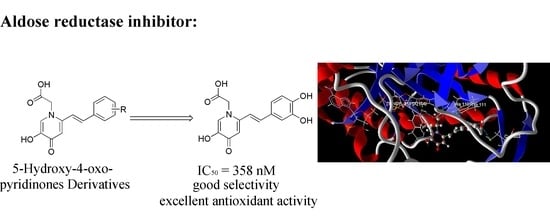
(5-Hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid Derivatives as Multifunctional Aldose Reductase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
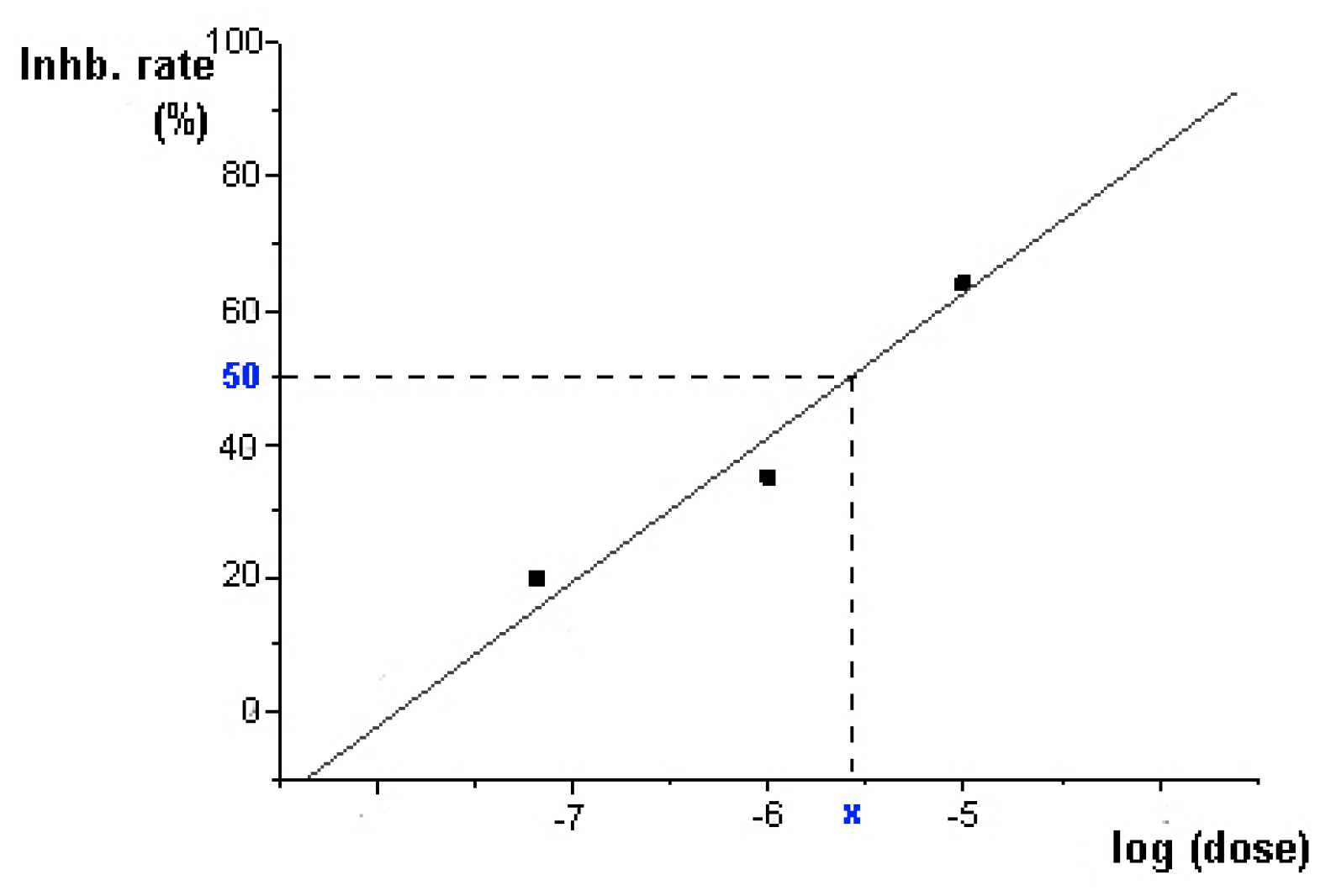
2.2. Enzyme Inhibition
2.3. Antioxidant Activity
2.3.1. DPPH Radical Scavenging Activity
2.3.2. Lipid Peroxidation Suppression
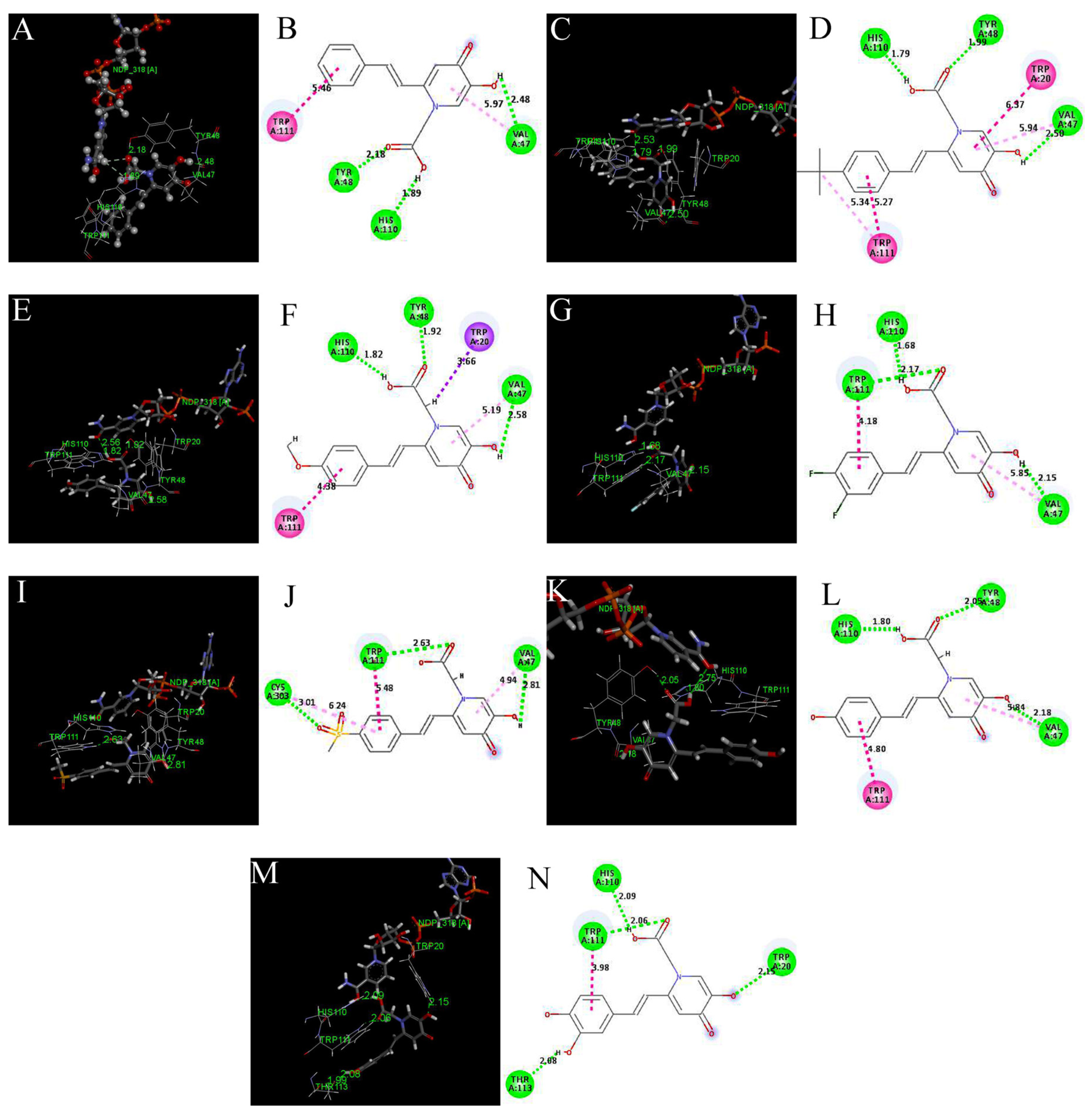
2.4. Molecular Docking
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures Preparing Title Compounds
3.2.1. Synthetic Procedure for 5-benzyloxy-2-hydroxymethyl-pyran-4-one (2)
3.2.2. Synthetic Procedure for 5-benzyloxy-2-chloromethyl-pyran-4-one (3)
3.2.3. Synthetic Procedure for ((5-(benzyloxy)-4-oxo-4H-pyran-2-yl)methyl)triphenylphosphonium chloride (4)
3.2.4. General Procedure Preparing 5-benzyloxy-2-styryl-pyran-4-one (5)
3.2.5. General Procedure Preparing (5-Benzyloxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid (6)
3.2.6. General Procedure Preparing (5-hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid (7a–l)
3.3. Enzyme Inhibition Assays
3.4. Antioxidant Assays
3.5. Molecular Docking Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosconati, S.; Marinelli, L.; Motta, C.L.; Sartini, S.; Settimo, F.D.; Olson, A.J.; Novellino, E. Pursuing aldose reductase inhibitors through in situ cross-docking and similarity-based virtual screening. J. Med. Chem. 2009, 52, 5578–5581. [Google Scholar] [CrossRef]
- Gabbay, K.H.; Merola, L.O.R.; Field, A. Sorbitol pathway: Presence in nerve and cord with substrate accumulation in diabetes. Science 1966, 151, 209–210. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ramana, K.V.; Bhatnagar, A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr. Rev. 2005, 26, 380–392. [Google Scholar] [CrossRef]
