Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis
Abstract
1. Introduction
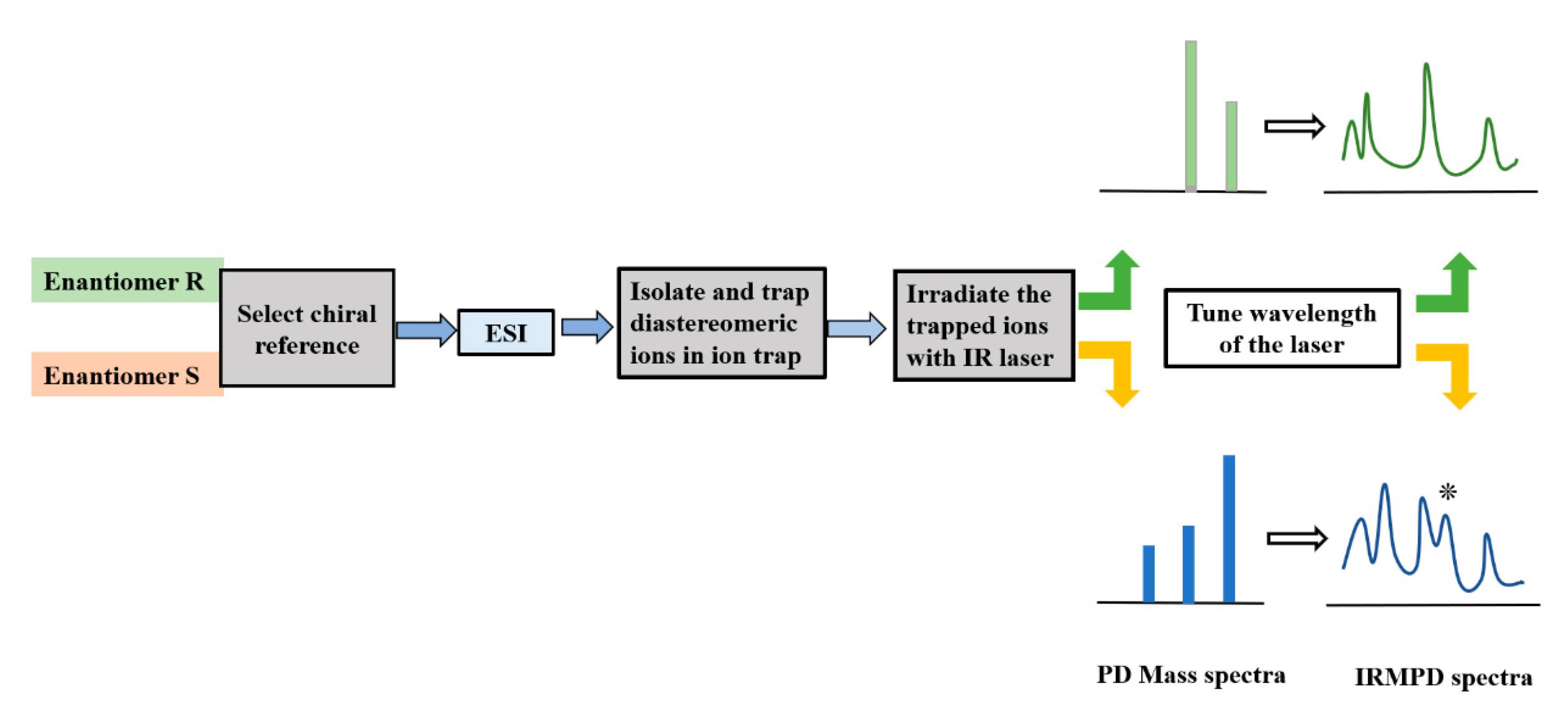
2. IRMPD Spectroscopy: A Brief Description of the Technique
3. Application of IRMPD Mass Spectrometry and Spectroscopy in Chiral Analysis
3.1. Calixarene Complexes
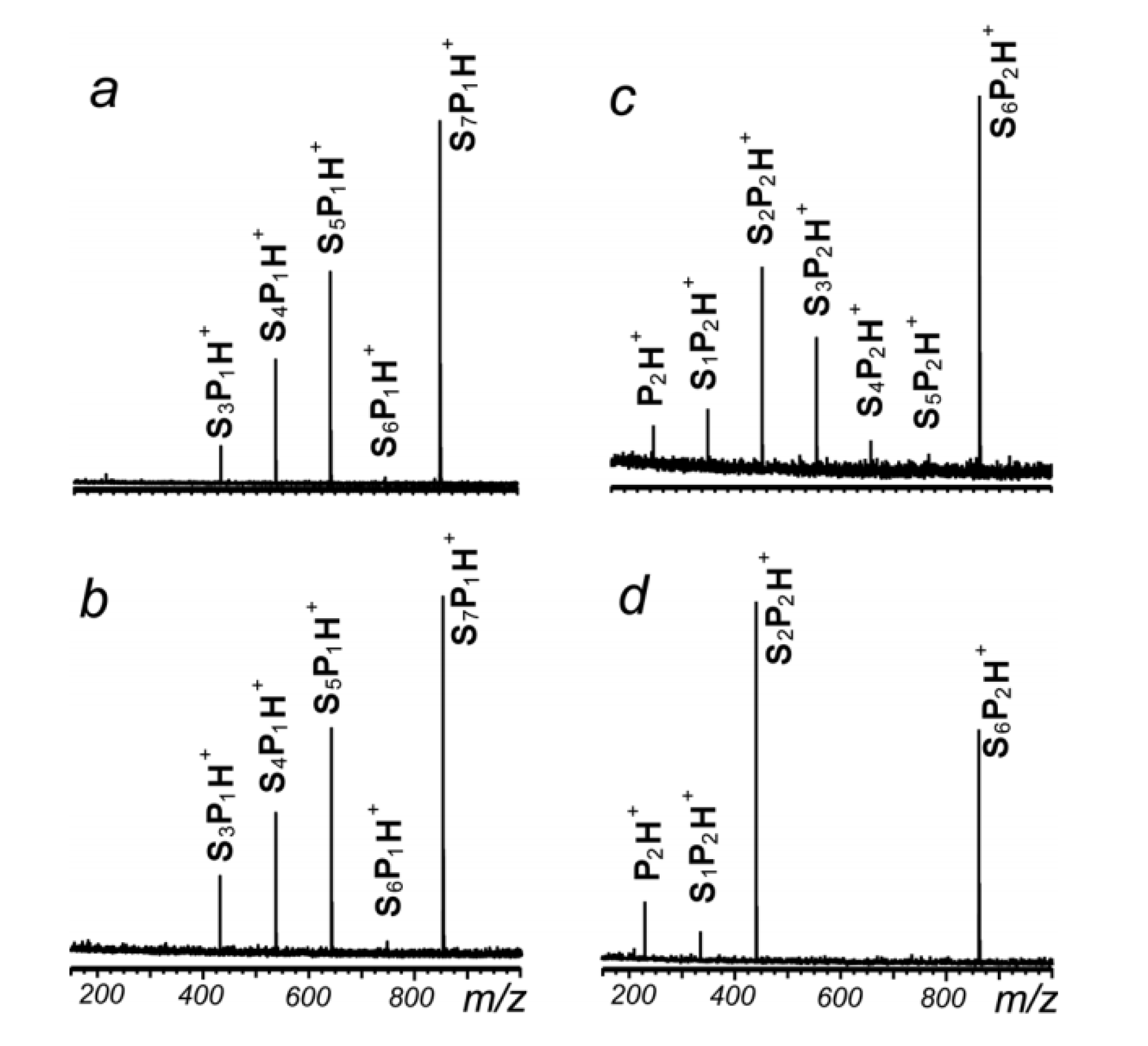
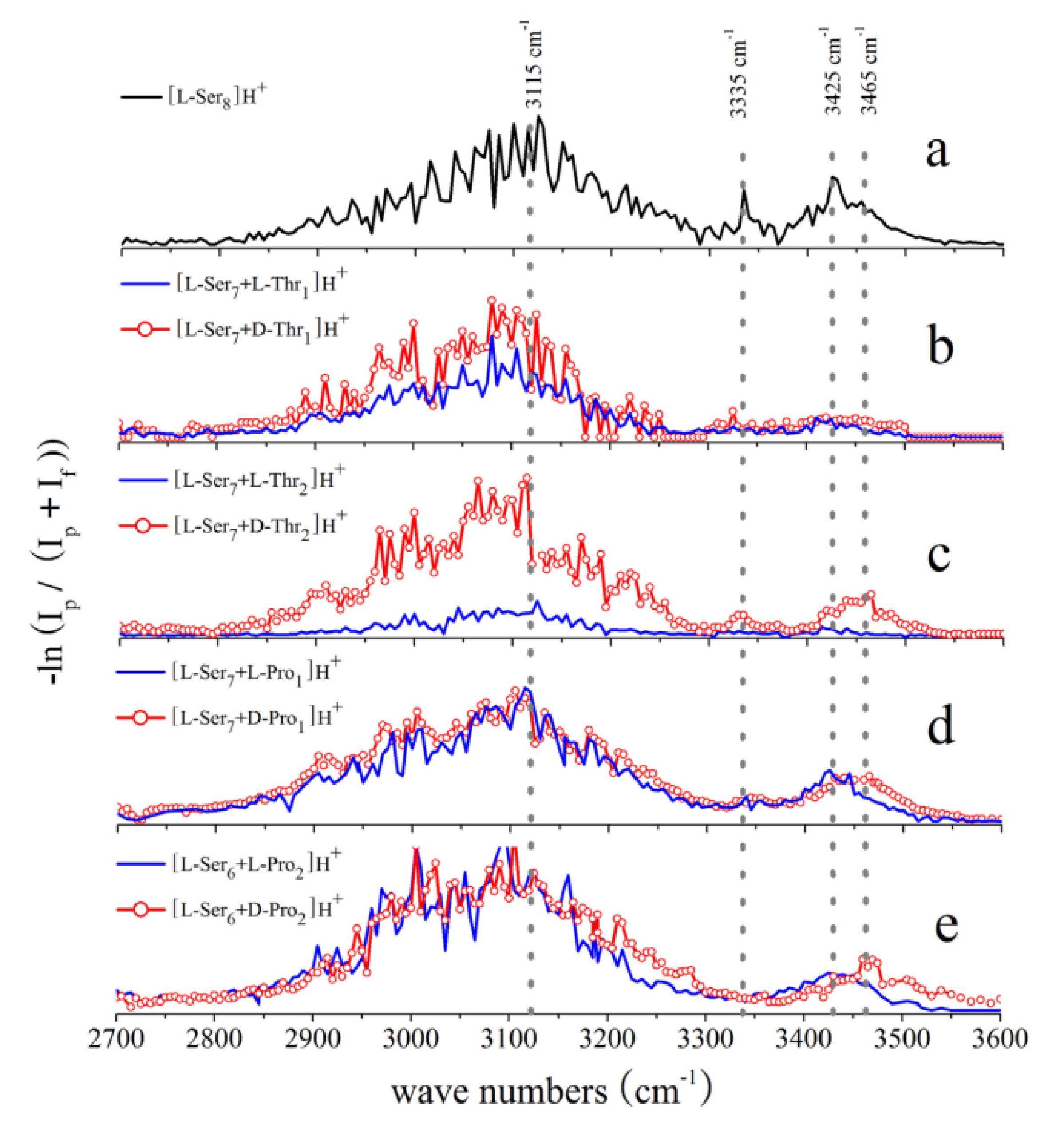
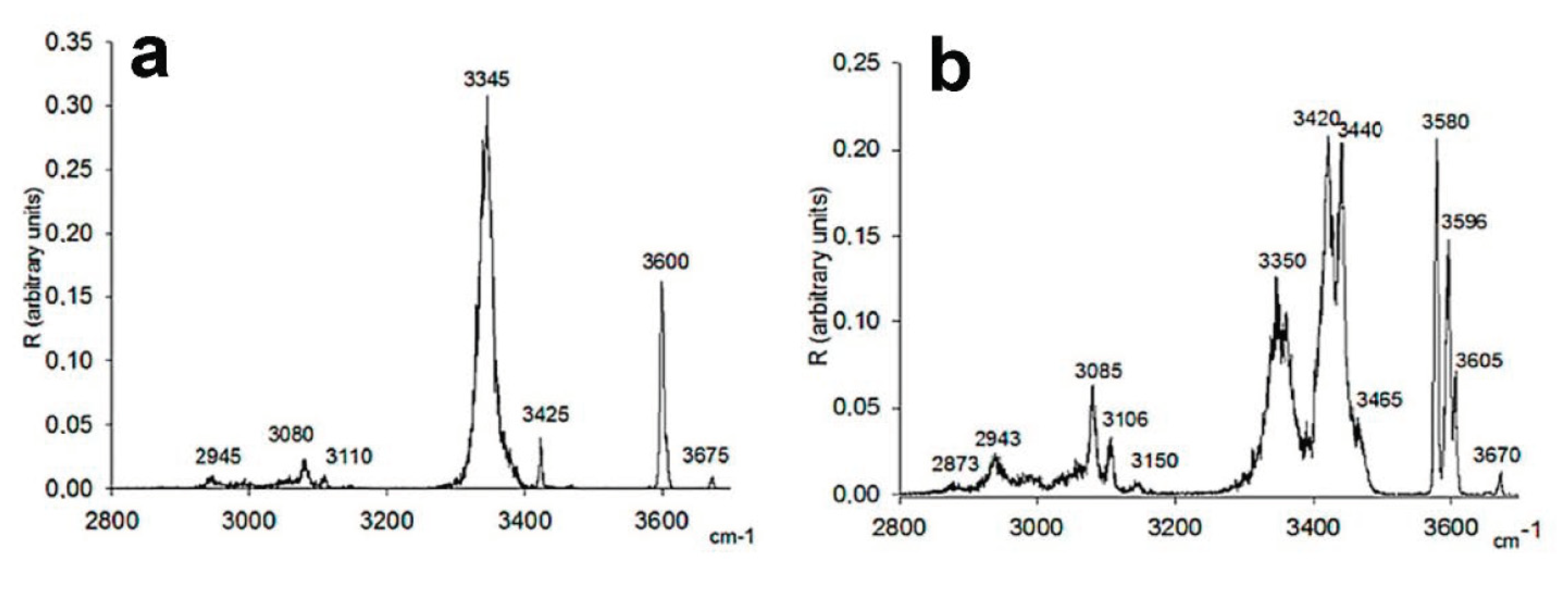
3.2. Serine Octamer Clusters
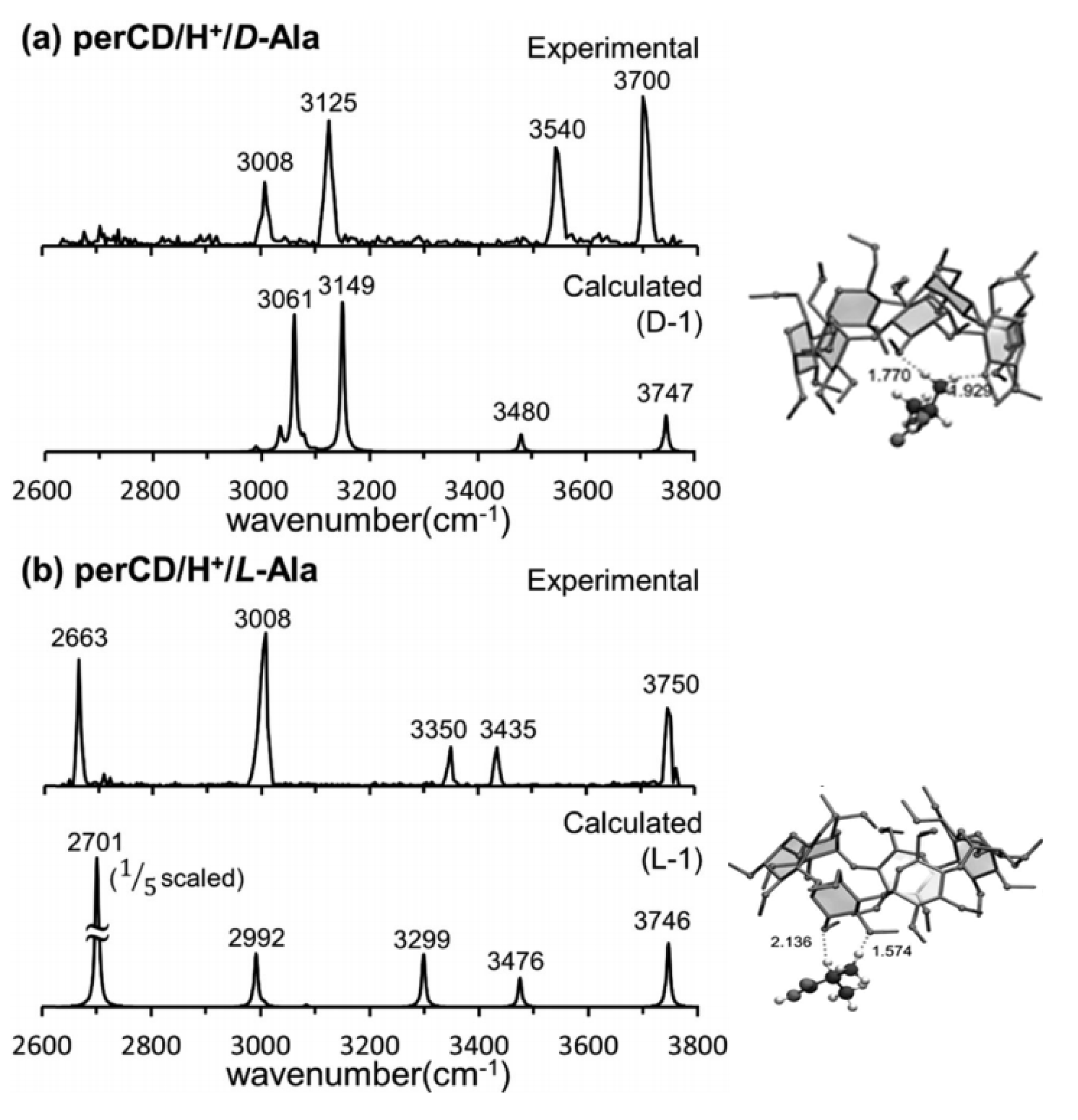
3.3. β-Cyclodextrin Complexes
3.4. Compexes with Axially Chiral Ligand
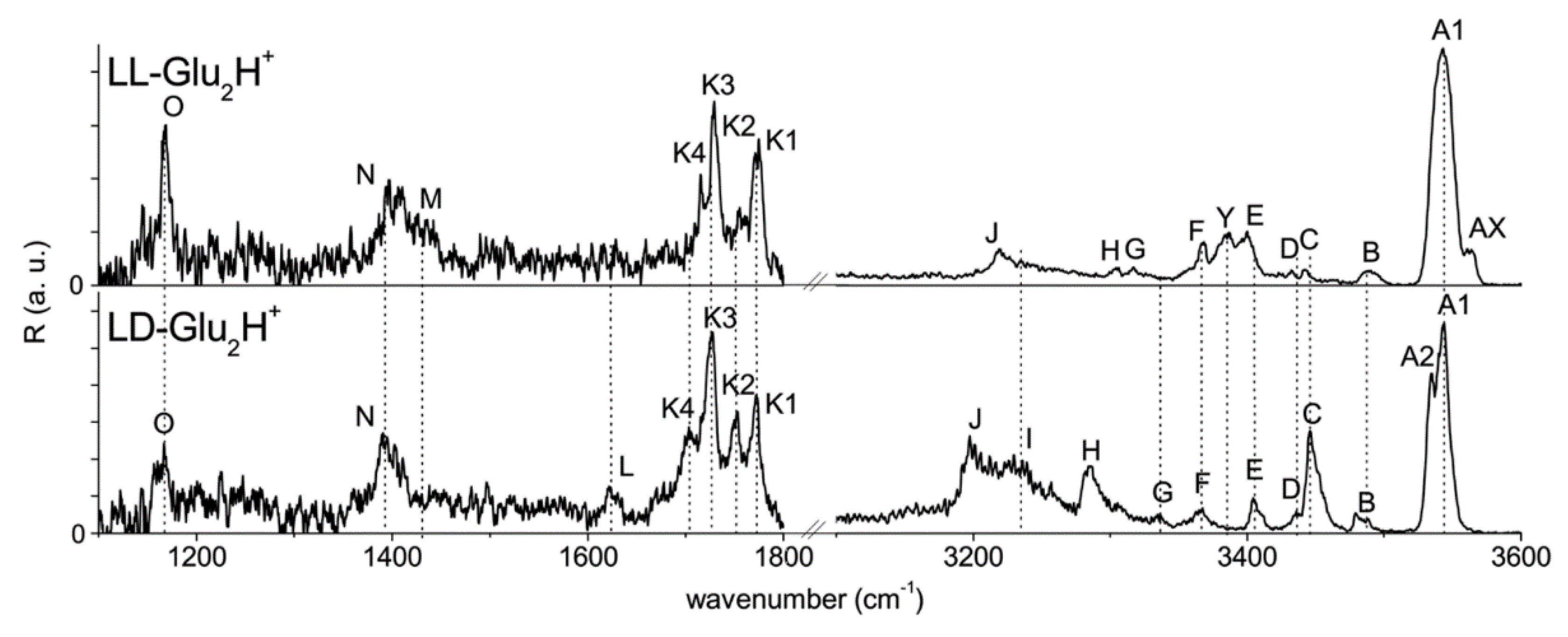
3.5. Dimers of the Chiral Molecules
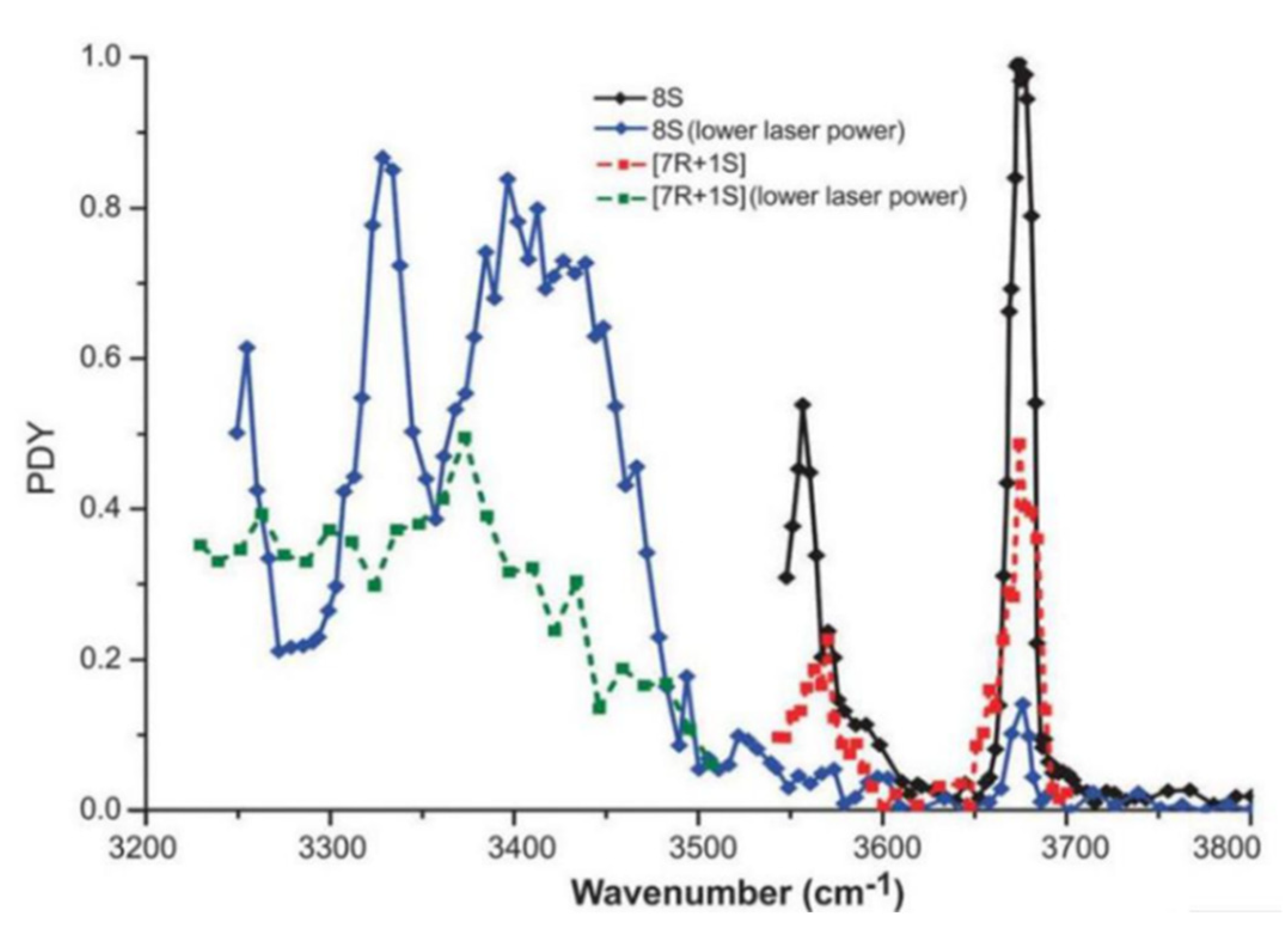
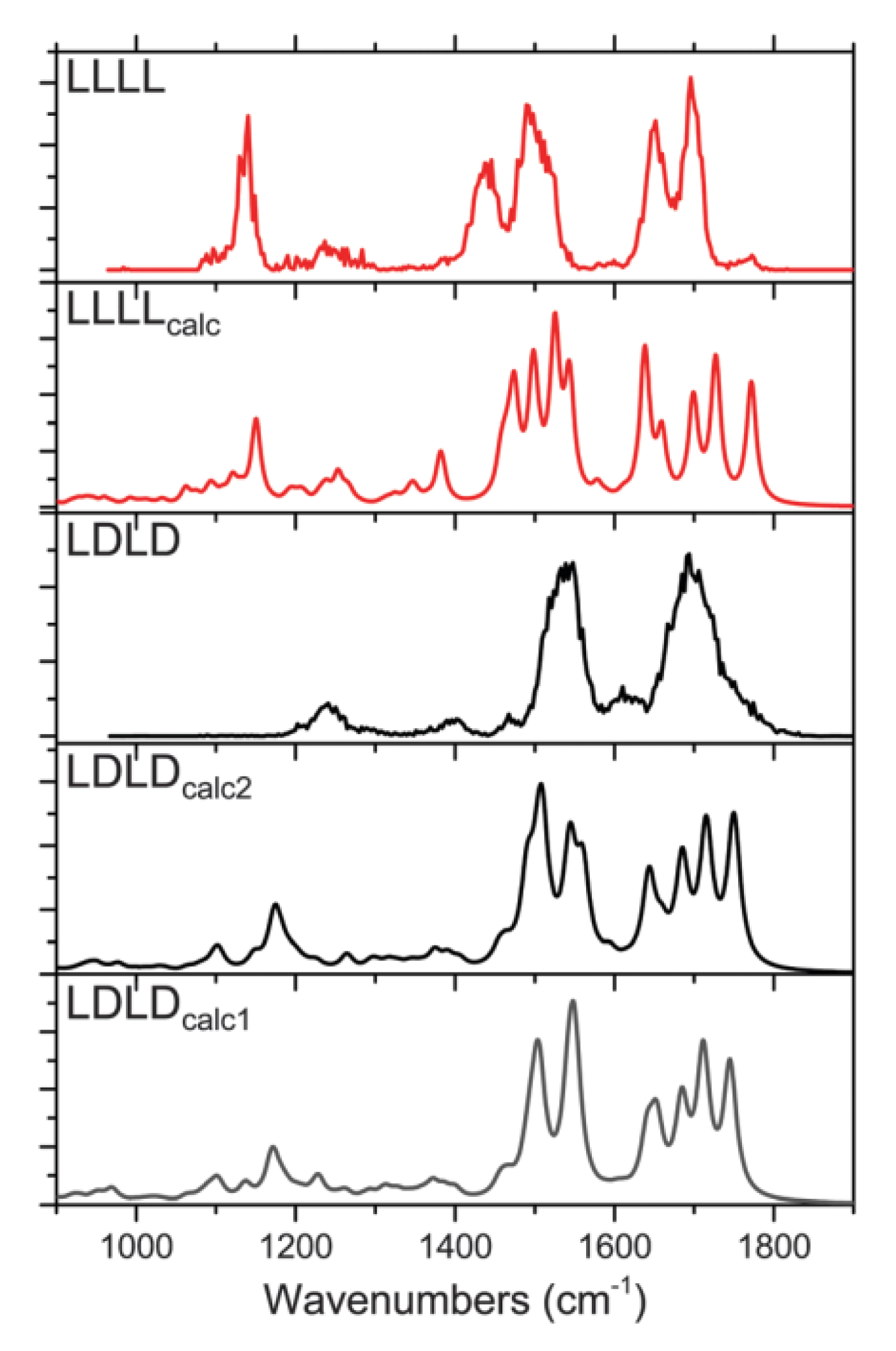
3.6. Molecules of Diastereomers
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Ward, T.J.; Baker, B.A. Chiral Separations. Anal. Chem. 2008, 80, 4363–4372. [Google Scholar] [CrossRef]
- Wenzel, T.J. Differentiation of Chiral Compounds Using NMR Spectroscopy, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Lanucara, F.; Holman, S.W.; Gray, C.J.; Eyers, C.E. The power of ion mobility-mass for structural characterization and the study of conformational dynamics. Nat. Chem. 2014, 6, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.A.; Cooks, R.G. Chiral analysis by MS. Anal. Chem. 2003, 75, 25A–31A. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Vog, F.G. A review of recent advances in mass spectrometric methods for gas-phase chiral analysis of pharmaceutical and biological compounds. Pharm. Biomed. Anal. 2012, 69, 133–147. [Google Scholar] [CrossRef]
- Awad, H.; El-Aneed, A. Enantioselectivity of mass spectrometry: Challenges and promises. Mass Spectrom. Rev. 2013, 32, 466–483. [Google Scholar] [CrossRef]
- Yu, X.; Yao, Z.P. Chiral recognition and determination of enantiometric excess by mass spectrometry: A review. Anal. Chim. Acta 2017, 968, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Han, D.Q.; Yao, Z.P. Chiral mass spectrometry: An overview. Trends Anal. Chem. 2020, 123, 115763. [Google Scholar] [CrossRef]
- Okumura, M.; Yeh, L.; Lee, Y.T. The vibrational predissociation spectroscopy of hydrogen cluster ions. J. Chem. Phys. 1985, 83, 3705–3706. [Google Scholar] [CrossRef]
