Abstract
A new modified ion-selective electrode with membranes of LaF3 single crystals with different internal contacts (solid steel or electrolyte) and with FexOy nanoparticles as loading was developed. The best response characteristic with linear potential change was found in the fluoride concentration range from 10−1 to 3.98 × 10−7 M. The detection limit for the electrolyte contact was determined at 7.41 × 10−8 M with a regression coefficient of 0.9932, while the regression coefficient for the solid contact was 0.9969. The potential change per concentration decade ranged from 50.3 to 62.4 mV, depending on whether the contact was solid or electrolytic. The prepared modified electrode has a long lifetime, as well as the possibility of application in different positions (solid contact), and it can also be used for the determination of iron ions. The electrode characterization was performed with scanning electron microscopy and elemental analysis with the technique of electron-dispersive X-ray spectroscopy.
1. Introduction
Detection of fluoride and its complexes plays an important role in understanding the benefits, as well as the potential toxicity, of fluoride natural sources [1]. The fluoride ion-selective electrode (FISE) with LaF3 membrane is probably the most widely used ion-selective electrode (ISE) for practical measurements [2,3,4]. The electrode was described first by Frant and Ross [5]. Commercially available models, including the Orion Model 94-09, are constructed in the conventional ISE way, with the membrane arranged symmetrically between two solutions. Often, the life of the electrode is shortened because a reference electrode has lost contact with the membrane.
In many applications, such as online process analysis and clinical analysis, it is advantageous to replace the internal reference solution with a fixed contact. Solid contacts allow the construction of electrodes that can withstand high temperatures and pressures (e.g., autoclaving). To achieve the desired electrode quality in terms of sensitivity, response time, and stability, it is essential that the contact materials are in thermodynamic equilibrium at zero current. If equilibrium is not reached, potential instability and long-term potential drift can generally be expected. These effects depend on the speed of the relaxation processes, for example, at the blocked interface between an electronic and an ionic conductor [6].
Previously, internal contacts, based on the use of Ag2S, between the LaF3 membrane and a stainless-steel disc of the multipurpose solid-state electrode body [7], the redox reference system [8], and the Cu(II) ISE [9] were described. Bralic et al [6] described FISE with a simple solid contact using a laboratory version of the ISE body and a stainless-steel disc. In this instrument, the LaF3 membrane was built into a multipurpose electrode body, and contact with the instrument was made with a stainless-steel disc and coaxial cable. An ion-selective solid-state fluoride electrode was developed consisting of 70% Ag2S, 10% Cu2S, and 20% CaF2 [10].
The production of a novel FISE is necessary for several reasons; fluoride is important for trade and technology, its small quantities are vital for the human body although larger quantities are toxic, and its determination using previous techniques is difficult.
In recent years, much attention has been paid to the study of different types of cheap and efficient materials, such as different clays, for the removal of fluoride from the environment, especially from drinking and wastewater. Recently, fluoride ions have been investigated by adsorption on synthesized Fe2O3 nanoparticles [11,12,13].
Nanomaterials play an important role in the production of chemo- and biosensors, especially due to their unique physical and chemical properties, such as high surface/volume ratio, good conductivity, excellent electrocatalytic activity, and high mechanical strength. In recent years, nanomaterials have been gradually introduced into potentiometric sensors precisely because of their properties. For example, due to their exceptional electrical properties and good hydrophobicity, nanomaterials are suitable for ISE use in the solid state, since they can be dispersed directly in ion-selective membranes [14].
The manufacture of a gold-based ion sensor coated with Fe2O3 nanoparticles for the determination of fluoride is also described [15].
In reviewing the literature, we have not found recent work describing an FISE with metal nanoparticles. This paper describes an FISE with LaF3 membranes of different thickness and with different Eu ratios, to which iron oxide nanoparticles (FexOy NPs) were loaded. The purpose of FexOy NP loading was to improve the response characteristics of the prepared electrode in relation to the commercial FISE. The LaF3 membrane was mounted in a multipurpose electrode body, and contact with the instrument was made with a stainless-steel disc and coaxial cable or an internal electrolyte contact and an Ag/AgCl internal electrode. The electrode surfaces were recorded using scanning electron microscopy (SEM) and an elemental analysis was performed using electron-dispersive X-ray spectroscopy (EDS).
