Role of Inositols and Inositol Phosphates in Energy Metabolism
Abstract
:1. Introduction
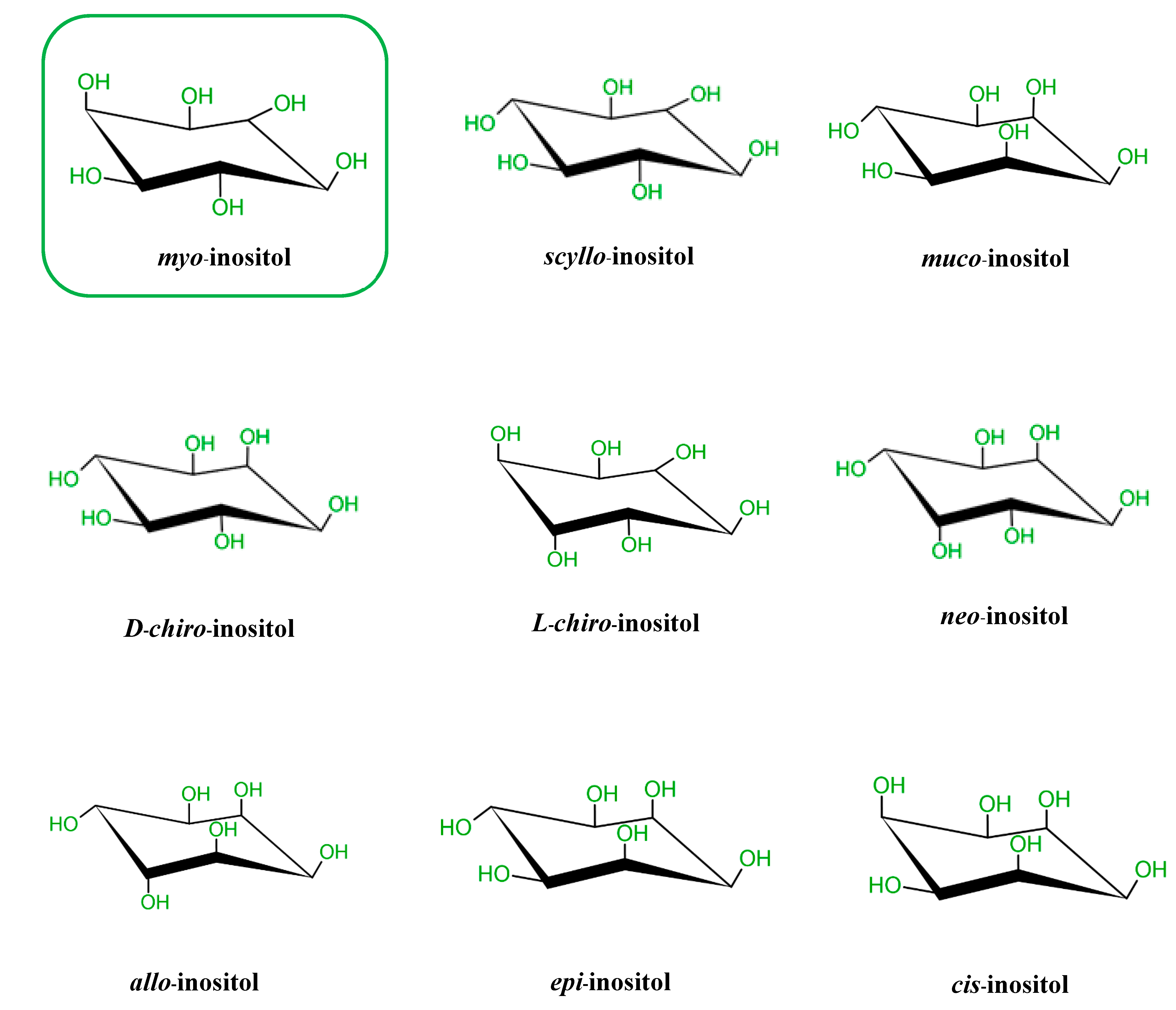
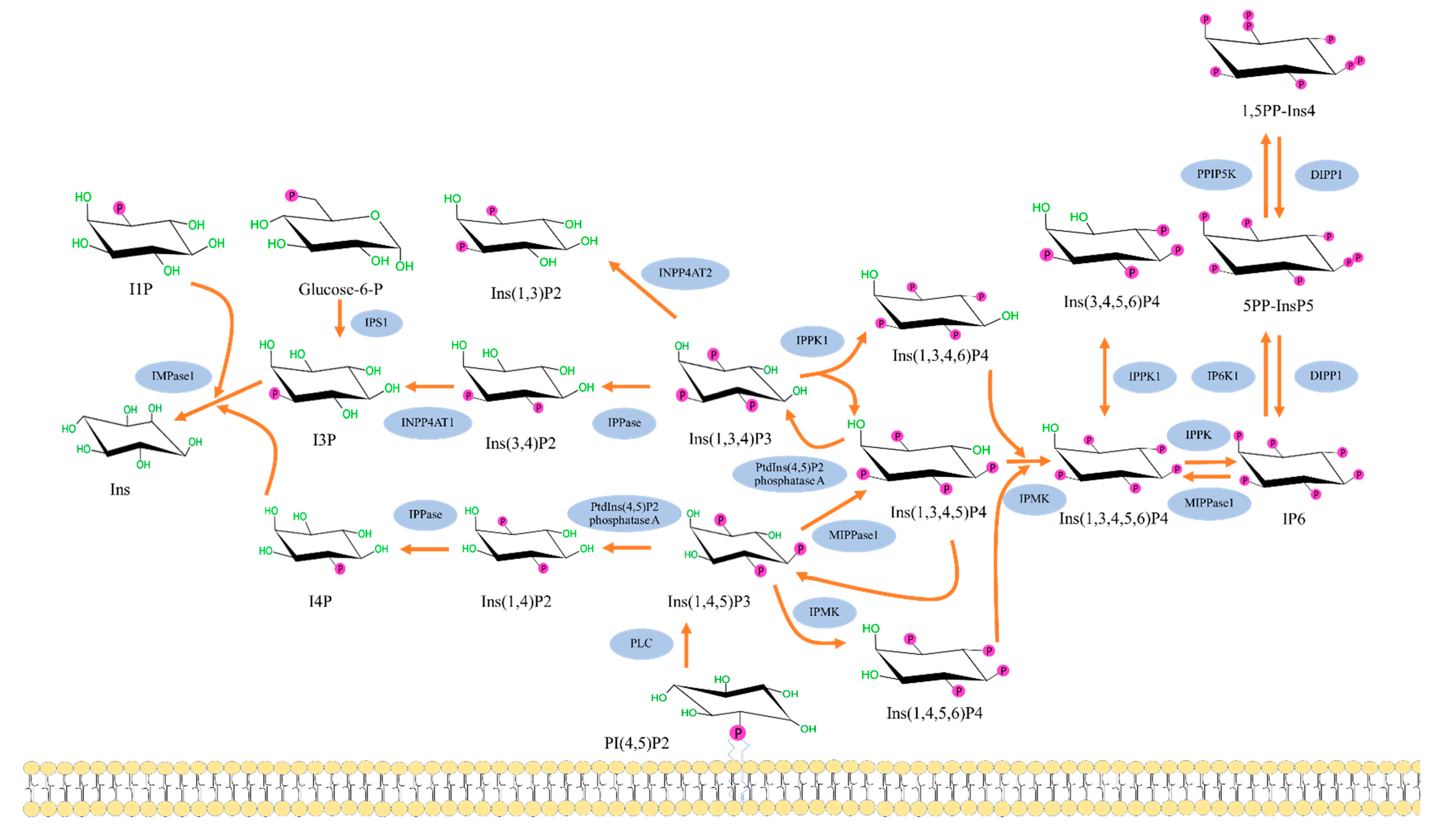
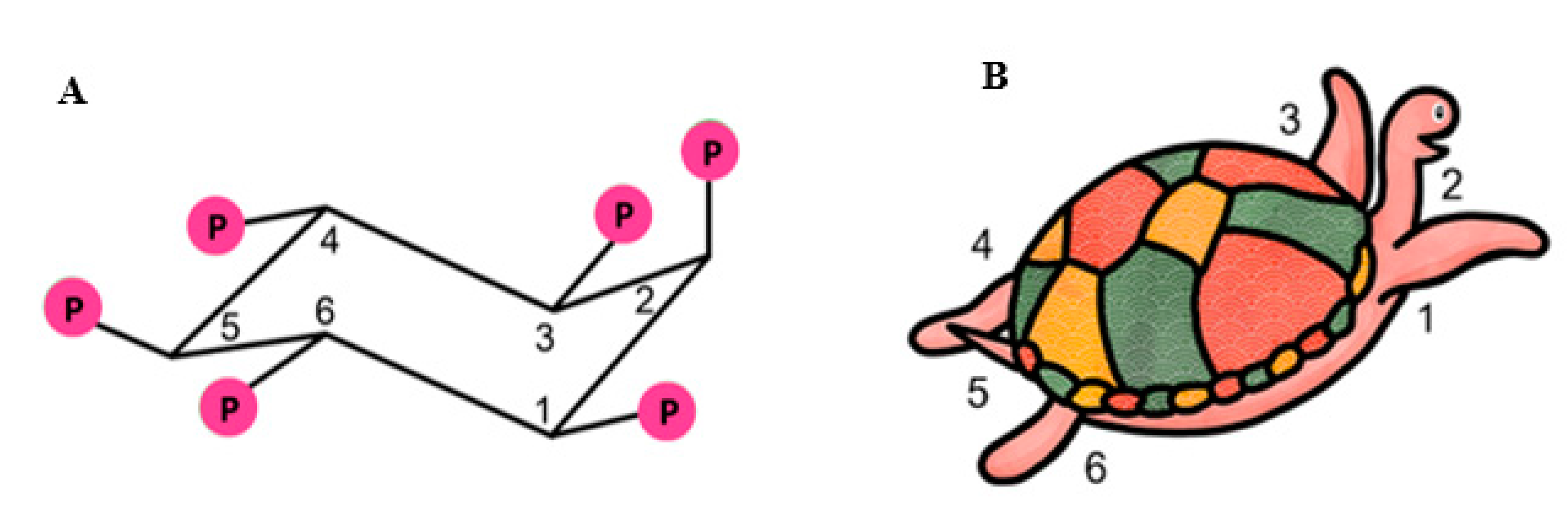
2. Biochemistry of Inositols and Inositol Phosphates
3. Biological Roles and Activities
4. Effects on Insulin Resistance and Energy Metabolism
4.1. Basic Pathophysiology of Insulin Resistance
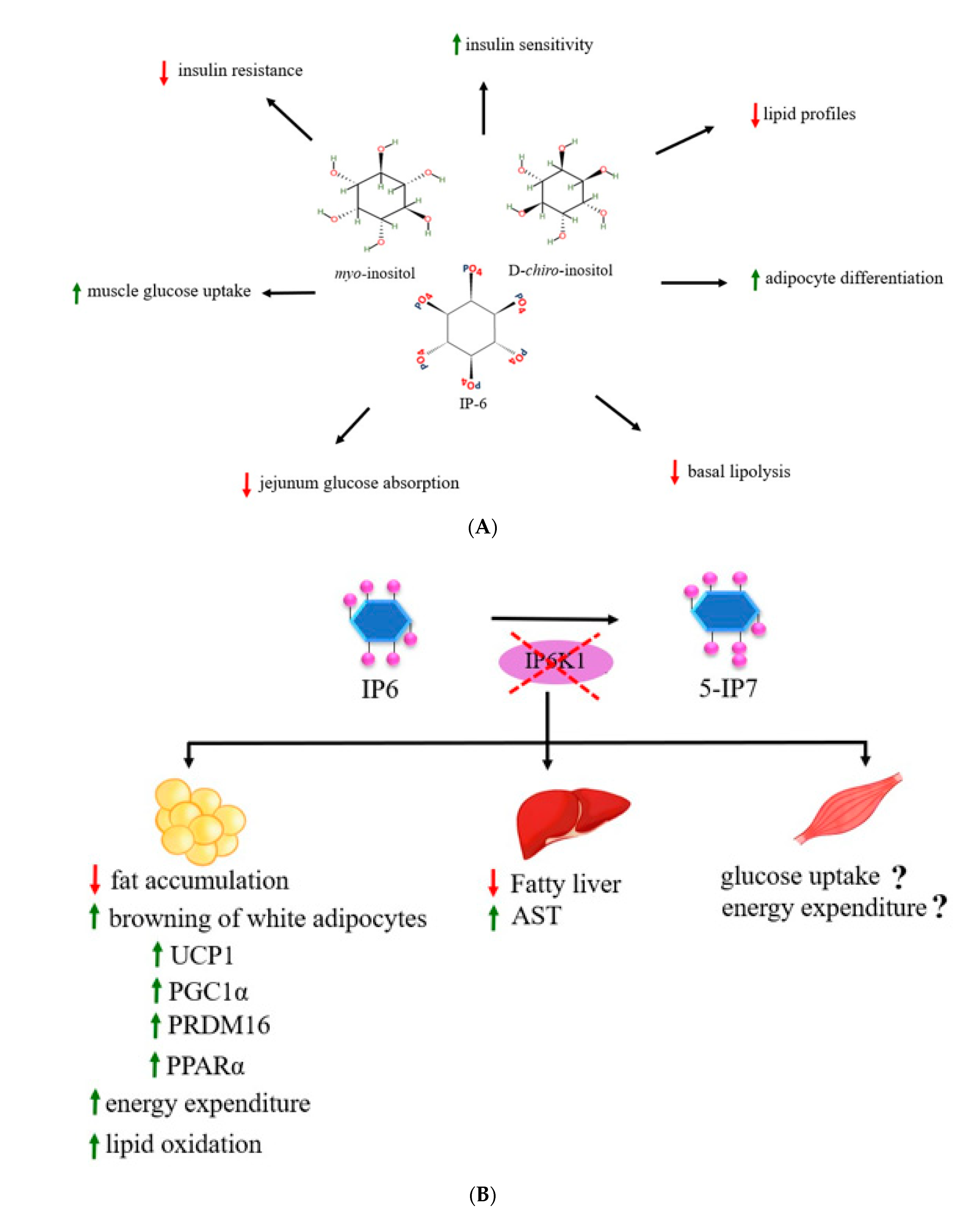
4.2. Inositols, Inositol Phosphates and Insulin Resistance
4.3. Inositol Phosphates on Obesity and Metabolic Parameters
4.4. Inositols and Inositol Phosphates in Energy Metabolism
5. Other Health-Beneficial Effects
6. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Irvine, R.F. A short history of inositol lipids. J. Lipid Res. 2016, 57, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, A.K.; Bose, S. IP6 (Inositol Hexaphosphate) as a Signaling Molecule. Curr. Cancer Rev. 2012, 7, 289–304. [Google Scholar] [CrossRef]
- Biswas, S.; Maity, I.B.; Chakrabarti, S.; Biswas, B.B. Purification and characterization of myo-Inositol hexaphosphate-adenosine diphosphate phosphotransferase from Phaseolus aureus. Arch. Biochem. Biophys. 1978, 185, 557–566. [Google Scholar] [CrossRef]
- Vucenik, I. Anticancer Properties of Inositol Hexaphosphate and Inositol: An Overview. J. Nutr. Sci. Vitam. (Tokyo) 2019, 65, S18–S22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, R.K.; Raison, J.K. A complete intracellular unit for incorporation of amino-acid into storage protein utilizing adenosine triphosphate generated from phytate. Nature 1963, 200, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F. Editorial-Update on Inositol(s). Eur. Rev. Med. Pharm. Sci. 2017, 21, 1–3. [Google Scholar]
- Thomas, M.P.; Mills, S.J.; Potter, B.V. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in myo-Inositol Chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650. [Google Scholar] [CrossRef] [Green Version]
- Al-Suod, H.; Ligor, M.; Rațiu, I.-A.; Rafińska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Tanaka, K.; Natsume, A.; Ishikawa, S.; Takenaka, S.; Yoshida, K.I. A new-generation of Bacillus subtilis cell factory for further elevated scyllo-inositol production. Microb. Cell Fact 2017, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, A.; Nandy Datta, P. Myo-inositol 1-phosphate synthase-The chosen path of evolution. Biotechnologia 2016, 97, 95–108. [Google Scholar] [CrossRef]
- Agranoff, B.W. Turtles All the Way: Reflections on myo-Inositol. J. Biol. Chem. 2009, 284, 21121–21126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desfougeres, Y.; Wilson, M.S.C.; Laha, D.; Miller, G.J.; Saiardi, A. ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 24551–24561. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Ganguli, S.; Sen, J.; Bhandari, R. Inositol Pyrophosphates: Energetic, Omnipresent and Versatile Signalling Molecules. J. Indian Inst. Sci. 2017, 97, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, A.M.; Vucenik, I.; Cole, K.E. IP6: A novel anti-cancer agent. Life Sci. 1997, 61, 343–354. [Google Scholar] [CrossRef]