- Oomens, J.; van Roij, A.J.A.; Meuer, G.; von Helden, G. Gas-phase infrared photodissociation spectroscopy of cationic polyaromatic hydrocarbons. Astrophys. J. 2000, 542, 404–410. [Google Scholar] [CrossRef]
- Oh, H.; Breuker, K.; Sze, S.K.; Ge, Y.; Carpenter, B.K.; McLafferty, F.W. Secondary and tertiary structures of gaseous protein ions characterized by electron capture dissociation mass spectrometry and photofragment spectroscopy. Proc. Natl. Acad. Sci. USA 2002, 99, 15863–15868. [Google Scholar] [CrossRef]
- Oh, H.B.; Lin, C.; Hwang, H.Y.; Zhai, H.L.; Breuker, K.; Zabrouskov, V.; Carpenter, B.K.; McLafferty, F.W. Infrared Photodissociation Spectroscopy of Electrosprayed Ions in a Fourier Transform Mass Spectrometer. J. Am. Chem. Soc. 2005, 127, 4076–4083. [Google Scholar] [CrossRef] [PubMed]
- Polfer, N.C.; Oomens, J. Vibrational spectroscopy of bare and solvated ionic complexes of biological relevance. J. Mass Spectrom. Rev. 2009, 28, 468–494. [Google Scholar] [CrossRef]
- Eyler, J.R. Infrared multiple photon dissociation spectroscopy of ions in Penning traps. Mass Spectrom. Rev. 2009, 28, 448–467. [Google Scholar] [CrossRef]
- Brodbelt, S. Infrared Multiphoton Dissociation in Quadrupole Time-of-Flight Mass Spectrometry: Top-Down Characterization of Proteins. Chem. Soc. Rev. 2014, 43, 2757–2783. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liao, G.H.; Kong, X.L. Charge-state Resolved Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy of Ubiquitin Ions in the Gas Phase. Sci. Rep. 2017, 7, 16592. [Google Scholar] [CrossRef] [PubMed]
- Maitre, P.; Scuderi, D.; Corinti, D.; Chiavarino, B.; Crestoni, M.E.; Fornarini, S. Applications of infrared multiple photon dissociation (IRMPD) to the detection of posttranslational modifications. Chem. Rev. 2020, 120, 3261–3295. [Google Scholar] [CrossRef]
- Zehnacker, A.; Suhm, M.A. Chirality Recognition between Neutral Molecules in the Gas Phase. Angew. Chem. Int. Ed. 2008, 47, 6970–6992. [Google Scholar] [CrossRef]
- Le Barbu-Debus, K.; Broquier, M.; Mahjoub, A.; Zehnacker-Rentien, A. Chiral recognition in jet-cooled complexes of (1R,2S)-(+)-cis-1-amino-2-indanol and methyl lactate: On the importance of the CH···π interaction. Phys. Chem. Chem. Phys. 2009, 11, 7589–7598. [Google Scholar] [CrossRef]