2. Results and Discussion
2.1. Potentiometric Measurements
The potential response of the FISE was measured using a two-electrode system. The solution was stirred and monitored throughout successive additions of known amounts of sodium fluoride. The FISE response to fluoride ion concentration is given by the Nernst equation.
where E, E′, and S denote the cell potential after addition of sodium fluoride, a conditional standard cell potential, and experimental slope, respectively.
Electrodes with membranes that were not treated with FexOy NPs showed linearity in the range within two concentration decades with a slope in the range from 10.4 to 33.9 mV for internal solid contact or in the range from 21.2 to 47.5 mV when the internal contact was electrolyte (Figures S1 and S2, Supplementary Materials; Table 1).

Table 1.
The response characteristics of electrodes without FexOy nanoparticles (NPs).
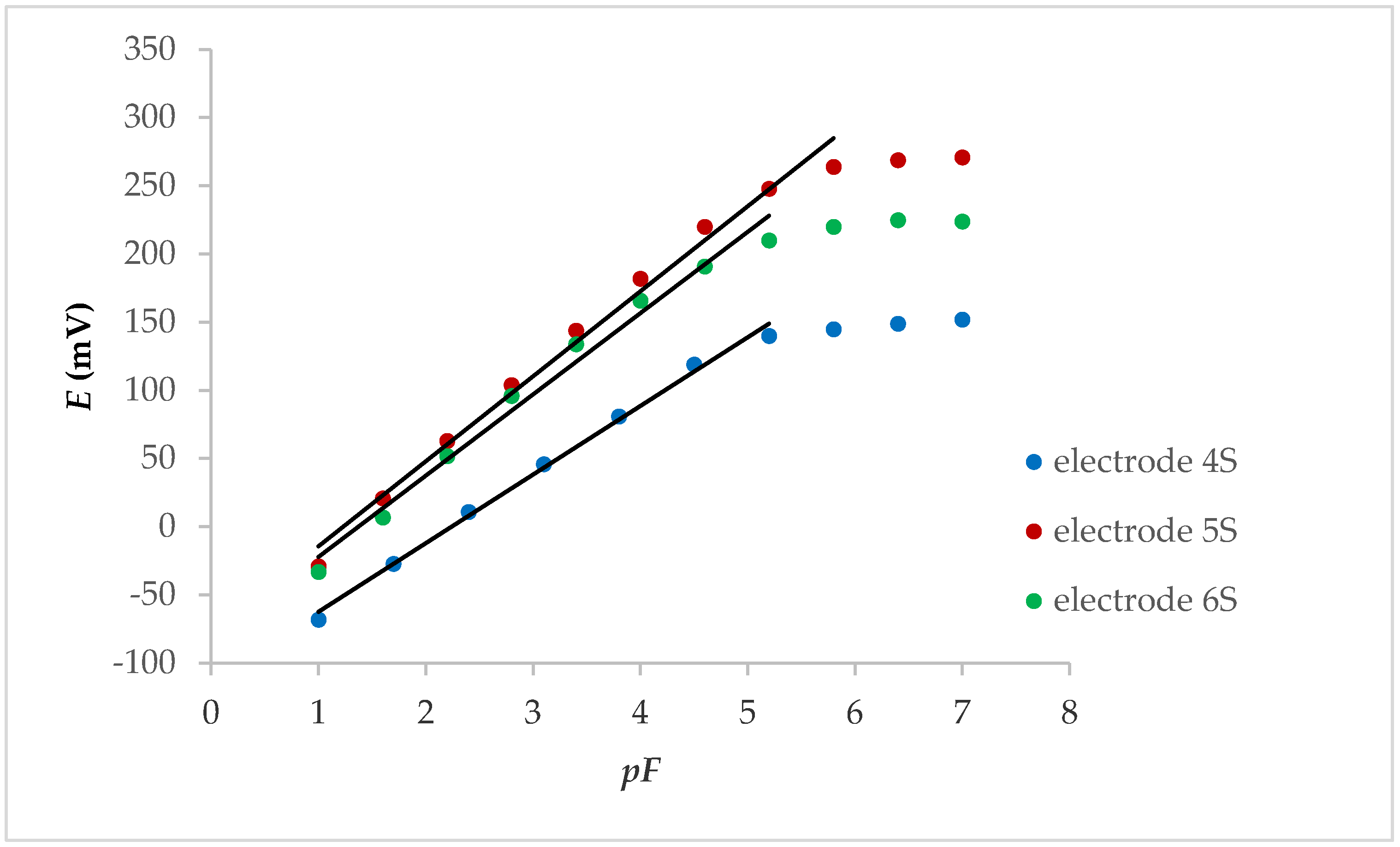
After preliminary measurements (without FexOy NPs), each of the electrodes mentioned above was tested after the FexOy NP loading on membranes. All three tested electrodes showed a linear potential change that was lower than the fluoride concentration of 1.00 × 10−5 M, while the potential changes per concentration decade at the internal solid contact were between 50.3 and 62.4 mV (Figure 1).