- Brehm, M.A.; Schenk, T.M.; Zhou, X.; Fanick, W.; Lin, H.; Windhorst, S.; Nalaskowski, M.M.; Kobras, M.; Shears, S.B.; Mayr, G.W. Intracellular localization of human Ins(1,3,4,5,6)P5 2-kinase. Biochem. J. 2007, 408, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Li, C.; Marcu, A.; Badran, H.; Pon, A.; Budinski, Z.; Patron, J.; Lipton, D.; Cao, X.; Oler, E.; et al. PathBank: A comprehensive pathway database for model organisms. Nucleic Acids Res. 2020, 48, D470–D478. [Google Scholar] [CrossRef]
- Mukherjee, S.; Haubner, J.; Chakraborty, A. Targeting the Inositol Pyrophosphate Biosynthetic Enzymes in Metabolic Diseases. Molecules 2020, 25, 1403. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, S.S.; Kim, J.; Gaboardi, G.-C.; Gromada, J.; Shears, S.B.; Dos Santos, K.T.; Nolasco, E.L.; Ferreira, S.D.S.; Illies, C.; Köhler, M.; et al. Inositol hexakisphosphate kinase 1 is a metabolic sensor in pancreatic β-cells. Cell Signal. 2018, 46, 120–128. [Google Scholar] [CrossRef]
- Chakraborty, A.; Koldobskiy, M.A.; Bello, N.T.; Maxwell, M.; Potter, J.J.; Juluri, K.R.; Maag, D.; Kim, S.; Huang, A.S.; Dailey, M.J.; et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 2010, 143, 897–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Ghoshal, S.; Rodrigues, A.; Gao, S.; Asterian, A.; Kamenecka, T.M.; Barrow, J.C.; Chakraborty, A. Adipocyte-specific deletion of Ip6k1 reduces diet-induced obesity by enhancing AMPK-mediated thermogenesis. J. Clin. Investig. 2016, 126, 4273–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szijgyarto, Z.; Garedew, A.; Azevedo, C.; Saiardi, A. Influence of inositol pyrophosphates on cellular energy dynamics. Science 2011, 334, 802–805. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in legumes and cereals. Adv. Food Res. 1982, 28, 1–92. [Google Scholar]
- Vucenik, I.; Shamsuddin, A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: From laboratory to clinic. J. Nutr. 2003, 133, 3778s–3784s. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Irvine, R.F. Inositide evolution-towards turtle domination? J. Physiol. 2005, 566, 295–300. [Google Scholar] [CrossRef]
- Scherer, P.C.; Ding, Y.; Liu, Z.; Xu, J.; Mao, H.; Barrow, J.C.; Wei, N.; Zheng, N.; Snyder, S.H.; Rao, F. Inositol hexakisphosphate (IP6) generated by IP5K mediates cullin-COP9 signalosome interactions and CRL function. Proc. Natl. Acad. Sci. USA 2016, 113, 3503–3508. [Google Scholar] [CrossRef] [Green Version]
- Hokin, M.R.; Hokin, L.E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J. Biol. Chem. 1953, 203, 967–977. [Google Scholar] [CrossRef]
- Europe-Finner, G.N.; Gammon, B.; Newell, P.C. Accumulation of [3H]-inositol into inositol polyphosphates during development of Dictyostelium. Biochem. Biophys. Res. Commun. 1991, 181, 191–196. [Google Scholar] [CrossRef]
