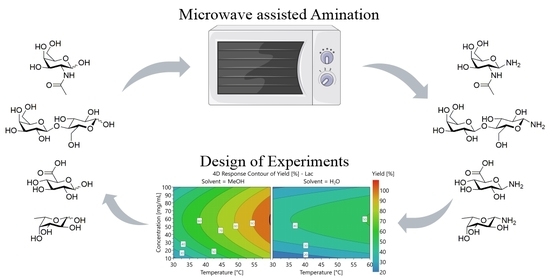
Optimization of the Microwave Assisted Glycosylamines Synthesis Based on a Statistical Design of Experiments Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimizing the Amination of Oligosaccharides
2.2. Design of Experiment Approach
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Design of Experiments (DoE)
3.2.2. Nuclear Magnetic Resonance (NMR)
3.2.3. Electrospray Ionization Mass Spectrometry (ESI-MS)
3.2.4. Synthesis of Glycosylamines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Krasnova, L.; Wong, C.-H. Understanding the Chemistry and Biology of Glycosylation with Glycan Synthesis. Annu. Rev. Biochem. 2016, 85, 599–630. [Google Scholar] [CrossRef]
- Gabius, H.-J. The Sugar Code, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9783527320899. [Google Scholar]
- Varki, A.; Lowe, J.B. Biological Roles of Glycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. (Eds.) Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Van Breedam, W.; Pöhlmann, S.; Favoreel, H.W.; de Groot, R.J.; Nauwynck, H.J. Bitter-sweet symphony: Glycan-lectin interactions in virus biology. FEMS Microbiol. Rev. 2014, 38, 598–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ielasi, F.S.; Alioscha-Perez, M.; Donohue, D.; Claes, S.; Sahli, H.; Schols, D.; Willaert, R.G. Lectin-Glycan Interaction Network-Based Identification of Host Receptors of Microbial Pathogenic Adhesins. mBio 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Rambaruth, N.D.S.; Dwek, M.V. Cell surface glycan-lectin interactions in tumor metastasis. Acta Histochem. 2011, 113, 591–600. [Google Scholar] [CrossRef]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef] [Green Version]
- Puri, A.; Neelamegham, S. Understanding glycomechanics using mathematical modeling: A review of current approaches to simulate cellular glycosylation reaction networks. Ann. Biomed. Eng. 2012, 40, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Bojarová, P.; Rosencrantz, R.R.; Elling, L.; Křen, V. Enzymatic glycosylation of multivalent scaffolds. Chem. Soc. Rev. 2013, 42, 4774–4797. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hauck, D.; Hoffmann, M.; Sommer, R.; Joachim, I.; Müller, R.; Imberty, A.; Varrot, A.; Titz, A. Covalent Lectin Inhibition and Application in Bacterial Biofilm Imaging. Angew. Chem. Int. Ed. Engl. 2017, 56, 16559–16564. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, A.H.; Alalawi, Z.; Mirandola, L.; Rakhshanda, R.; Dahlbeck, S.; Nguyen, D.; Jenkins, M.; Grizzi, F.; Cobos, E.; Figueroa, J.A.; et al. Galectins in cancer: Carcinogenesis, diagnosis and therapy. Ann. Trans. Med. 2014, 2, 88. [Google Scholar] [CrossRef]
- Ng, S.; Lin, E.; Kitov, P.I.; Tjhung, K.F.; Gerlits, O.O.; Deng, L.; Kasper, B.; Sood, A.; Paschal, B.M.; Zhang, P.; et al. Genetically encoded fragment-based discovery of glycopeptide ligands for carbohydrate-binding proteins. J. Am. Chem. Soc. 2015, 137, 5248–5251. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Gu, Y.; Feng, R.; Brash, J.; Eissa, A.M.; Haddleton, D.M.; Chen, G.; Chen, H. Synthesis of glycopolymers with specificity for bacterial strains via bacteria-guided polymerization. Chem. Sci. 2019, 10, 5251–5257. [Google Scholar] [CrossRef] [Green Version]