- Bouchet, A.; Klyne, J.; Piani, G.; Dopfer, O.; Zehnacker, A. Diastereo-specific conformational properties of neutral, protonated and radical cation forms of (1R,2S)-cis- and (1R,2R)-trans-amino-indanol by gas phase spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 25809–25821. [Google Scholar] [CrossRef]
- Barbu-Debus, K.; Broquier, M.; Mahjoub, A.; Zehnacker-Rentien, A. Chiral Recognition between r-Hydroxylesters: A Double-Resonance IR/UV Study of the Complexes of Methyl Mandelate with Methyl Glycolate and Methyl Lactate. J. Phys. Chem. A 2008, 112, 9731–9741. [Google Scholar] [CrossRef]
- Albrecht, M.; Soloshonok, V.A.; Schrader, L.; Yasumoto, M.; Suhm, M.A. Chirality-dependent sublimation of α-(trifluoromethyl)-lactic acid: Relative vapor pressures of racemic, eutectic, and enantiomerically pure forms, and vibrational spectroscopy of isolated (s,s) and (s,r) dimers. J. Fluor. Chem. 2010, 131, 495–504. [Google Scholar] [CrossRef]
- Filippi, A.; Fraschetti, C.; Piccirillo, S.; Rondino, F.; Botta, B.; D’Acquarica, I.; Calcaterra, A.; Speranza, M. Chirality Effects on the IRMPD Spectra of Basket Resorcinarene/Nucleoside Complexes. Chem. Eur. J. 2012, 18, 8320–8328. [Google Scholar] [CrossRef]
- Rondino, F.; Ciavardini, A.; Satta, M.; Paladini, A.; Fraschetti, C.; Filippi, A.; Botta, B.; Calcaterra, A.; Speranza, M.; Giardini, A.; et al. Ultraviolet and infrared spectroscopy of neutral and ionic non-covalent diastereomeric complexes in the gas phase. Rend. Fis. Acc. Lincei 2013, 24, 259–267. [Google Scholar] [CrossRef]
- Cooks, R.G.; Zhang, D.; Koch, K.J.; Gozzo, F.C.; Eberlin, M.N. Chiroselective Self-Directed Octamerization of Serine: Implications for Homochirogenesis. Anal. Chem. 2001, 73, 3646–3655. [Google Scholar] [CrossRef]
- Counterman, A.E.; Clemmer, D.E. Magic Number Clusters of Serine in the Gas Phase. J. Phys. Chem. B 2001, 105, 8092–8096. [Google Scholar] [CrossRef]
- Nanita, S.C.; Takats, Z.; Cooks, R.G.; Myung, S.; Clemmer, D.E. Chiral Enrichment of Serine via Formation, Dissociation, and Soft-Landing of Octameric Cluster Ions. J. Am. Soc. Mass. Spectrom. 2004, 15, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.J.; Gozzo, F.C.; Nanita, S.C.; Takats, Z.; Eberlin, M.N.; Cooks, R.G. Chiral Transmission between Amino Acids: Chirally Selective Amino Acid Substitution in the Serine Octamer as a Possible Step in Homochirogenesis. Angew. Chem. Int. Ed. 2002, 114, 1797–1800. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Roussel, C.; Kitagawa, O.; Sorochinsky, A.E. Self-disproportionation of enantiomers via achiral chromatography: A warning and an extra dimension in optical purifications. Chem. Soc. Rev. 2012, 41, 4180–4188. [Google Scholar] [CrossRef]
- Han, J.; Wzorek, A.; Kwiatkowska, M.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE) of amino acids and their derivatives. Amino Acids 2019, 51, 865–889. [Google Scholar] [CrossRef]
- Takats, Z.; Nanita, S.C.; Cooks, R.G. Serine Octamer Reactions: Indicators of Prebiotic Relevance. Angew. Chem. Int. Ed. 2010, 42, 3521–3523. [Google Scholar] [CrossRef]
- Ren, J.; Bian, S.; Wang, Y.; Kong, X. Magic-number Cluster of Serine Octamer: Structure and Chiral Characteristics. Prog. Chem. 2018, 30, 383–397. [Google Scholar]
- Kong, X.; Tsai, I.A.; Sabu, S.; Han, C.C.; Lee, Y.T.; Chang, H.C.; Tu, S.Y.; Kung, A.H.; Wu, C.-C. Progressive stabilization of zwitterionic structures in [H(Ser)2–8]+ studied by infrared photodissociation spectroscopy. Angew. Chem. Int. Ed. 2006, 45, 4130–4134. [Google Scholar] [CrossRef]
- Kong, X.; Lin, C.; Infusini, G.; Oh, H.B.; Jiang, H.; Breuker, K.; Wu, C.C.; Charkin, O.P.; Chang, H.C.; McLafferty, F.W. Numerous isomers of serine octamer ions characterized by infrared photodissociation spectroscopy. Chem. Phys. Chem. 2009, 10, 2603–2606. [Google Scholar] [CrossRef]
- Seo, J.; Warnke, S.; Pagel, K.; Bowers, M.T.; von Helden, G. Infrared spectrum and structure of the homochiral serine octamer-dichloride complex. Nat. Chem. 2017, 9, 1263–1268. [Google Scholar] [CrossRef]
- Scutelnic, V.; Perez, M.A.S.; Marianski, M.; Warnke, S.; Gregor, A.; Rothlisberger, U.; Bowers, M.T.; Baldauf, C.; von Helden, G.; Rizzo, T.R.; et al. The Structure of the Protonated Serine Octamer. J. Am. Chem. Soc. 2018, 140, 7554–7560. [Google Scholar] [CrossRef] [PubMed]
- Sunahori, F.X.; Yang, G.; Kitova, E.N.; Klassen, J.S.; Xu, Y.J. Chirality Recognition of the Protonated Serine Dimer and Octamer by Infrared Multiphoton Dissociation Spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 1873–1886. [Google Scholar] [CrossRef]
