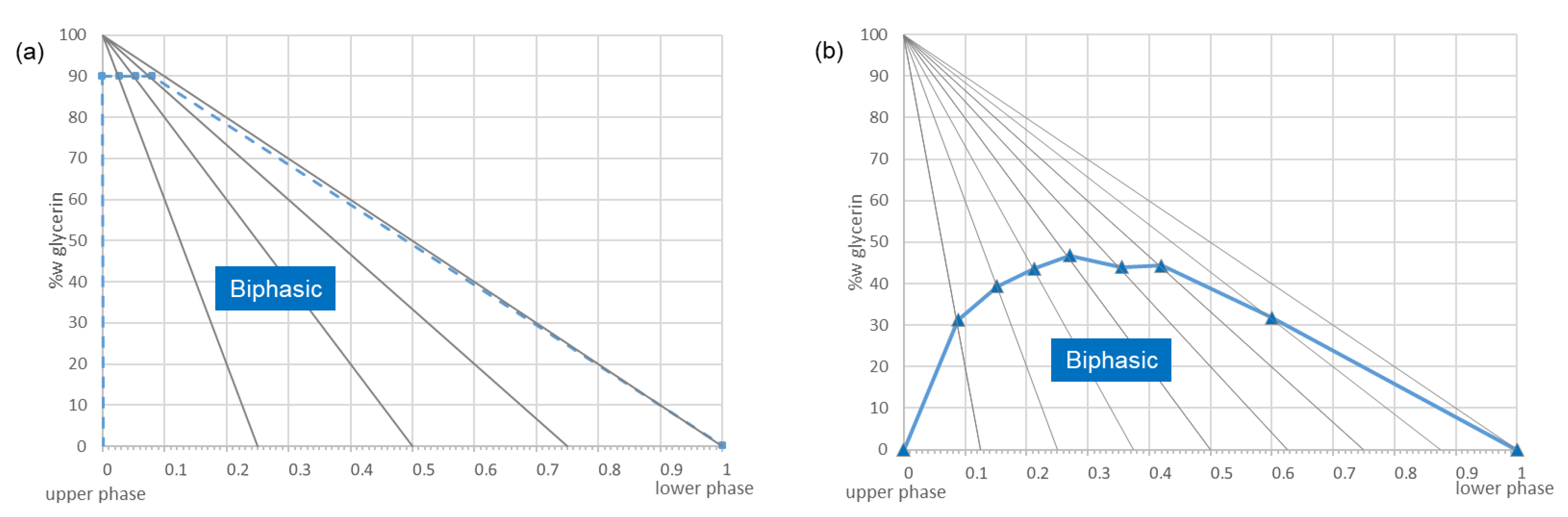
2.2. Proof of Concept on a Model Mixture
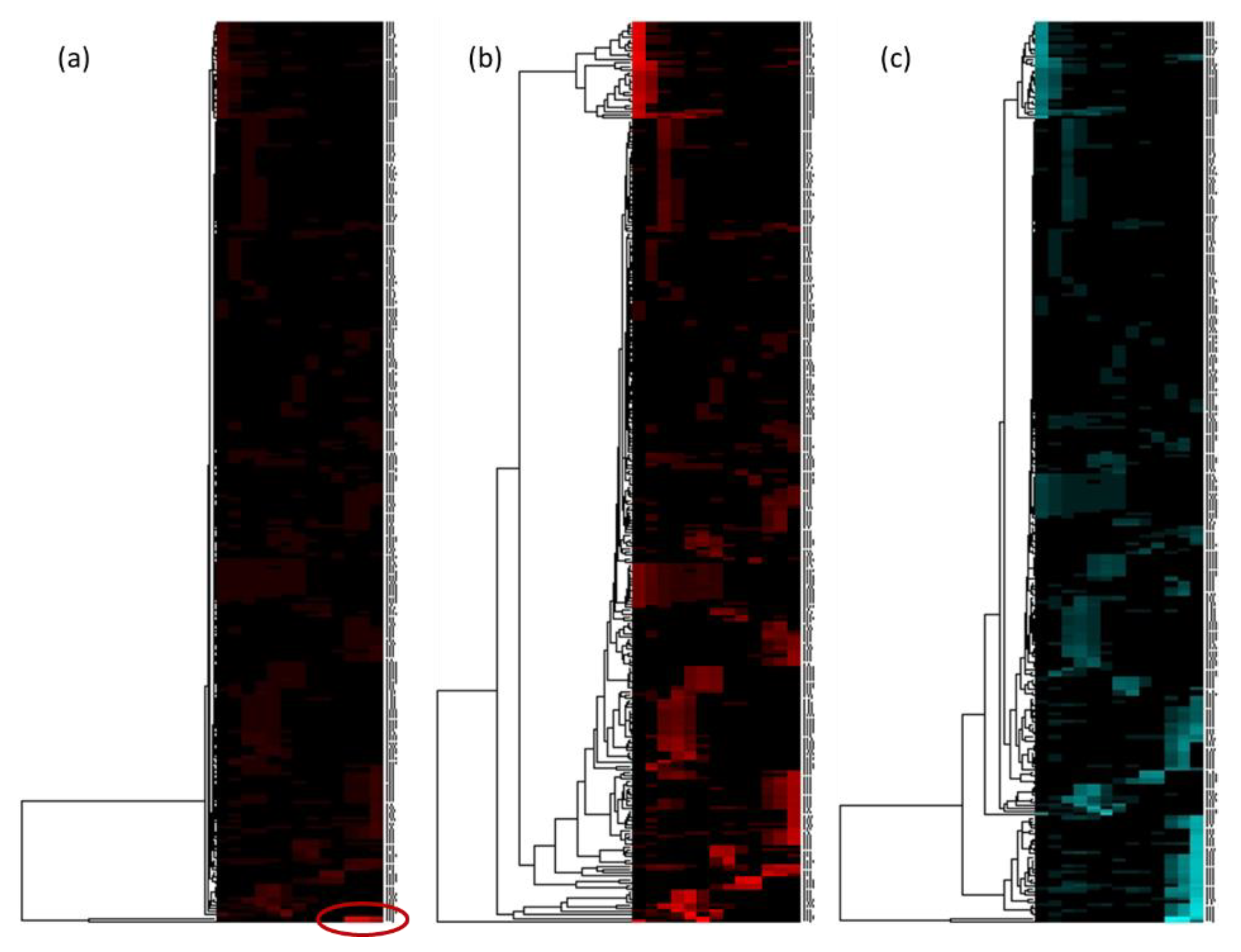
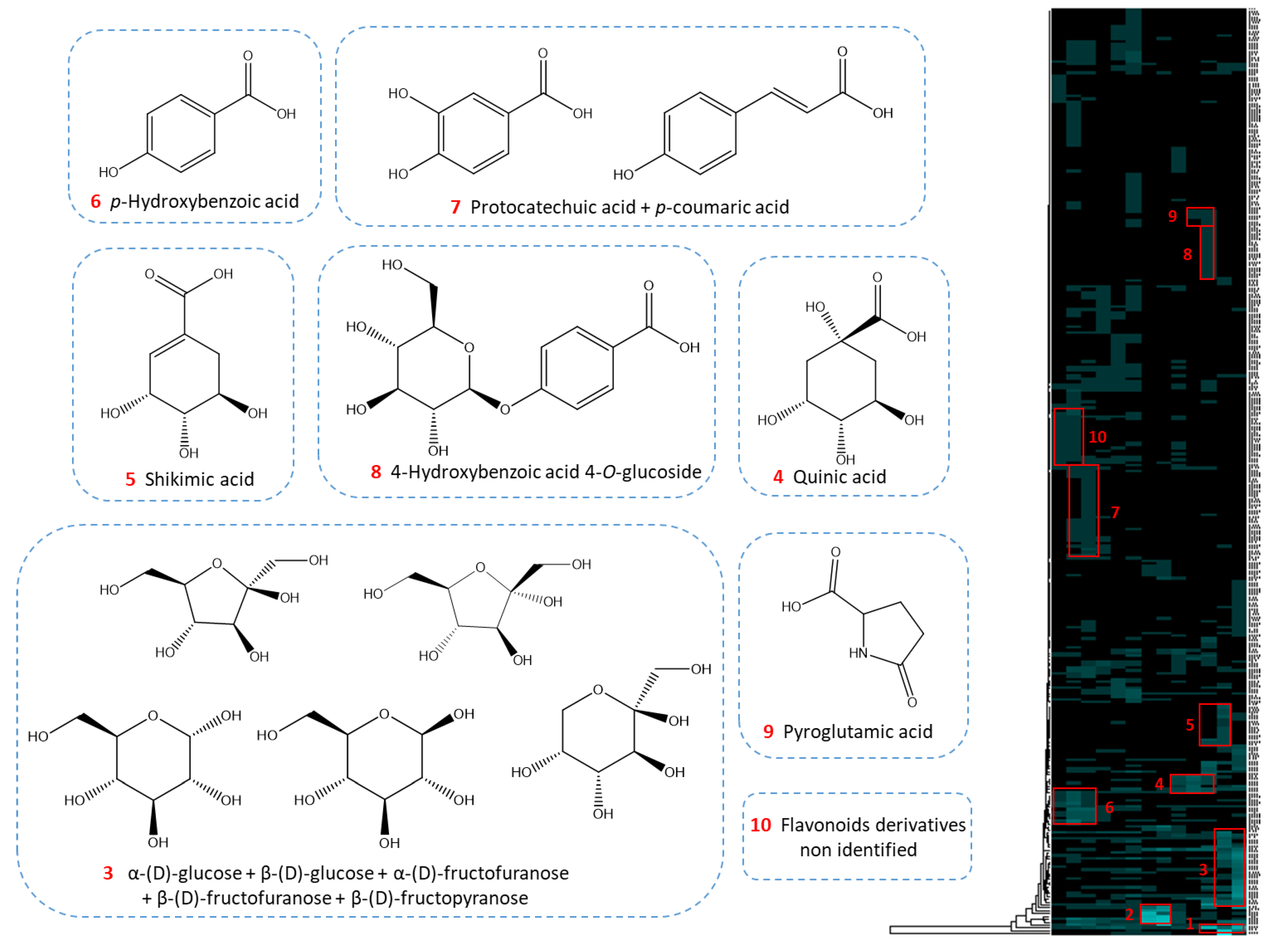
2.3. Dereplication of a Genuine Natural Extract Diluted in Glycerin
3. Materials and Methods
3.1. Chemical and Natural Extract
3.2. Distribution Coefficients Determination
3.3. LC-MS Analyses
3.4. Centrifugal Partition Chromatography
3.5. Nuclear Magnetic Resonance
3.6. Data Processing
3.7. High-Performance Thin-Layer Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kusumawati, I.; Indrayanto, G. Natural Antioxidants in Cosmetics. Stud. Nat. Prod. Chem. 2013, 15, 485–505. [Google Scholar] [CrossRef]
- Hughes, K.; Ho, R.; Butaud, J.-F.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. A selection of eleven plants used as traditional Polynesian cosmetics and their development potential as anti-aging ingredients, hair growth promoters and whitening products. J. Ethnopharmacol. 2019, 245, 112159. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- European Commission. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 9th Revision; Adopted at the 11th Plenary Meeting of SCCS; European Commission: Luxembourg, 2016. [Google Scholar]
- Fernandez, X.; Michel, T.; Kerdudo, A. Analyse des principes actifs et substances réglementées en cosmétique. Technique de l’ingénieur. 2015; Ref J3300 V1. 1–30. (In French) [Google Scholar]
- Demirci, F. Natural products isolation. In Methods in Biotechnology, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Hubert, J.; Nuzillard, J.-M.; Renault, J.-H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 2015, 16, 55–95. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nuzillard, J.-M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.-H. Identification of Natural Metabolites in Mixture: A Pattern Recognition Strategy Based on 13C NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef]