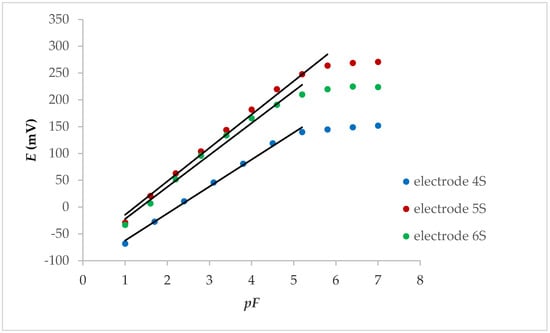
Figure 1.
Potentiometric response of internal solid contact LaF3 electrodes with FexOy NPs.
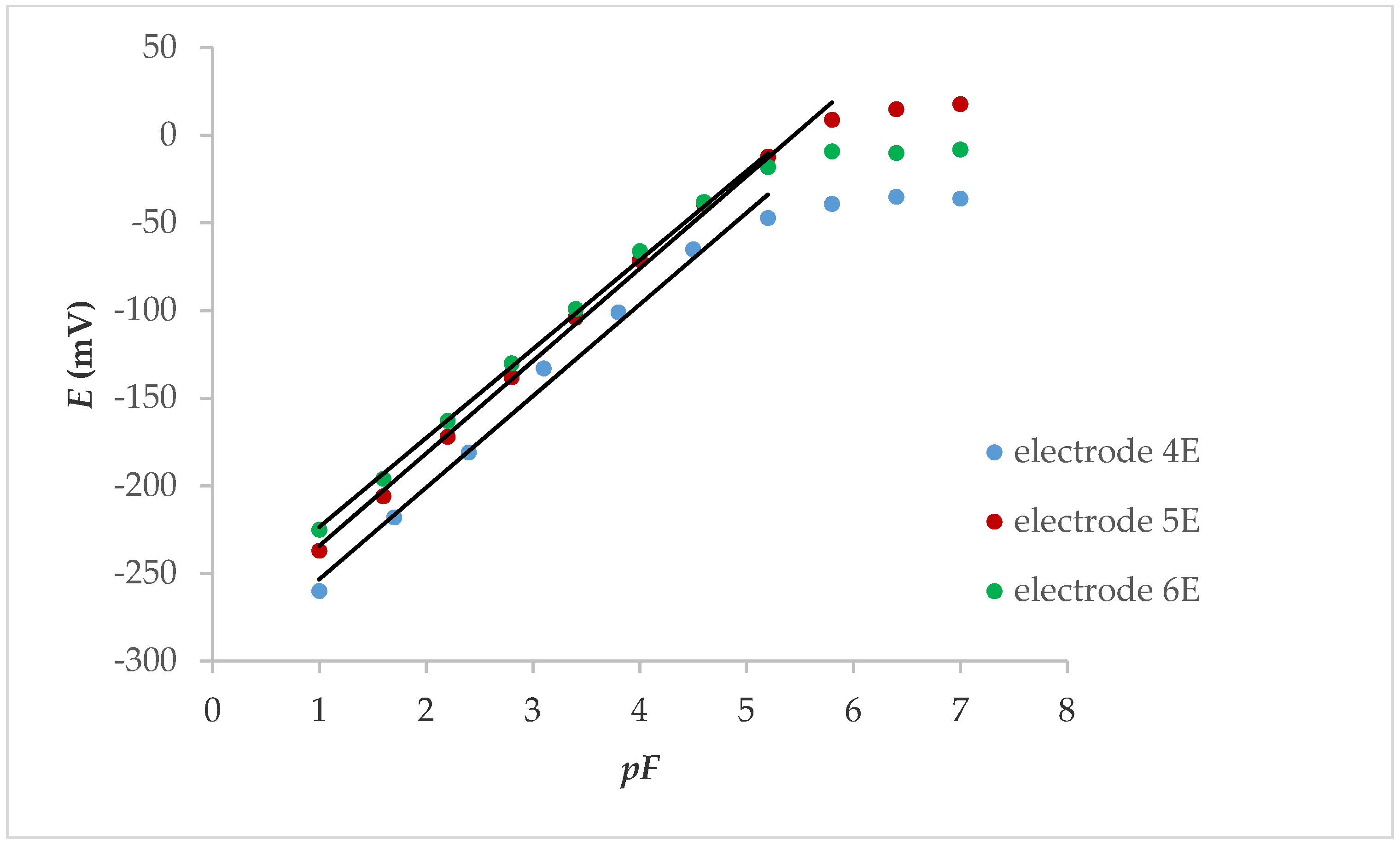
If the internal contact was an electrolyte (Figure 2), the linear response was also in the concentration range below 1.00 × 10−5 M, while the slope was between 50.8 and 52.7 mV, depending on whether the electrode was conditioned (24 h in 0.001 M KNO3 solution) or not. The response characteristics of the electrodes are shown in Table 2.
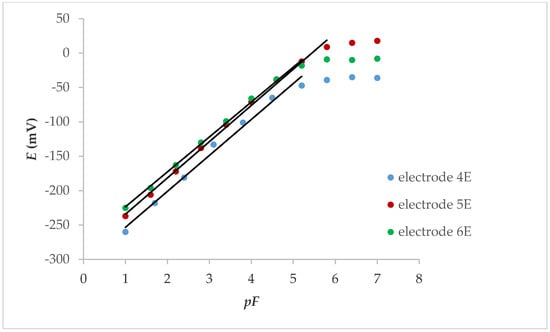
Figure 2.
Potentiometric response of internal electrolyte contact LaF3 electrodes with FexOy NPs.

Table 2.
The response characteristics of electrodes with FexOy NPs.
After the measurements described above, the FexOy NPs were washed off the membrane surface so that the membranes were left in 1 M nitric acid solution for 24 h. The response of the electrodes was tested, and it was observed that, after washing the FexOy NPs out from the surface, the electrodes showed an even wider linear range. The potential change per concentration decade was in the range of 52.9 to 57.3 mV for solid-state contact, while it was in the range of 44.1 to 54.3 mV for electrolyte contact (Figures S3 and S4, Supplementary Materials; Table 3).

Table 3.
The response characteristics of electrodes after washing the FexOy NPs out from surface.
Furthemore, the response of the electrode 7E to fluoride ions was compared with the response of the commercial electrode (Figure S5, Supplementary Materials).
2.1.1. pH Effect on the Electrode Response
Hydroxide ions are known to have great influence on fluoride determination by electrode with an LaF3 membrane. The penetration of OH− ions into the LaF3 crystal lattice plays an important role. The consequence is the release of F− ions from the lattice, their diffusion into solution, and a change in potential.