- Von der Ehe, C.; Buś, T.; Weber, C.; Stumpf, S.; Bellstedt, P.; Hartlieb, M.; Schubert, U.S.; Gottschaldt, M. Glycopolymer-Functionalized Cryogels as Catch and Release Devices for the Pre-Enrichment of Pathogens. ACS Macro Lett. 2016, 5, 326–331. [Google Scholar] [CrossRef]
- Filipová, M.; Bojarová, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunár, K.; Křen, V.; Etrych, T.; Janoušková, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy Using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef]
- Brun, M.A.; Disney, M.D.; Seeberger, P.H. Miniaturization of microwave-assisted carbohydrate functionalization to create oligosaccharide microarrays. ChemBioChem 2006, 7, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Puvirajesinghe, T.M.; Turnbull, J.E. Glycoarray Technologies: Deciphering Interactions from Proteins to Live Cell Responses. Microarrays 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateescu, A.; Ye, J.; Narain, R.; Vamvakaki, M. Synthesis and characterization of novel glycosurfaces by ATRP. Soft Matter 2009, 5, 1621–1629. [Google Scholar] [CrossRef]
- Pacholski, C.; Rosencrantz, S.; Rosencrantz, R.R.; Balderas-Valadez, R.F. Plasmonic biosensors fabricated by galvanic displacement reactions for monitoring biomolecular interactions in real time. Anal. Bioanal. Chem. 2020, 412, 3433–3445. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Osseili, C.; Kleinert, M.; Keil, N.; Rosencrantz, R.R. Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators. Biosensors 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barras, A.; Martin, F.A.; Bande, O.; Baumann, J.-S.; Ghigo, J.-M.; Boukherroub, R.; Beloin, C.; Siriwardena, A.; Szunerits, S. Glycan-functionalized diamond nanoparticles as potent E. coli anti-adhesives. Nanoscale 2013, 5, 2307–2316. [Google Scholar] [CrossRef]
- Poonthiyil, V.; Nagesh, P.T.; Husain, M.; Golovko, V.B.; Fairbanks, A.J. Gold Nanoparticles Decorated with Sialic Acid Terminated Bi-antennary N-Glycans for the Detection of Influenza Virus at Nanomolar Concentrations. ChemistryOpen 2015, 4, 708–716. [Google Scholar] [CrossRef]
- Witten, K.G.; Rech, C.; Eckert, T.; Charrak, S.; Richtering, W.; Elling, L.; Simon, U. Glyco-DNA-gold nanoparticles: Lectin-mediated assembly and dual-stimuli response. Small 2011, 7, 1954–1960. [Google Scholar] [CrossRef]
- Böcker, S.; Laaf, D.; Elling, L. Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3. Biomolecules 2015, 5, 1671–1696. [Google Scholar] [CrossRef] [Green Version]
- Böcker, S.; Elling, L. Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3. Bioengineering 2017, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Dimde, M.; Haag, R. Multivalent glycoconjugates as vaccines and potential drug candidates. Med. Chem. Commun. 2014, 5, 862–878. [Google Scholar] [CrossRef] [Green Version]