- Letcher, A.J.; Schell, M.J.; Irvine, R.F. Do mammals make all their own inositol hexakisphosphate? Biochem. J. 2008, 416, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Zhang, X.; Liu, L.; Fu, Q.; Zang, C.; Ding, Y.; Su, Y.; Xu, Z.; He, S.; Yang, X.; et al. Basis for metabolite-dependent Cullin-RING ligase deneddylation by the COP9 signalosome. Proc. Natl. Acad. Sci. USA 2020, 117, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, M.R.; Schubert, H.L.; Vandemark, A.P.; Lingam, A.T.; Hill, C.P.; Bass, B.L. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science 2005, 309, 1534–1539. [Google Scholar] [CrossRef] [Green Version]
- Okamura, M.; Yamanaka, Y.; Shigemoto, M.; Kitadani, Y.; Kobayashi, Y.; Kambe, T.; Nagao, M.; Kobayashi, I.; Okumura, K.; Masuda, S. Depletion of mRNA export regulator DBP5/DDX19, GLE1 or IPPK that is a key enzyme for the production of IP6, resulting in differentially altered cytoplasmic mRNA expression and specific cell defect. PLoS ONE 2018, 13, e0197165, reprinted in PLoS ONE 2019, 14, e0220511. [Google Scholar] [CrossRef]
- Folkmann, A.W.; Noble, K.N.; Cole, C.N.; Wente, S.R. Dbp5, Gle1-IP6 and Nup159: A working model for mRNP export. Nucleus 2011, 2, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe, V.O.; Savill, J.M.; Chavali, S.; Jonsdottir, A.B.; Rajendra, E.; Grüner, T.; Laskey, R.A.; Babu, M.M.; Venkitaraman, A.R. Human inositol polyphosphate multikinase regulates transcript-selective nuclear mRNA export to preserve genome integrity. Mol. Cell 2013, 51, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Byrum, J.; Jordan, S.; Safrany, S.T.; Rodgers, W. Visualization of inositol phosphate-dependent mobility of Ku: Depletion of the DNA-PK cofactor InsP6 inhibits Ku mobility. Nucleic Acids Res. 2004, 32, 2776–2784. [Google Scholar] [CrossRef] [Green Version]
- Watson, P.J.; Fairall, L.; Santos, G.M.; Schwabe, J.W.R. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 2012, 481, 335–340. [Google Scholar] [CrossRef]
- Millard, C.J.; Watson, P.J.; Celardo, I.; Gordiyenko, Y.; Cowley, S.M.; Robinson, C.V.; Fairall, L.; Schwabe, J.W.R. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol. Cell 2013, 51, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Marcum, R.D.; Radhakrishnan, I. Inositol phosphates and core subunits of the Sin3L/Rpd3L histone deacetylase (HDAC) complex up-regulate deacetylase activity. J. Biol. Chem. 2019, 294, 13928–13938. [Google Scholar] [CrossRef] [PubMed]
- Toste Rêgo, A.; da Fonseca, P.C.A. Characterization of Fully Recombinant Human 20S and 20S-PA200 Proteasome Complexes. Mol. Cell 2019, 76, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Wang, Y.; Yu, T.; Huang, Y.; Li, M.; Saeed, A.; Perčulija, V.; Li, D.; Xiao, J.; Wang, D.; et al. Cryo-EM structures of the human PA200 and PA200-20S complex reveal regulation of proteasome gate opening and two PA200 apertures. PLoS Biol. 2020, 18, e3000654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.S.; Jessen, H.J.; Saiardi, A. The inositol hexakisphosphate kinases IP6K1 and -2 regulate human cellular phosphate homeostasis, including XPR1-mediated phosphate export. J. Biol. Chem. 2019, 294, 11597–11608. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.L.; Mason, A.C.; Glass, L.; Aditi; Wente, S.R. Nup42 and IP (6) coordinate Gle1 stimulation of Dbp5/DDX19B for mRNA export in yeast and human cells. Traffic 2017, 18, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Aryanpur, P.P.; Regan, C.A.; Collins, J.M.; Mittelmeier, T.M.; Renner, D.M.; Vergara, A.M.; Brown, N.P.; Bolger, T.A. Gle1 Regulates RNA Binding of the DEAD-Box Helicase Ded1 in Its Complex Role in Translation Initiation. Mol. Cell Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Lieber, M.R. Binding of inositol hexakisphosphate (IP6) to Ku but not to DNA-PKcs. J. Biol. Chem. 2002, 277, 10756–10759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanakahi, L.A.; Bartlet-Jones, M.; Chappell, C.; Pappin, D.; West, S.C. Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell 2000, 102, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gu, C.; Hostachy, S.; Sahu, S.; Wittwer, C.; Jessen, H.J.; Fiedler, D.; Wang, H.; Shears, S.B. Control of XPR1-dependent cellular phosphate efflux by InsP(8) is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3568–3574. [Google Scholar] [CrossRef]
- López-Sánchez, U.; Tury, S.; Nicolas, G.; Wilson, M.S.; Jurici, S.; Ayrignac, X.; Courgnaud, V.; Saiardi, A.; Sitbon, M.; Battini, J.L. Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J. Biol. Chem. 2020, 295, 9366–9378. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Bizzarri, M.; Dinicola, S.; Cucina, A. Modulation of both Insulin Resistance and Cancer Growth by Inositol. Curr. Pharm. Des. 2017, 23, 5200–5210. [Google Scholar] [CrossRef]
- Segre, G.; Turco, G.L.; Vercellone, G. Modeling blood glucose and insulin kinetics in normal, diabetic and obese subjects. Diabetes 1973, 22, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Tsao, Y.-C.; Tzeng, I.S.; Chuang, H.-H.; Li, W.-C.; Tung, T.-H.; Chen, J.-Y. Body mass index and waist circumference are better predictors of insulin resistance than total body fat percentage in middle-aged and elderly Taiwanese. Medicine 2017, 96, e8126. [Google Scholar] [CrossRef]
- Cerf, M. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R.; Cuatrecasas, P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc. Natl. Acad. Sci. USA 1986, 83, 5793–5797. [Google Scholar] [CrossRef] [Green Version]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharm. 2012, 165, 1288–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawa, J.M.; Taylor, C.G.; Przybylski, R. Buckwheat concentrate reduces serum glucose in streptozotocin-diabetic rats. J. Agric. Food Chem. 2003, 51, 7287–7291. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, M.; Nordio, M.; Pajalich, R. The Combined therapy myo-inositol plus D-Chiro-inositol, in a physiological ratio, reduces the cardiovascular risk by improving the lipid profile in PCOS patients. Eur. Rev. Med. Pharm. Sci. 2013, 17, 537–540. [Google Scholar]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Myoinositol and D-Chiro Inositol in Improving Insulin Resistance in Obese Male Children: Preliminary Data. Int. J. Endocrinol. 2016, 2016, 8720342. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, A.; Bizzarri, M. Inositols in Insulin Signaling and Glucose Metabolism. Int. J. Endocrinol. 2018, 2018, 1968450. [Google Scholar] [CrossRef] [Green Version]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of Myo-Inositol and D-Chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef] [Green Version]