- Liao, G.; Yang, Y.; Kong, X. Chirality Effects on Proline-Substituted Serine Octamers Revealed by Infrared Photodissociation Spectroscopy. Phys. Chem. Chem. Phys. 2013, 16, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Y.Y.; Feng, R.X.; Kong, X.L. Investigation of L/D-Threonine Substituted L-Serine Octamer Ions by Mass Spectrometry and Infrared Photodissociation Spectroscopy. Chin. Chem. Lett. 2017, 28, 57–60. [Google Scholar] [CrossRef]
- Ren, J.; Ma, Y.; Li, S.Q.; Kong, X.L. Infrared photodissociation spectra of phenylalanine-substituted serine octamer ion and its application in chiral analysis. Chin. J. Anal. Test. 2018, 37, 1269–1273. [Google Scholar]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Xiao, Y.; Ng, S.C.; Tan, T.T.Y.; Wang, Y. Recent Development of Cyclodextrin Chiral Stationary Phases and their Applications in Chromatography. J. Chromatogr. A 2012, 1269, 52–68. [Google Scholar] [CrossRef]
- Ramirez, J.; Ahn, S.H.; Grigorean, G.; Lebrilla, C.B. Evidence for the formation of gasphase inclusion complexes with cyclodextrins and amino acids. J. Am. Chem. Soc. 2000, 122, 6884–6890. [Google Scholar] [CrossRef]
- Lebrilla, C.B. The gas-phase chemistry of cyclodextrin inclusion complexes. Acc. Chem. Res. 2001, 34, 653–661. [Google Scholar] [CrossRef]
- Elena, A. Molecular Simulation of the Separation of Isoleucine Enantiomers by β-Cyclodextrin. Molecules 2019, 24, 1021. [Google Scholar]
- Wang, S.Y.; Li, L.; Xiao, Y.; Wang, Y. Recent advances in cyclodextrins-based chiral-recognizing platforms. Trends Anal. Chem. 2019, 121, 115691. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, S.; Hong, Y.; Lee, J.U.; Kim, J.H.; Yoon, D.; Kong, X.L.; Lee, S.; Oh, H.B. Chiral Differentiation of D- and L-Alanine by Permethylated β-Cyclodextrin: IRMPD Spectroscopy and DFT Methods. Phys. Chem. Chem. Phys. 2017, 19, 14729–14737. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, J.U.; Oh, J.h.; Park, S.; Hong, Y.; Min, B.K.; Lee, H.H.L.; Kim, H.I.; Kong, X.L.; Lee, S.; et al. Chiral Differentiation of D- and L-Isoleucine Using Permethylated β-Cyclodextrin: Infrared Multiple Photon Dissociation Spectroscopy, Ion-Mobility Mass Spectrometry, and DFT Calculations. Phys. Chem. Chem. Phys. 2018, 20, 30428–30436. [Google Scholar] [CrossRef]