- Alexiou, P.; Pegklidou, K.; Chatzopoulou, M.; Nicolaou, I.; Demopoulos, V. Aldose reductase enzyme and its implication to major health problems of the 21st century. J. Curr. Med. Chem. 2009, 16, 734–752. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, J.H.; Nishimura, C. The involvement of aldose reductase in diabetic complications. Diabetes Metab. Rev. 1988, 4, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Chung, S.K. Genetic analysis of aldose reductase in diabetic complications. Curr. Med. Chem. 2003, 10, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, K.H.N. The sorbitol pathway and the complications of diabetes. Engl. J. Med. 1973, 288, 831–836. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Hers, H.G. Le mécanisme de la transformation de glucose en fructose par les vésicules séminales. Biochim. Biophys. Acta. 1956, 22, 202–203. [Google Scholar] [CrossRef]
- Suzen, S.; Buyukbingol, E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr. Med. Chem. 2003, 10, 1329–1352. [Google Scholar] [CrossRef]
- El-Kabbani, O.; Darmanin, C.; Schneider, T.R.; Hazemann, I.; Ruiz, F.M.; Oka, M.; Joachimiak, A.; Schulze-Briese, C.; Tomizaki, T.; Mitschler, A.; et al. Ultrahigh resolution drug design. II. Atomic resolution structures of human aldose reductase holoenzyme complexed with fidarestat and minalrestat: Implications for the binding of cyclic imide inhibitors. Proteins 2004, 55, 805–813. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Brownlee, M. A single unifying mechanism for diabetic complications. Kidney Int. 2000, 58, S26–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, H.L.; Hotchkiss, J.H. Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res. 1995, 339, 73–89. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Berliner, L.J. Manganese complexes of curcumin analogues: Evaluation of hydroxyl radical scavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radical Res. 2004, 38, 303–314. [Google Scholar] [CrossRef]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Bohren, K.M.; Grimshaw, C.E.; Gabbay, K.H. Stable expression of chicken type-VI collagen α1, α2 and α3 cDNAs in murine NIH/3T3 cells. J. Biol. Chem. 1992, 209, 785–792. [Google Scholar] [CrossRef]