- Chukwuma, C.I.; Ibrahim, M.A.; Islam, M.S. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: A dual approach study. J. Physiol. Biochem. 2016, 72, 791–801. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar] [CrossRef]
- Santamaria, A.; Alibrandi, A.; Di Benedetto, A.; Pintaudi, B.; Corrado, F.; Facchinetti, F.; D’Anna, R. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: A secondary analysis from 3 RCTs. Am. J. Obs. Gynecol. 2018, 219, e1–e300. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F.; Orru, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.U.; Button, C.L.; Jenkins, D.J. Phytic acid and calcium affect the in vitro rate of navy bean starch digestion and blood glucose response in humans. Am. J. Clin. Nutr. 1987, 46, 467–473. [Google Scholar] [CrossRef]
- Foster, S.R.; Omoruyi, F.O.; Bustamante, J.; Lindo, R.L.; Dilworth, L.L. The effect of combined inositol hexakisphosphate and inositol supplement in streptozotocin-induced type 2 diabetic rats. Int. J. Exp. Pathol. 2016, 97, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Han, S.N.; Kim, H.K. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2014, 34, 723–731. [Google Scholar] [CrossRef]
- Alvarez, L.; Avila, M.A.; Mato, J.M.; Castaño, J.G.; Varela-Nieto, I. Insulin-like effects of inositol phosphate-glycan on messenger RNA expression in rat hepatocytes. Mol. Endocrinol. 1991, 5, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Efanov, A.M.; Zaitsev, S.V.; Berggren, P.O. Inositol hexakisphosphate stimulates non-Ca2+-mediated and primes Ca2+-mediated exocytosis of insulin by activation of protein kinase C. Proc. Natl. Acad. Sci. USA 1997, 94, 4435–4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høy, M.; Efanov, A.M.; Bertorello, A.M.; Zaitsev, S.V.; Olsen, H.L.; Bokvist, K.; Leibiger, B.; Leibiger, I.B.; Zwiller, J.; Berggren, P.O.; et al. Inositol hexakisphosphate promotes dynamin I- mediated endocytosis. Proc. Natl. Acad. Sci. USA 2002, 99, 6773–6777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37–43. [Google Scholar] [CrossRef]
- Bhandari, R.; Juluri, K.R.; Resnick, A.C.; Snyder, S.H. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 2349–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaraldi, T.P.; Oh, D.K.; Christiansen, L.; Nikoulina, S.E.; Kong, A.P.; Baxi, S.; Mudaliar, S.; Henry, R.R. Tissue-specific expression and regulation of GSK-3 in human skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E891–E898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brondani, L.A.; Assmann, T.S.; Duarte, G.C.; Gross, J.L.; Canani, L.H.; Crispim, D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metab. 2012, 56, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosell, M.; Kaforou, M.; Frontini, A.; Okolo, A.; Chan, Y.W.; Nikolopoulou, E.; Millership, S.; Fenech, M.E.; MacIntyre, D.; Turner, J.O.; et al. Brown and white adipose tissues: Intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E945–E964. [Google Scholar] [CrossRef] [Green Version]