- Beutler, J.A.; Alvarado, A.B.; Schaufelberger, D.E.; Andrews, P.; McCloud, T.G. Dereplication of Phorbol Bioactives: Lyngbya majuscula and Croton cuneatus. J. Nat. Prod. 1990, 53, 867–874. [Google Scholar] [CrossRef]
- Corley, D.G.; Durley, R.C. Strategies for Database Dereplication of Natural Products. J. Nat. Prod. 1994, 57, 1484–1490. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef]
- Hubert, J.; Angelis, A.; Aligiannis, N.; Rosalia, M.; Abedini, A.; Bakiri, A.; Reynaud, R.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, L.A.; et al. In Vitro Dermo-Cosmetic Evaluation of Bark Extracts from Common Temperate Trees. Planta Medica 2016, 82, 1351–1358. [Google Scholar] [CrossRef]
- Tisserant, L.-P.; Hubert, J.; Lequart, M.; Borie, N.; Maurin, N.; Pilard, S.; Jeandet, P.; Aziz, A.; Renault, J.-H.; Nuzillard, J.-M.; et al. 13C NMR and LC-MS Profiling of Stilbenes from Elicited Grapevine Hairy Root Cultures. J. Nat. Prod. 2016, 79, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Martiny, L.; Courot, E.; Jeandet, P.; Tarpin, M.; Renault, J.-H.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Herman, A. Antimicrobial Ingredients as Preservative Booster and Components of Self-Preserving Cosmetic Products. Curr. Microbiol. 2018, 76, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Oh, J.Y.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2014, 37, 31–40. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Ardi, M.; Aroua, M.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Canton, M.; Roe, R.; Poigny, S.; Renault, J.-H.; Nuzillard, J.-M. Multiple solvent signal presaturation and decoupling artifact removal in 13C{1H} nuclear magnetic resonance. Magn. Reson. 2020, 1, 155–164. [Google Scholar] [CrossRef]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent Separation of Natural Products: An Update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; Hazekamp, A.; Hoek, A.C.; Balconi, S.; Verpoorte, R. Application of centrifugal partition chromatography in a general separation and dereplication procedure for plant extracts. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2195–2208. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Counter-current chromatography as a preparative tool—applications and perspectives. J. Chromatogr. A 1994, 658, 315–341. [Google Scholar] [CrossRef]
- Hubert, J.; Chollet, S.; Purson, S.; Reynaud, R.; Harakat, D.; Martinez, A.; Nuzillard, J.-M.; Renault, J.-H. Exploiting the Complementarity between Dereplication and Computer-Assisted Structure Elucidation for the Chemical Profiling of Natural Cosmetic Ingredients: Tephrosia purpurea as a Case Study. J. Nat. Prod. 2015, 78, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Lehbili, M.; Magid, A.A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef]
- Schmitt, M.; Magid, A.A.; Hubert, J.; Etique, N.; Duca, L.; Voutquenne-Nazabadioko, L. Bio-guided isolation of new phenolic compounds from Hippocrepis emerus flowers and investigation of their antioxidant, tyrosinase and elastase inhibitory activities. Phytochem. Lett. 2020, 35, 28–36. [Google Scholar] [CrossRef]
- Marchal, L.; Intes, O.; Foucault, A.; Legrand, J.; Nuzillard, J.-M.; Renault, J.-H. Rational improvement of centrifugal partition chromatographic settings for the production of 5-n-alkylresorcinols from wheat bran lipid extract I. Flooding conditions—Optimizing the injection step. J. Chromatogr. A 2003, 1, 51–62. [Google Scholar] [CrossRef]
- Renault, J.-H.; Nuzillard, J.-M.; Intes, O.; Maciuk, A. Chapter 3: Solvent systems. In Comprehensive Analytical Chromatography: Countercurrent Chromatography; Elsevier BV: Amsterdam, The Netherlands, 2002; Volume 38, pp. 49–83. [Google Scholar]
- Berthod, A.; Carda-Broch, S. Determination of liquid–liquid partition coefficients by separation methods. J. Chromatogr. A 2004, 1037, 3–14. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Ho, C.-T.; YuJun, L.; Sun, L.; Liu, Y. Effects of thermal treatments on 10 major phenolics and their antioxidant contributions in Acer truncatum leaves and flowers. R. Soc. Open Sci. 2018, 5, 180364. [Google Scholar] [CrossRef]