- Kiran, P.; Kumari, S.; Dernedde, J.; Haag, R.; Bhatia, S. Synthesis and comparison of linear and hyperbranched multivalent glycosides for C-type lectin binding. New J. Chem. 2019, 43, 16012–16016. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The cluster glycoside effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- Lee, R.T.; Lee, Y.C. Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconj. J. 2000, 17, 543–551. [Google Scholar] [CrossRef]
- Ginsburg, V. Complex. Carbohydrates; Academic Press: Orlando, FL, USA, 1987; ISBN 9780121820381. [Google Scholar]
- Hoffmann, M.; Gau, E.; Braun, S.; Pich, A.; Elling, L. Enzymatic Synthesis of 2-(β-Galactosyl)-ethyl Methacrylate by β-Galactosidase from Pyrococcus woesei and Application for Glycopolymer Synthesis and Lectin Studies. Biomacromolecules 2020, 21, 974–987. [Google Scholar] [CrossRef]
- Adharis, A.; Loos, K. Green Synthesis of Glycopolymers Using an Enzymatic Approach. Macromol. Chem. Phys. 2019, 220, 1900219. [Google Scholar] [CrossRef]
- Kwase, Y.A.; Cochran, M.; Nitz, M. Protecting-Group-Free Glycoconjugate Synthesis: Hydrazide and Oxyamine Derivatives in N-Glycoside Formation; Wiley: Hoboken, NJ, USA, 2013; ISBN 9783527658947. [Google Scholar]
- Vidal, S. (Ed.) Protecting Groups. Strategies and Applications in Carbohydrate Chemistry; Wiley-VCH: Weinheim, Germany, 2019; ISBN 3527697012. [Google Scholar]
- Tang, J.S.J.; Rosencrantz, S.; Tepper, L.; Chea, S.; Klöpzig, S.; Krüger-Genge, A.; Storsberg, J.; Rosencrantz, R.R. Functional Glyco-Nanogels for Multivalent Interaction with Lectins. Molecules 2019, 24, 1865. [Google Scholar] [CrossRef] [Green Version]
- Rosencrantz, S.; Tang, J.S.J.; Schulte-Osseili, C.; Böker, A.; Rosencrantz, R.R. Glycopolymers by RAFT Polymerization as Functional Surfaces for Galectin-3. Macromol. Chem. Phys. 2019, 220, 1900293. [Google Scholar] [CrossRef] [Green Version]
- Likhoshertov, L.M.; Novikova, O.S.; Derevitskaja, V.A.; Kochetkov, N.K. A new simple synthesis of amino sugar b-D-glycosylamines. Carbohydr. Res. 1986, 146, C1–C5. [Google Scholar] [CrossRef]
- Likhoshertov, L.M.; Novikova, O.S.; Shibaev, V.N. New Efficient Synthesis of b-Glucosylamines of Mono- and Disaccharides with the Use of Ammonium Carbamate. Dokl. Chem. 2002, 4, 500–503. [Google Scholar]
- Likhoshertov, L.M.; Novikova, O.S.; Shibaev, V.N. New Synthesis of b-Glycosylamines of D-Mannose, 2- and 6-Deoxysugars, and D-Glucuronic Acid with the Use of Ammonium Carbamate. Dokl. Chem. 2003, 4, 482–485. [Google Scholar]
- Likhoshertov, L.M.; Novikova, O.S.; Zheltova, A.O.; Shibaev, V.N. An improved procedure for the synthesis of N-bromoacetyl-b-glycopyranosylamines, derivatives of mono- and disaccharides. Russ. Chem. Bull. 2004, 3, 709–713. [Google Scholar] [CrossRef]
- Bejugam, M.; Flitsch, S.L. An efficient synthetic route to glycoamino acid building blocks for glycopeptide synthesis. Org. Lett. 2004, 6, 4001–4004. [Google Scholar] [CrossRef]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-48735-7. [Google Scholar]
- Yates, F. The Design and Analysis of Factorial Experiments; Imperial Bureau of Soil Science: Harpenden, UK, 1937. [Google Scholar]