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus white adipose tissue: A mini-review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef]
- Townsend, K.; Tseng, Y.-H. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 2012, 1, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Ghoshal, S.; Tyagi, R.; Chakraborty, A. Global IP6K1 deletion enhances temperature modulated energy expenditure which reduces carbohydrate and fat induced weight gain. Mol. Metab. 2017, 6, 73–85. [Google Scholar] [CrossRef]
- Kalra, B.; Kalra, S.; Sharma, J.B. The inositols and polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2016, 20, 720–724. [Google Scholar] [CrossRef]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. d-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef]
- Fan, C.; Liang, W.; Wei, M.; Gou, X.; Han, S.; Bai, J. Effects of D-Chiro-Inositol on Glucose Metabolism in db/db Mice and the Associated Underlying Mechanisms. Front. Pharm. 2020, 11, 354. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Goode, J.; Paz, J.C.; Ouyang, K.; Screaton, R.; Fischer, W.H.; Chen, J.; Tabas, I.; Montminy, M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 2012, 485, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.D. Insulin signaling: Inositol phosphates get into the Akt. Cell 2010, 143, 861–863. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A. The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1203–1227. [Google Scholar] [CrossRef]
- Ghoshal, S.; Tyagi, R.; Zhu, Q.; Chakraborty, A. Inositol hexakisphosphate kinase-1 interacts with perilipin1 to modulate lipolysis. Int. J. Biochem. Cell Biol. 2016, 78, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.S.; de Maré, S.; Jones, H.A.; Göransson, O.; Lindkvist-Petersson, K. Visualization of lipid directed dynamics of perilipin 1 in human primary adipocytes. Sci. Rep. 2017, 7, 15011. [Google Scholar] [CrossRef]
- Moritoh, Y.; Oka, M.; Yasuhara, Y.; Hozumi, H.; Iwachidow, K.; Fuse, H.; Tozawa, R. Inositol Hexakisphosphate Kinase 3 Regulates Metabolism and Lifespan in Mice. Sci. Rep. 2016, 6, 32072. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Gorecki, R.J.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols-A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [Green Version]
- McLaurin, J.; Kierstead, M.E.; Brown, M.E.; Hawkes, C.A.; Lambermon, M.H.; Phinney, A.L.; Darabie, A.A.; Cousins, J.E.; French, J.E.; Lan, M.F.; et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006, 12, 801–808. [Google Scholar] [CrossRef]