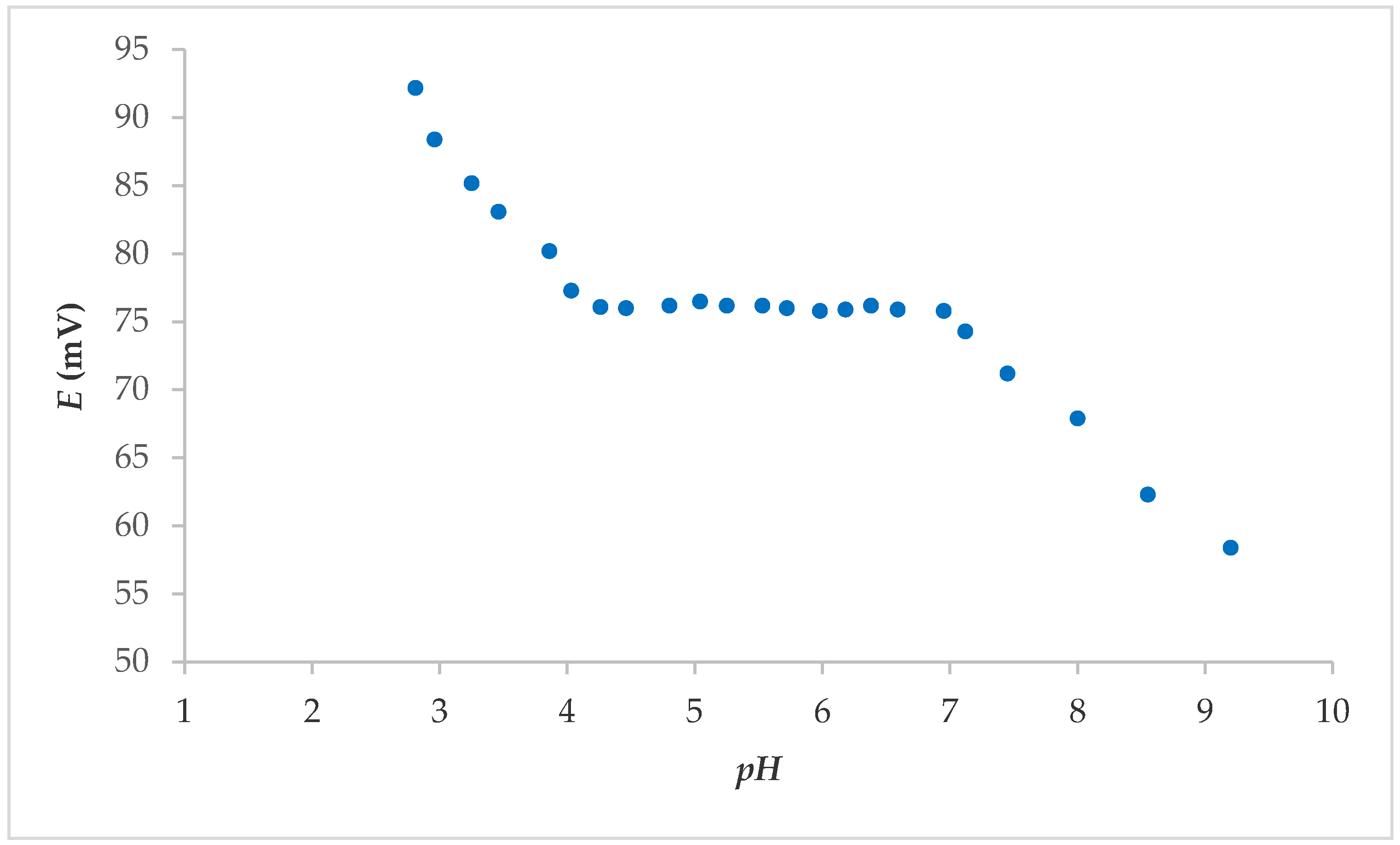
From the above, it was concluded that electrodes 7S and 7E showed the best properties overall; thus, it was further tested. The effect of the pH was determined by studying the fabricated electrode in solutions with an F− concentration of 1.00 × 10−3 M. The pH value was varied from 3 to 9 with the addition of NaOH. The potential change was a function of the pH value. The pH influence of electrode 7S is shown in Figure 3. As shown, the reaction of the sensors in the range 4–7 was independent of the pH influence. No visible interference from H3O+ or OH− ions was observed in this pH range. The pronounced influence of pH on the FISE is usually in the pH range below 4 and above 9 [16].
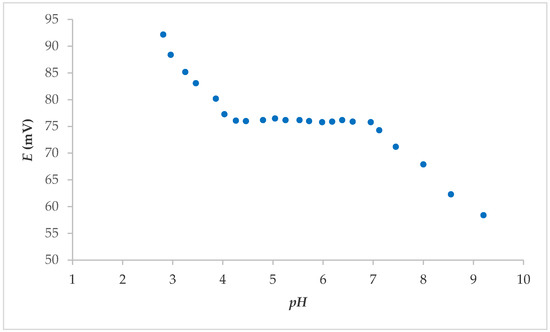
Figure 3.
pH effect on the electrode 7S response in F− solution (concentration: 1.00 × 10−3 M), after washing FexOy NPs from surface.