- Rodríguez, H.; Rivas, B.D.L.; Gómez-Cordovés, C.; Muñoz, R. Degradation of tannic acid by cell-free extracts of Lactobacillus plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Douros, A.; Hadjipavlou-Litina, D.; Nikolaou, K.; Skaltsa, H. The Occurrence of Flavonoids and Related Compounds in Cedrus brevifolia A. Henry ex Elwes & A. Henry Needles. Inhibitory Potencies on Lipoxygenase, Linoleic Acid Lipid Peroxidation and Antioxidant Activity. Plants 2017, 7, 1. [Google Scholar] [CrossRef]
- Dakir, M.; El Hanbali, F.; Mellouki, F.; Akssira, M.; Benharref, A.; Del Moral, J.F.Q.; Barrero, A.F.; Del Moral, J.F.Q. Antibacterial diterpenoids from Cedrus atlantica. Nat. Prod. Res. 2005, 19, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Quilezdelmoral, J.; Herrador, M.M.; Arteaga, J.F.; Akssira, M.; Benharref, A.; Dakir, M. Abietane diterpenes from the cones of Cedrus atlantica. Phytochemistry 2005, 66, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.-M.; Paoli, M.; Castola, V.; Casanova, J.; Bighelli, A. Identification and quantitative determination of lignans in Cedrus atlantica resins using 13C NMR spectroscopy. Nat. Prod. Commun. 2011, 6. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Compound | Chemical Family | Formula | Rf | Retention Time (min) | Exact Mass (g/mol) | Scan Mode | Individual KD by LC-MS | KD in Mixture |
|---|---|---|---|---|---|---|---|---|
| Caffeine | Alkaloid | C8H10N4O2 | 0.39 | 3.63 | 194.0804 | ESI+ | 1.13 ± 0.09 | 1.03 ± 0.01 |
| Chlorogenic acid | Hydroxycinnamic acid | C16H18O9 | 0.11 | 3.69 | 354.0951 | ESI− | 1.11 ± 0.09 | 1.23 ± 0.01 |
| Ferulic acid | Hydroxycinnamic acid | C10H10O4 | 0.66 | 5.42 | 194.0579 | ESI− | 0.14 ± 0.01 | 0.17 ± 0.01 |
| Glycyrrhizin | Saponin | C42H62O16 | 0.04 | 10.15 | 822.4038 | ESI− | 2.09 ± 0.13 | 0.78 ± 0.02 |