- Yoshida, K.I.; Ishikawa, S. Production of scyllo-Inositol: Conversion of Rice Bran into a Promising Disease-Modifying Therapeutic Agent for Alzheimer’s Disease. J. Nutr. Sci. Vitam. 2019, 65, S139–S142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, J.; Thorner, M.; Brautigan, D.; Larner, J.; Klein, W.L. Protection against the synaptic targeting and toxicity of Alzheimer’s-associated Aβ oligomers by insulin mimetic chiro-inositols. Faseb. J. 2013, 27, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anekonda, T.S.; Wadsworth, T.L.; Sabin, R.; Frahler, K.; Harris, C.; Petriko, B.; Ralle, M.; Woltjer, R.; Quinn, J.F. Phytic acid as a potential treatment for alzheimer’s pathology: Evidence from animal and in vitro models. J. Alzheimers Dis. 2011, 23, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Vassar, R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.K.; Taniguchi, M. Identification of myo-inositol hexakisphosphate (IP6) as a β-secretase 1 (BACE1) inhibitory molecule in rice grain extract and digest. FEBS Open Bio 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, T.; Kishi, T.; Matsuda, Y.; Iwata, N. A meta-analysis of inositol for depression and anxiety disorders. Hum. Psychopharmacol. 2014, 29, 55–63. [Google Scholar] [CrossRef]
- Taylor, M.J.; Wilder, H.; Bhagwagar, Z.; Geddes, J. Inositol for depressive disorders. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Chiappelli, J.; Rowland, L.M.; Wijtenburg, S.A.; Muellerklein, F.; Tagamets, M.; McMahon, R.P.; Gaston, F.; Kochunov, P.; Hong, L.E. Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology 2015, 40, 2157–2164. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, P.M.; di Fiore, J.M. Myo-inositol Effects on the Developing Respiratory Neural Control System. Adv. Exp. Med. Biol. 2018, 1071, 159–166. [Google Scholar]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Benvenga, S.; Micali, A.; Pallio, G.; Vita, R.; Malta, C.; Puzzolo, D.; Irrera, N.; Squadrito, F.; Altavilla, D.; Minutoli, L. Effects of Myo-inositol Alone and in Combination with Seleno-Lmethionine on Cadmium-Induced Testicular Damage in Mice. Curr. Mol. Pharm. 2019, 12, 311–323. [Google Scholar] [CrossRef]
- Benvenga, S.; Marini, H.R.; Micali, A.; Freni, J.; Pallio, G.; Irrera, N.; Squadrito, F.; Altavilla, D.; Antonelli, A.; Ferrari, S.M.; et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients 2020, 12, 1222. [Google Scholar] [CrossRef]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vucenik, I.; Ramakrishna, G.; Tantivejkul, K.; Anderson, L.M.; Ramljak, D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res. Treat 2005, 91, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, A.A.; Grases, F.; Monroy, N.; Mari, B.; Vicente-Herrero, M.T.; Tur, F.; Perello, J. Protective effect of myo-inositol hexaphosphate (phytate) on bone mass loss in postmenopausal women. Eur. J. Nutr. 2013, 52, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Arriero, M.D.M.; Ramis, J.M.; Perelló, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [Green Version]