2.1.2. Response Time and Electrode Characteristics
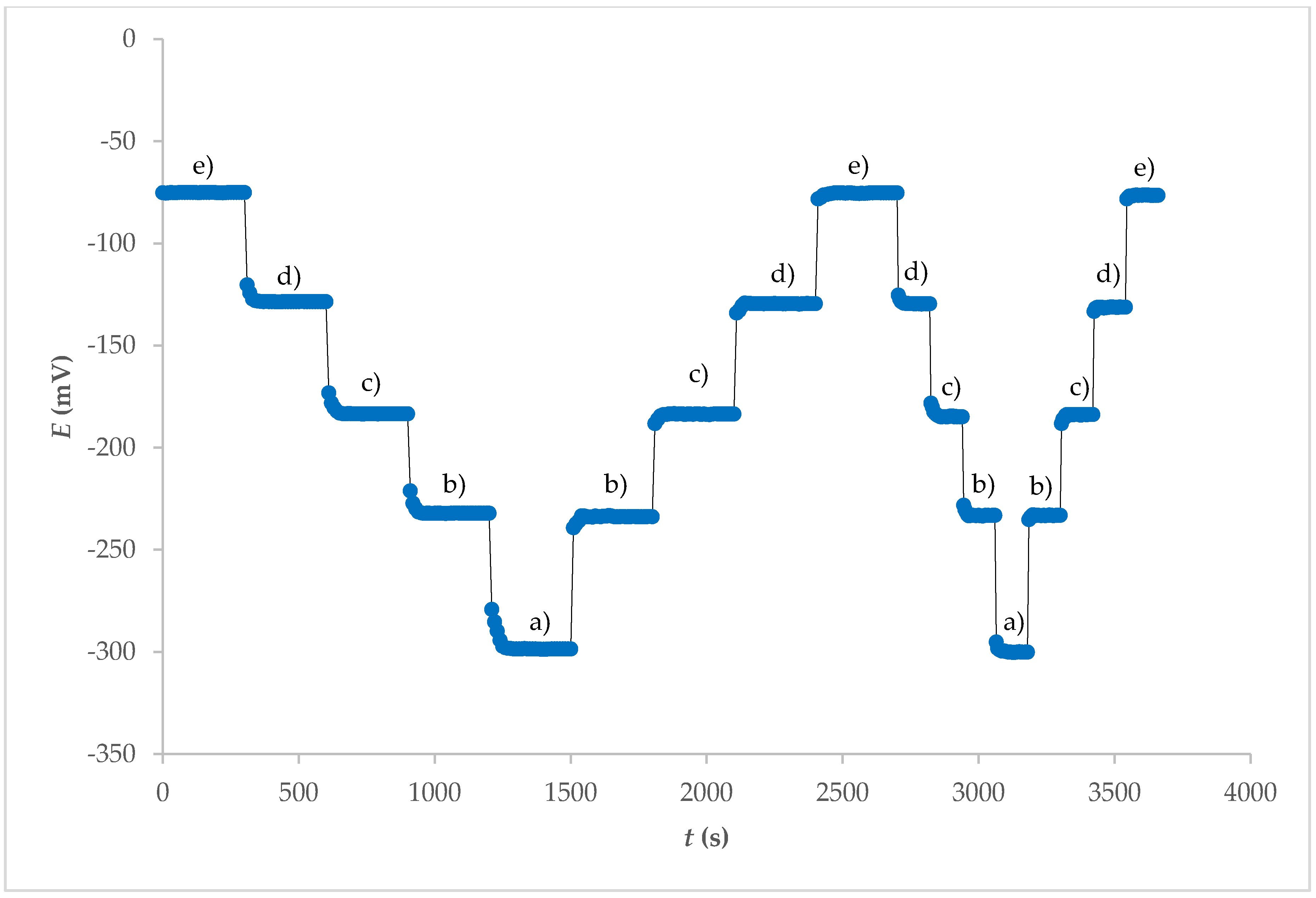
The response time of an ISE is also an important factor for any analytical application. Experimental conditions such as stirring, ionic strength, and composition of the test solution, as well as the concentration and composition of the solution to which the electrode was exposed, can have an influence on the experimental response time of a sensor. Before the experimental measurements are carried out, any previous use or preconditioning of the electrode and the test temperature can also have an influence on the response time [17]. The potential–time response curve of the electrode obtained from the internal electrolyte contact for different concentration ranges of F− ions is shown in Figure 4. The stationary potential was reached within 1 min. A similar response time, but in a smaller concentration range, was observed with fluoride ion sensors based on a crystal cadmium (II) Schiff base complex [18].