- El-Kabbani, O.; Podjarny, A. Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites. Cell. Mol. Life Sci. 2007, 64, 1970–1978. [Google Scholar] [CrossRef]
- Balestri, F.; Cappiello, M.; Moschini, R.; Rotondo, R.; Abate, M.; Del-Corso, A.; Mura, U. Modulation of aldose reductase activity by aldose hemiacetals. BBA-Gen. Subj. 2015, 1850, 2329–2339. [Google Scholar] [CrossRef] [Green Version]
- Kucerova-Chlupacova, M.; Halakova, D.; Majekova, M.; Treml, J.; Stefek, M.; Prnova, M.S. (4-Oxo-2-thioxothiazolidin-3-yl)acetic acids as potent and selective aldose reductase inhibitors. Chem.-Biol Interact. 2020, 332, 109286. [Google Scholar] [CrossRef] [PubMed]
- Celestina, S.K.; Sundaram, K.; Ravi, S. In vitro studies of potent aldose reductase inhibitors: Synthesis, characterization, biological evaluation and docking analysis of rhodanine-3-hippuric acid derivatives. Bioorg. Chem. 2020, 97, 103640. [Google Scholar] [CrossRef]
- Hao, X.; Qi, G.; Ma, H.; Zhu, C.; Han, Z. Novel 2-phenoxypyrido[3,2-b]pyrazin-3(4H)-one derivatives as potent and selective aldose reductase inhibitors with antioxidant activity. J. Enzym. Inhib. Med. Ch. 2019, 34, 1368–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlavac, M.; Kovacikova, L.; Prnova, M.S.; Šramel, P.; Addova, G.; Majekova, M.; Hanquet, G.; Bohac, A.; Stefek, M. Development of Novel Oxotriazinoindole Inhibitors of Aldose Reductase: Isosteric Sulfur/Oxygen Replacement in the Thioxotriazinoindole Cemtirestat Markedly Improved Inhibition Selectivity. J. Med. Chem. 2020, 63, 369–381. [Google Scholar] [CrossRef]