- Sun, L.; Huang, F.; Liu, W.; Lin, L.; Hong, Y.; Kong, X.L. Chiral Differentiation of L- and D-penicillamine by β-Cyclodextrin: Investigated by IRMPD Spectroscopy and Theoretical Simulations. Spectrochim. Acta A 2020, 241, 118653. [Google Scholar] [CrossRef]
- Hirata, K.; Mori, Y.; Ishiuchi, S.; Fujii, M.; Zehnacker, A. Chiral discrimination between tyrosine and β-cyclodextrin revealed by cryogenic ion trap infrared spectroscopy. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef]
- Filippi, A.; Fraschetti, C.; Guarcini, L.; Zazza, C.; Ema, T.; Speranza, M. Spectroscopic Discrimination of Diastereomeric Complexes Involving an Axially Chiral Receptor. Chem. Phys. Chem. 2017, 18, 2475–2481. [Google Scholar] [CrossRef]
- Klyne, J.; Bouchet, A.; Ishiuchi, S.; Fujii, M.; Schneider, M.; Baldaufcd, C.; Dopfer, O. Probing chirality recognition of protonated glutamic acid dimers by gas-phase vibrational spectroscopy and first-principles simulations. Phys. Chem. Chem. Phys. 2018, 20, 28452–28464. [Google Scholar] [CrossRef]
- Poline, M.; Rebrov, O.; Larsson, M.; Zhaunerchyk, V. Theoretical studies of infrared signatures of proton-bound amino acid dimers with homochiral and heterochiral moieties. Chirality 2020, 32, 359–369. [Google Scholar] [CrossRef]
- Barbu-Debus, K.; Scuderi, D.; Lepere, V.; Zehnacker, A. Homochiral vs. heterochiral sodium core dimers of tartaric acid esters:A mass spectrometry and vibrational spectroscopy study. J. Mol. Struct. 2020, 1205, 127583. [Google Scholar] [CrossRef]
- Robert, C.D.; Jeffrey, D.S.; Jos, O. Chirality-Induced Conformational Preferences in Peptide-Metal Ion Binding Revealed by IR Spectroscopy. J. Am. Chem. Soc. 2011, 133, 1212–1215. [Google Scholar]
- Crestoni, M.E.; Chiavarino, B.; Scuderi, D.; Di Marzio, A.; Fornarini, S. Discrimination of 4-Hydroxyproline Diastereomers by Vibrational Spectroscopy of the Gaseous Protonated Species. J. Phys. Chem. B 2012, 116, 8771–8779. [Google Scholar] [CrossRef]
- Alata, I.; Pérez-Mellor, A.F.; Nasr, F.B.; Scuderi, D.; Steinmetz, V.; Gobert, F.; Nejm-Eddine, J.; Zehnacker-Rentien, A. Does the Residues Chirality Modify the Conformation of a Cyclo-Dipeptide? Vibrational Spectroscopy of Protonated Cyclo-diphenylalanine in the Gas Phase. J. Phys. Chem. A 2017, 121, 7130–7138. [Google Scholar] [CrossRef]