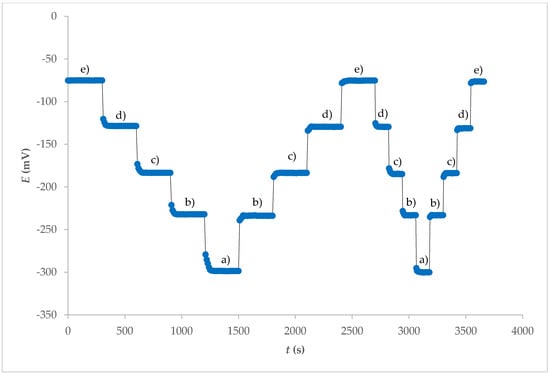
Figure 4.
Dynamic response and reproducibility of electrode 7E for different concentrations of F− at pH = 5 after washing FexOy NPs out from surface: (a) 1 × 10−1 M; (b) 1 × 10−2 M; (c) 1 × 10−3 M; (d) 1 × 10−4 M; (e) 1 × 10−5 M.
After the removal of FexOy NPs from the membrane surface, the response properties were improved for all electrodes tested. It is possible that iron from the oxide reacted with the F− ions (reaction 2) from the solution to form an FeF2+ or FeF2+ complex [19], which influenced the electrode reaction or contributed to the improved conductivity, since Fe was embedded in the membrane itself, as shown by the elemental analysis of the membrane (Table 4) and the changes observed on the membrane of the electrode surface.

Table 4.
Elemental analysis before FexOy NP loading.
Furthermore, it is obvious that the thickness of the membrane and the ratio of Eu influence the reaction properties. Specifically, the 1 mm thick membrane with 1% Eu showed the best response characteristics.
Compared to some of the FISEs described above [10], the electrode in this study showed a lower detection limit and easier replacement of the internal contacts. Moreover, the electrode described in this paper was much easier to prepare than the electrodes in the article mentioned.