| Linoleic acid | Fatty acid | C18H32O2 | 0.81 | 14.67 | 280.2402 | ESI− | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Polydatin | Stilbene | C20H22O8 | 0.21 | 5.55 | 390.1315 | ESI− | 0.62 ± 0.02 | 0.72 ± 0.01 |
| Quercetin | Flavonoid | C15H10O7 | 0.63 | 7.81 | 302.0427 | ESI+ | 0.02 ± 0.01 | 0.05 ± 0.01 |
| Rutin | Flavonoid | C27H30O16 | 0.04 | 5.61 | 610.1534 | ESI+ | 5.28 ± 0.34 | 2.52 ± 0.14 |
| Succinic acid | Organic acid | C4H6O4 | - | 0.80 | 118.0266 | ESI− | 1.18 ± 0.09 | 1.24 ± 0.14 |
| Vanillin | Other phenolic | C8H8O3 | 0.66 | 4.55 | 152.0473 | ESI+ | 0.08 ± 0.01 | 0.17 ± 0.01 |
| Compound | δ in F12 | δ in F13 | Δδ |
|---|---|---|---|
| α-(D)-glucose | 92.94 | 92.71 | 0.23 |
| β-(D)-glucose | 97.51 | 97.35 | 0.16 |
| α-(D)-fructofuranose | 104.89 | 104.65 | 0.24 |
| β-(D)-fructopyranose | 102.65 | 102.47 | 0.18 |
| β-(D)-fructopyranose | 98.79 | 98.59 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canton, M.; Hubert, J.; Poigny, S.; Roe, R.; Brunel, Y.; Nuzillard, J.-M.; Renault, J.-H. Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy. Molecules 2020, 25, 5061. https://doi.org/10.3390/molecules25215061
Canton M, Hubert J, Poigny S, Roe R, Brunel Y, Nuzillard J-M, Renault J-H. Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy. Molecules. 2020; 25(21):5061. https://doi.org/10.3390/molecules25215061
Chicago/Turabian StyleCanton, Marine, Jane Hubert, Stéphane Poigny, Richard Roe, Yves Brunel, Jean-Marc Nuzillard, and Jean-Hugues Renault. 2020. "Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy" Molecules 25, no. 21: 5061. https://doi.org/10.3390/molecules25215061
APA StyleCanton, M., Hubert, J., Poigny, S., Roe, R., Brunel, Y., Nuzillard, J.-M., & Renault, J.-H. (2020). Dereplication of Natural Extracts Diluted in Glycerin: Physical Suppression of Glycerin by Centrifugal Partition Chromatography Combined with Presaturation of Solvent Signals in 13C-Nuclear Magnetic Resonance Spectroscopy. Molecules, 25(21), 5061. https://doi.org/10.3390/molecules25215061