- Tye, H. Application of statistical ’design of experiments’ methods in drug discovery. Drug Discov. Today 2004, 9, 485–491. [Google Scholar] [CrossRef]
- Lendrem, D.; Lendrem, B.C.; Woods, D.; Rowland-Jones, R.C.; Burke, M.; Chatfield, M.J.; Isaacs, J.; Owen, M. Lost in space: Design of experiments and scientific exploration in a Hogarth Universe. Drug Discov. Today 2015. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Bowden, G.D.; Pichler, B.J.; Maurer, A. A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes. Sci. Rep. 2019, 9, 11370. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.M.; Bellany, F.; Benhamou, L.; Bučar, D.-K.; Tabor, A.B.; Sheppard, T.D. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org. Biomol. Chem. 2016, 14, 2373–2384. [Google Scholar] [CrossRef] [Green Version]
- Tye, H.; Whittaker, M. Use of a Design of Experiments approach for the optimisation of a microwave assisted Ugi reaction. Org. Biomol. Chem. 2004, 2, 813–815. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Gao, P.; Zhang, Z. Application of the factorial design of experiments to hydrothermal synthesis of lithium iron phosphate. Int. J. Appl. Ceram. Technol. 2020, 17, 1231–1240. [Google Scholar] [CrossRef]
- Hemmati, S.; Barkey, D.P. Parametric Study, Sensitivity Analysis, and Optimization of Polyol Synthesis of Silver Nanowires. ECS J. Solid State Sci. Technol. 2017, 6, P132. [Google Scholar] [CrossRef]
- Karim, M.R.; Ferrandon, M.; Medina, S.; Sture, E.; Kariuki, N.; Myers, D.J.; Holby, E.F.; Zelenay, P.; Ahmed, T. Coupling High-Throughput Experiments and Regression Algorithms to Optimize PGM-Free ORR Electrocatalyst Synthesis. ACS Appl. Energy Mater. 2020, 3, 9083–9088. [Google Scholar] [CrossRef]
- Ghadban, A.; Albertin, L.; Moussavou Mounguengui, R.W.; Peruchon, A.; Heyraud, A. Synthesis of β-D-glucopyranuronosylamine in aqueous solution: Kinetic study and synthetic potential. Carbohydr. Res. 2011, 346, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.A.; Townsend, S.D. Bioorthogonal human milk oligosaccharide probes for antimicrobial target identification within Streptococcus agalactiae. Carbohydr. Res. 2020, 488, 107895. [Google Scholar] [CrossRef]
- Vetter, D.; Gallop, M.A. Strategies for the Synthesis and Screening of Glycoconjugates. 1. A Library of Glycosylamines. Bioconj. Chem. 1995, 6, 316–318. [Google Scholar] [CrossRef]
- Ghadban, A.; Albertin, L.; Condamine, E.; Mounguengui, R.W.M.; Heyraud, A. NMR and MS study of the formation of β-D-glucopyranosylamine uronic acid in aqueous solution. Can. J. Chem. 2011, 89, 987–1000. [Google Scholar] [CrossRef]
- Hackenberger, C.P.R.; O’Reilly, M.K.; Imperiali, B. Improving glycopeptide synthesis: A convenient protocol for the preparation of b-glycosylamines and the synthesis of glycopeptides. J. Org. Chem. 2005, 70, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Chan, K.; Li, J. Microwave-assisted Kochetkov amination followed by permanent charge derivatization: A facile strategy for glycomics. Chem. Commun. 2010, 46, 7424–7426. [Google Scholar] [CrossRef] [PubMed]