- Lepere, V.; Barbu-Debus, K.; Clavaguéra, C.; Scuderi, D.; Piani, G.; Simon, A.L.; Chirot, F.; MacAleese, L.; Dugourde, P.; Zehnacker, A. Chirality-dependent structuration of protonated or sodiated polyphenylalanines: IRMPD and ion mobility studies. Phys. Chem. Chem. Phys. 2016, 18, 1807–1817. [Google Scholar] [CrossRef]
- Scuderi, D.; Barbu-Debus, K.; Zehnacker, A. The role of weak hydrogen bonds in chiral recognition. Phys. Chem. Chem. Phys. 2011, 13, 17916–17929. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, U.J.; Rizzo, T.R. Multiple isomers and protonation sites of the phenylalanine/serine dimer. J. Am. Chem. Soc. 2012, 134, 11053–11055. [Google Scholar] [CrossRef] [PubMed]
- Kong, X. Reinvestigation of the Structure of Protonated Lysine Dimer. J. Am. Soc. Mass. Spectrom. 2014, 25, 422–426. [Google Scholar] [CrossRef]
- Ma, L.; Ren, J.; Feng, R.; Zhang, K.; Kong, X. Structural Characterizations of Protonated Homodimers of Amino Acids: Revealed by Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy and Theoretical Calculations. Chin. Chem. Lett. 2018, 29, 1333–1339. [Google Scholar] [CrossRef]
- Tao, W.A.; Zhang, D.X.; Nikolaev, E.N.; Cooks, R.G. Copper(II)-assisted enantiomeric analysis of D,L-amino acids using the kinetic method: Chiral recognition and quantification in the gas phase. J. Am. Chem. Soc. 2000, 122, 10598–10609. [Google Scholar] [CrossRef]
- Tao, W.A.; Gozzo, F.C.; Cooks, R.G. Mass spectrometric quantitation of chiral drugs by the kinetic method. Anal. Chem. 2001, 73, 1692–1698. [Google Scholar] [CrossRef]
- Wu, L.M.; Cooks, R.G. Chiral analysis using the kinetic method with optimized fixed ligands: Applications to some antibiotics. Anal. Chem. 2003, 75, 678–684. [Google Scholar] [CrossRef]
- Yao, Z.P.; Wan, T.S.M.; Kwong, K.P.; Che, C.T. Chiral recognition of amino acids by electrospray ionisation mass spectrometry/mass spectrometry. Chem. Commun. 1999, 20, 2119–2120. [Google Scholar] [CrossRef]
- Yao, Z.P.; Wan, T.S.M.; Kwong, K.P.; Che, C.T. Chiral analysis by electrospray ionization mass spectrometry/mass spectrometry. 1. Chiral recognition of 19 common amino acids. Anal. Chem. 2000, 72, 5383–5393. [Google Scholar] [CrossRef]
- Yao, Z.P.; Wan, T.S.M.; Kwong, K.P.; Che, C.T. Chiral analysis by electrospray ionization mass spectrometry/mass spectrometry. 2. Determination of enantiomeric excess of amino acids. Anal. Chem. 2000, 72, 5394–5401. [Google Scholar] [CrossRef]