- Carlo, G. Depigmenting Compositions. WO 20010117497A1, 15 March 2001. Available online: https://patents.google.com/patent/WO2001017497A1/en (accessed on 5 October 2020).
- Cohen, S.M.; Credille, C.V.; Puerta, D.T. Compositions and methods for inhibiting influenza RNA polymerase endonuclease. U.S. Patent 2019106398, 11 April 2019. [Google Scholar]
- Zou, C.B.; Liu, X.G.; Xie, R.J.; Bao, Y.S.; Jin, Q.; Jia, X.B.; Li, L.; Liu, R.C. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochem. Bioph. Res. Co. 2017, 486, 930–936. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Zhang, M.X.; Dong, X.W.; Hu, Y.Z.; Dai, X.Y.; Wei, X.Y.; Hider, R.C.; Zhang, J.C.; Zhou, T. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3103–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.D. Solution chemistry of vanadium-(IV) and-(V) with the bidentate ligand 1, 2-dimethyl-3-hydroxy-4 (1H)-pyridinone: Relevance to the treatment of diabetes. Chem. Commun. 1996, 3, 405–406. [Google Scholar] [CrossRef]
- Katoh, A.; Yamaguchi, M.; Taguchi, K.; Saito, R.; Adachi, Y.; Yoshikawa, Y.; Sakurai, H. Oxovanadium (IV) and (V) Complexes with 3-Hydroxy-4 (1H)-pyridinones and 1-Hydroxy-2 (1H)-pyrimidinones—Synthesis, Structural Characteristics, and Their Insulin-mimetic Activities. Biomed. Res. Trace Elem. 2006, 17, 1–10. [Google Scholar]
- Rangel, M.; Tamura, A.; Fukushima, C.; Sakurai, H. In vitro study of the insulin-like action of vanadyl-pyrone and-pyridinone complexes with a VO (O 4) coordination mode. J. Biol. Inorg. Chem. 2001, 6, 128–132. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front Biosci. 2014, 19, 862–885. [Google Scholar] [CrossRef] [Green Version]
- Bebbington, D.; Gaur, S. 3-Hydroxy-2(1H)-pyridinone or 3-hydroxy-4(1H)-pyridinone Derivatives Useful as Reactive Oxygen Species (ROS) Scavengers. EP1006112A1, 7 June 2000. Available online: https://patents.google.com/patent/EP1006112A1/en (accessed on 5 October 2020).