Sample Availability:
Samples of the compounds are not available from the authors. |







| Exp No | T (°C) | (mg/mL) | Salt | Solvent | Yield (%) | |||
|---|---|---|---|---|---|---|---|---|
| Am-I GalNAcNH2 | Am-II LacNH2 | Am-III GlcANH2 | Am-IV FucNH2 | |||||
| 01 | 60 | 10 | (NH4)2CO3 | MeOH | 64.2 | 83.6 | 7 | 60.5 |
| 02 | 30 | 100 | (NH4)2CO3 | MeOH | 53.7 | 33 | 0.9 | 12.4 |
| 03 | 60 | 100 | (NH4)2CO3 | MeOH | 42.2 | 68 | 33.6 | 21.8 |
| 04 | 30 | 40 | (NH4)2CO3 | MeOH | 43.1 | 46.4 | 2.1 | 45 |
| 05 | 40 | 10 | (NH4)2CO3 | MeOH | 30.9 | 20.8 | 1.6 | 25 |
| 06 | 30 | 10 | H2NCOONH4 | MeOH | 33.6 | 11.8 | 3.3 | 42.6 |
| 07 | 60 | 10 | H2NCOONH4 | MeOH | 51.6 | 81.4 | 12 | 69.8 |
| 08 | 30 | 100 | H2NCOONH4 | MeOH | 44.9 | 27.4 | 3 | 32.4 |
| 09 | 60 | 100 | H2NCOONH4 | MeOH | 41.9 | 79.2 | 23.6 | 38.8 |
| 10 | 45 | 55 | H2NCOONH4 | MeOH | 57.4 | 79.7 | 53.1 | 26 |
| 11 | 30 | 10 | (NH4)2CO3 | H2O | 39.1 | 16.7 | 16.8 | 16.2 |
| 12 | 60 | 10 | (NH4)2CO3 | H2O | 27.3 | 26.2 | 35.7 | 18.2 |
| 13 | 30 | 100 | (NH4)2CO3 | H2O | 26.5 | 11.5 | 37.3 | 9 |
| 14 | 60 | 70 | (NH4)2CO3 | H2O | 37.8 | 42.4 | 54.6 | 10.3 |
| 15 | 50 | 100 | (NH4)2CO3 | H2O | 20.4 | 44.3 | 51.9 | 8.4 |
| 16 | 30 | 10 | H2NCOONH4 | H2O | 41.2 | 8.8 | 18.3 | 6.9 |
| 17 | 60 | 100 | H2NCOONH4 | H2O | 50.5 | 30.2 | 46.8 | 12.4 |
| 18 | 30 | 70 | H2NCOONH4 | H2O | 29.4 | 13.5 | 47.8 | 8.7 |
| 19 | 60 | 40 | H2NCOONH4 | H2O | 44.2 | 21.5 | 44.4 | 17.1 |
| 20 | 50 | 10 | H2NCOONH4 | H2O | 30 | 20.1 | 46.1 | 11.1 |
| 21 | 40 | 100 | H2NCOONH4 | H2O | 34.4 | 24.7 | 40.3 | 8.7 |
| 22a | 45 | 55 | H2NCOONH4 | H2O | 17 | 32.5 | 77.7 | 33.3 |
| 22b | 45 | 55 | H2NCOONH4 | H2O | 20.4 | 74.1 | 77 | 41.8 |
| 22c | 45 | 55 | H2NCOONH4 | H2O | 18 | 62.5 | 81.6 | 31.2 |
| Exp No | T (°C) | (mg/mL) | Salt | Solvent | Predicted Yield (%) | Found Yield (%) |
|---|---|---|---|---|---|---|
| Am-I-0/-01 | 60 | 10 | (NH4)2CO3 | MeOH | 54.7 | 64.2 |
| Am-II-0 | 60 | 58 | (NH4)2CO3 | MeOH | 100.4 | 91.1 |
| Am-III-0 | 47 | 59 | H2NCOONH4 | H2O | 73.8 | 60.3 |
| Am-IV-0/-07 | 60 | 50 | H2NCOONH4 | MeOH | 63.4 | 69.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.S.J.; Schade, K.; Tepper, L.; Chea, S.; Ziegler, G.; Rosencrantz, R.R. Optimization of the Microwave Assisted Glycosylamines Synthesis Based on a Statistical Design of Experiments Approach. Molecules 2020, 25, 5121. https://doi.org/10.3390/molecules25215121
Tang JSJ, Schade K, Tepper L, Chea S, Ziegler G, Rosencrantz RR. Optimization of the Microwave Assisted Glycosylamines Synthesis Based on a Statistical Design of Experiments Approach. Molecules. 2020; 25(21):5121. https://doi.org/10.3390/molecules25215121
Chicago/Turabian StyleTang, Jo Sing Julia, Kristin Schade, Lucas Tepper, Sany Chea, Gregor Ziegler, and Ruben R. Rosencrantz. 2020. "Optimization of the Microwave Assisted Glycosylamines Synthesis Based on a Statistical Design of Experiments Approach" Molecules 25, no. 21: 5121. https://doi.org/10.3390/molecules25215121