- Kong, X.L. Chiral Differentiation of Amino Acids by the Kinetic Method by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry via a Different Dissociation Pathway. Rapid Commun. Mass Spectrom. 2012, 26, 870–873. [Google Scholar] [CrossRef]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Quantitative Chiral Analysis of Tryptophan Using Enantiomer-Selective Photolysis of Cold Non-Covalent Complexes in the Gas Phase. J. Mass Spectrom. 2015, 50, 451–453. [Google Scholar] [CrossRef]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Chiral Recognition Between L-alanine Peptides and Tryptophan Enantiomers Probed by Ultraviolet Photodissociation in the Gas Phase. J. Mass Spectrom. 2016, 51, 257–260. [Google Scholar] [CrossRef]
- Fujihara, A.; Maeda, N.; Doan, T.N.; Hayakawa, S. Enantiomeric Excess Determination for Monosaccharides Using Chiral Transmission to Cold Gas-Phase Tryptophan in Ultraviolet Photodissociation. J. Am. Soc. Mass Spectrom. 2017, 28, 224–228. [Google Scholar] [CrossRef]
- Fujihara, A.; Inoue, H.; Sogi, M.; Tajiria, M.; Wada, Y. Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase. Molecules 2018, 23, 162. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, M.; Zhang, K.; Ma, L.; Kong, X. Chiral Differentiation of Non-covalent Diastereomers Based on Multichannel Dissociation Induced by 213 nm Ultraviolet Photodissociation. J. Am. Soc. Mass. Spectrom. 2019, 30, 2297–2305. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Du, M.; Ren, J.; Zhang, K.; Xu, Y.; Kong, X. Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis. Molecules 2020, 25, 5152. https://doi.org/10.3390/molecules25215152
Shi Y, Du M, Ren J, Zhang K, Xu Y, Kong X. Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis. Molecules. 2020; 25(21):5152. https://doi.org/10.3390/molecules25215152
Chicago/Turabian StyleShi, Yingying, Mengying Du, Juan Ren, Kailing Zhang, Yicheng Xu, and Xianglei Kong. 2020. "Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis" Molecules 25, no. 21: 5152. https://doi.org/10.3390/molecules25215152
APA StyleShi, Y., Du, M., Ren, J., Zhang, K., Xu, Y., & Kong, X. (2020). Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis. Molecules, 25(21), 5152. https://doi.org/10.3390/molecules25215152