- Chen, T.D.; Ko, J.H.; Prendecki, M.; McAdoo, S.P.; Pusey, C.D.; Cook, H.T.; Behmoaras, J. Deferiprone, an iron chelator, is preventive and therapeutic in experimental crescentic glomerulonephritis. bioRxiv 2017, 225540. Available online: https://www.biorxiv.org/content/10.1101/225540v1.article-info (accessed on 27 November 2017). [CrossRef] [Green Version]
- Xu, P.; Zhang, M.; Sheng, R.; Ma, Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 174–186. [Google Scholar] [CrossRef]
- Iqbal, Z.; Morahan, G.; Arooj, M.; Sobolev, A.N.; Hameed, S. Synthesis of new arylsulfonylspiroimidazolidine-2ʹ,4ʹ-diones and study of their effect on stimulation of insulin release from MIN6 cell line, inhibition of human aldose reductase, sorbitol accumulations in various tissues and oxidative stress. Eur. J. Med. Chem. 2019, 168, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.W.; Fairooz, S.; Elson, J.L.; Hubscher-Bruder, V.; Brandel, J.; Soundararajan1, M.; Smith, D.; Dexter, D.T.; Tétard, D.; Pienaar, I.S. Novel 1-hydroxypyridin-2-one metal chelators prevent and rescue ubiquitin proteasomal-related neuronal injury in an in vitro model of Parkinson’s disease. Arch. Toxicol. 2020, 94, 813–831. [Google Scholar] [CrossRef] [Green Version]
- Chaves, S.; Canário, S.; Carrasco, M.P.; Mira, L.; Santos, M.A. Hydroxy(thio)pyrone and hydroxy(thio)pyridinone iron chelators: Physico-chemical properties and anti-oxidant activity. J. Inorg. Biochem. 2012, 114, 38–46. [Google Scholar] [CrossRef]
- El-Kabbani, O.; Carbone, V.; Darmanin, C.; Oka, M.; Mitschler, A.; Podjarny, A.; Schulze-Briese, C.; Chung, R.P. Structure of aldehyde reductase holoenzyme in complex with the potent aldose reductase inhibitor fidarestat: implications for inhibitor binding and selectivity. J. Med. Chem. 2005, 48, 5536–5542. [Google Scholar] [CrossRef]
- Costantino, L.; Ferrari, A.M.; Gamberini, M.C.; Rastelli, G. Nitrophenyl derivatives as aldose reductase inhibitors. Bioorg. Med. Chem. 2002, 10, 3923–3931. [Google Scholar] [CrossRef]
- Gallego, O.; Ruiz, F.X.; Ardèvol, A.; Domínguez, M.; Alvarez, R.; Lera, A.R.; Rovira, C.; Farrés, J.; Fita, I.; Parés, X. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc. Natl. Acad. Sci. USA 2007, 104, 20764–20769. [Google Scholar] [CrossRef] [Green Version]
- Cappiello, M.; Balestri, F.; Moschini, R.; Mura, U.; Del-Corso, A. Intra-site differential inhibition of multi-specific enzymes. J. Enzym. Inhib. Med. Chem. 2020, 35, 840–846. [Google Scholar] [CrossRef]
- Qin, X.Y.; Hao, X.; Han, H.; Zhu, S.J.; Yang, Y.C.; Wu, B.B.; Hussain, S.; Parveen, S.; Jing, C.J.; Ma, B. Design and synthesis of potent and multifunctional aldose reductase inhibitors based on quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef]


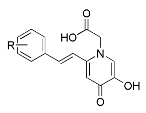
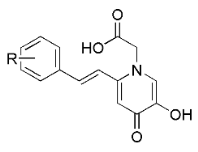 | |||||
|---|---|---|---|---|---|
| Comp. | R | IC50 for ALR2 (μM) 1 | ALR1 Inh. % 1,2 | IC50 for ALR1 (μM) 1 | SI 3 |
| 7a | H | 8.41 ± 0.63 | 38.78 ± 1.75 | >100 | |