- Boregowda, S.V.; Ghoshal, S.; Booker, C.N.; Krishnappa, V.; Chakraborty, A.; Phinney, D.G. IP6K1 Reduces Mesenchymal Stem/Stromal Cell Fitness and Potentiates High Fat Diet-Induced Skeletal Involution. Stem Cells 2017, 35, 1973–1983. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.O.; Bracarense, A.P. Phytic Acid: From Antinutritional to Multiple Protection Factor of Organic Systems. J. Food Sci. 2016, 81, R1357–R1362. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharm. 2017, 87, 443–450. [Google Scholar] [CrossRef]
- Claxson, A.; Morris, C.; Blake, D.; Sirén, M.; Halliwell, B.; Gustafsson, T.; Löfkvist, B.; Bergelin, I. The anti-inflammatory effects ofd-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Actions 1990, 29, 68–70. [Google Scholar] [CrossRef]
- Chen, C.; Chen, K.; Su, T.; Zhang, B.; Li, G.; Pan, J.; Si, M. Myo-inositol-1-phosphate synthase (Ino-1) functions as a protection mechanism in Corynebacterium glutamicum under oxidative stress. Microbiologyopen 2019, 8, e00721. [Google Scholar] [CrossRef]
- Bizzarri, M.; Laganà, A.S.; Aragona, D.; Unfer, V. Inositol and pulmonary function. Could myo-inositol treatment downregulate inflammation and cytokine release syndrome in SARS-CoV-2? Eur. Rev. Med. Pharm. Sci. 2020, 24, 3426–3432. [Google Scholar]
- Carlomagno, G.; Unfer, V. Inositol safety: Clinical evidences. Eur. Rev. Med. Pharm. Sci. 2011, 15, 931–936. [Google Scholar]
- Formoso, G.; Baldassarre, M.P.A.; Ginestra, F.; Carlucci, M.A.; Bucci, I.; Consoli, A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab. Res. Rev. 2019, 35, e3154. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Espinola, M.S.B.; Dewailly, D.; Ozay, A.C.; Prapas, N.; Vazquez-Levin, M.; Wdowiak, A.; Unfer, V. Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends Endocrinol. Metab. 2020, 31, 570–579. [Google Scholar] [CrossRef]
- Martino, A.; Giuliani, A.; Todde, V.; Bizzarri, M.; Rizzi, A. Metabolic networks classification and knowledge discovery by information granulation. Comput. Biol. Chem. 2020, 84, 107187. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nahm, S.; Nihlani, K.; House, J.S.; Maguire, R.L.; Skinner, H.G.; Hoyo, C. Associations between Maternal Cadmium Exposure with Risk of Preterm Birth and Low after Birth Weight Effect of Mediterranean Diet Adherence on Affected Prenatal Outcomes. Toxics 2020, 8, 90. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. https://doi.org/10.3390/molecules25215079
Chatree S, Thongmaen N, Tantivejkul K, Sitticharoon C, Vucenik I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules. 2020; 25(21):5079. https://doi.org/10.3390/molecules25215079
Chicago/Turabian StyleChatree, Saimai, Nanthaphop Thongmaen, Kwanchanit Tantivejkul, Chantacha Sitticharoon, and Ivana Vucenik. 2020. "Role of Inositols and Inositol Phosphates in Energy Metabolism" Molecules 25, no. 21: 5079. https://doi.org/10.3390/molecules25215079
APA StyleChatree, S., Thongmaen, N., Tantivejkul, K., Sitticharoon, C., & Vucenik, I. (2020). Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules, 25(21), 5079. https://doi.org/10.3390/molecules25215079