| 7b | p-Me | 5.30 ± 0.60 | |||
| 7c | p-tBu | 2.98 ± 0.73 4 | 49.41 ± 0.45 | >100 | |
| 7d | p-OMe | 6.78 ± 0.91 | |||
| 7e | m,p-(OMe)2 | 9.56 ± 0.98 | |||
| 7f | p-F | 6.64 ± 2.39 | |||
| 7g | m,p-F2 | 2.11 ± 0.34 4 | 35.83 ± 3.23 | >100 | |
| 7h | p-CF3 | 10.72 ± 1.47 | |||
| 7i | p-SO2Me | 17.11 ± 2.30 4 | |||
| 7j | p-NH2 | 8.19 ± 0.43 | |||
| 7k | p-OH | 2.36 ± 0.42 4,5 | 75.78 ± 2.34 | 15.00 ± 0.54 | 6.36 |
| 7l | m,p-(OH)2 | 0.789 ± 0.183 4 | 80.01 ± 3.59 | 19.91 ± 1.72 | 25.23 |
| epalrestat | 0.205 ± 0.026 | 80.80 ± 3.74 | 3.56 ± 0.18 | 17.37 | |
| epalrestat 6 | 0.62 ± 0.035 | ||||
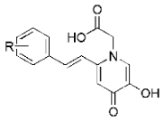 | |||||
|---|---|---|---|---|---|
| Comp. | R | % of Inhibition of Control Value 1 | |||
| 100 μM | 10 μM | 5 μM | 1 μM | ||
| 7a | H | 22.90 ± 3.30 | |||
| 7c | p-tBu | 25.44 ± 2.36 | |||
| 7g | m,p-F2 | 28.50 ± 0.95 | |||
| 7k | p-OH | 96.69 ± 0.72 | 89.31 ± 0.62 | 72.01 ± 1.80 | 33.08 ± 0.72 |
| 7l | m,p-(OH)2 | 97.96 ± 0.36 | 82.44 ± 1.65 | 67.18 ± 0.62 | 41.48 ± 0.95 |
| Deferiprone 2 | 63 | ||||
| Trolox | 92.32 ± 0.19 | 80.33 ± 1.22 | 45.53 ± 1.12 | 11.89 ± 1.39 | |
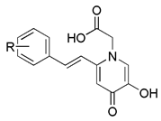 | ||
|---|---|---|
| Comp. | R | MDA inh. % 1 |
| 7a | H | 19.28 ± 0.98 |
| 7c | p-tBu | 26.10 ± 1.06 |
| 7g | m,p-F2 | 55.02 ± 3.00 |
| 7k | p-OH | 90.73 ± 3.97 |
| 7l | m,p-(OH)2 | 88.76 ± 2.04 |
| Deferiprone 2 | 25 | |
| Trolox | 74.82 ± 1.82 | |
 | |||||
|---|---|---|---|---|---|
| Comp. | R | ALR2 | ALR1 | ||
| Rerank Score (kcal/mol) | Ebinding (kcal/mol) | Ki (μM) | Rerank Score (kcal/mol) | ||
| 7a | H | −96.068 | −7.49 | 3.23 | −96.381 |
| 7c | p-tBu | −108.806 | −7.55 | 2.93 | −103.082 |
| 7d | p-OMe | −106.12 | −7.55 | 2.92 | |
| 7g | m,p-F2 | −112.514 | −7.54 | 2.98 | |
| 7k | p-OH | −112.86 | −7.63 | 2.54 | −97.062 |
| 7l | m,p-(OH)2 | −115.158 | −7.76 | 2.06 | −90.375 |
Sample Availability: Samples of the compounds 7a–l are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhang, X.; Zhang, X.; Liu, W.; Lei, Y.; Zhu, C.; Ma, B. (5-Hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid Derivatives as Multifunctional Aldose Reductase Inhibitors. Molecules 2020, 25, 5135. https://doi.org/10.3390/molecules25215135
Chen H, Zhang X, Zhang X, Liu W, Lei Y, Zhu C, Ma B. (5-Hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid Derivatives as Multifunctional Aldose Reductase Inhibitors. Molecules. 2020; 25(21):5135. https://doi.org/10.3390/molecules25215135
Chicago/Turabian StyleChen, Huan, Xin Zhang, Xiaonan Zhang, Wenchao Liu, Yanqi Lei, Changjin Zhu, and Bing Ma. 2020. "(5-Hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid Derivatives as Multifunctional Aldose Reductase Inhibitors" Molecules 25, no. 21: 5135. https://doi.org/10.3390/molecules25215135
APA StyleChen, H., Zhang, X., Zhang, X., Liu, W., Lei, Y., Zhu, C., & Ma, B. (2020). (5-Hydroxy-4-oxo-2-styryl-4H-pyridin-1-yl)-acetic Acid Derivatives as Multifunctional Aldose Reductase Inhibitors. Molecules, 25(21), 5135. https://doi.org/10.3390/molecules25215135