The described electrode showed a better response than the electrode based on the crystal cadmium (II) Schiff base complex [18] and a similar response to a gold-based electrode coated with β-Fe2O3 [15], but the regression coefficient was better in this work.
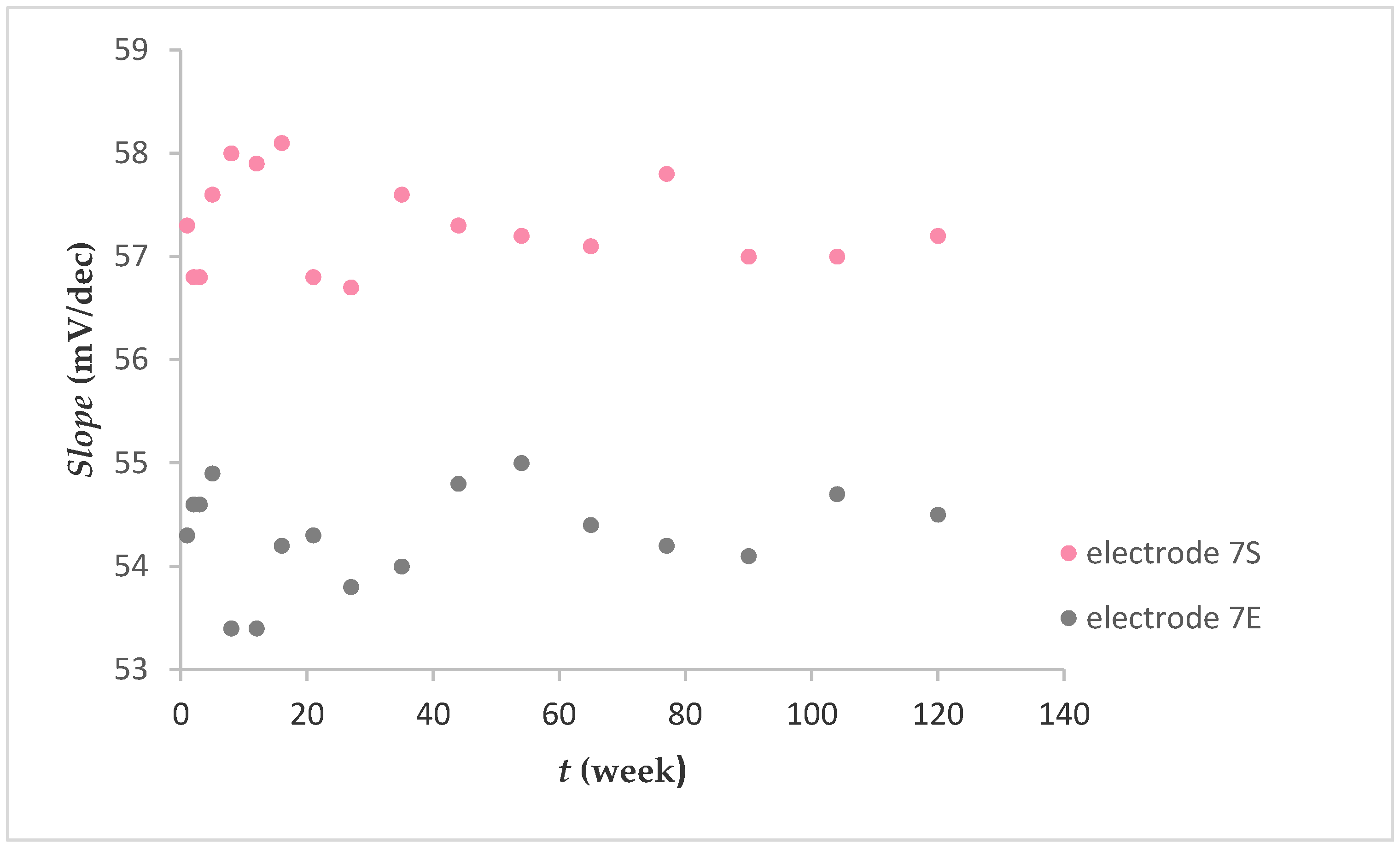
2.1.3. Lifetime of Electrode
Figure 5 shows the fluoride sensitivity expressed as mV per decade change in concentration (mV/dec) over a 120 week period. Electrodes 7S and 7E exhibited fluoride sensitivity with an average value of 54.3 ± 0.5 mV/dec and 57.3 ± 0.4 mV/dec, respectively. There were no noted losses in sensitivity. Generally, after preparing the electrodes and their use, they were stored in air. It was found that a prolonged dry storage of electrodes had no measurable effect on their responses.
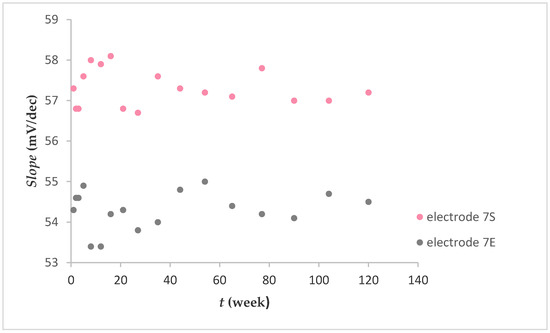
Figure 5.
Fluoride response over 120 weeks (after washing FexOy NPs out).
In contrast to the graphene-based FISE [20], where the lifetime of the electrode was limited, the lifetime of the electrode described in this paper (Figure 5) was almost unlimited (up to mechanical cracking).
2.1.4. Iron Ion Response Characteristics and Influence of the Interfering Ions
As it is known that fluorine forms stable complexes in water with a series of metal ions (most commonly with Al3+, Be2+, and Fe3+ ions), an electrode prepared in this way can be used to determine them [21].
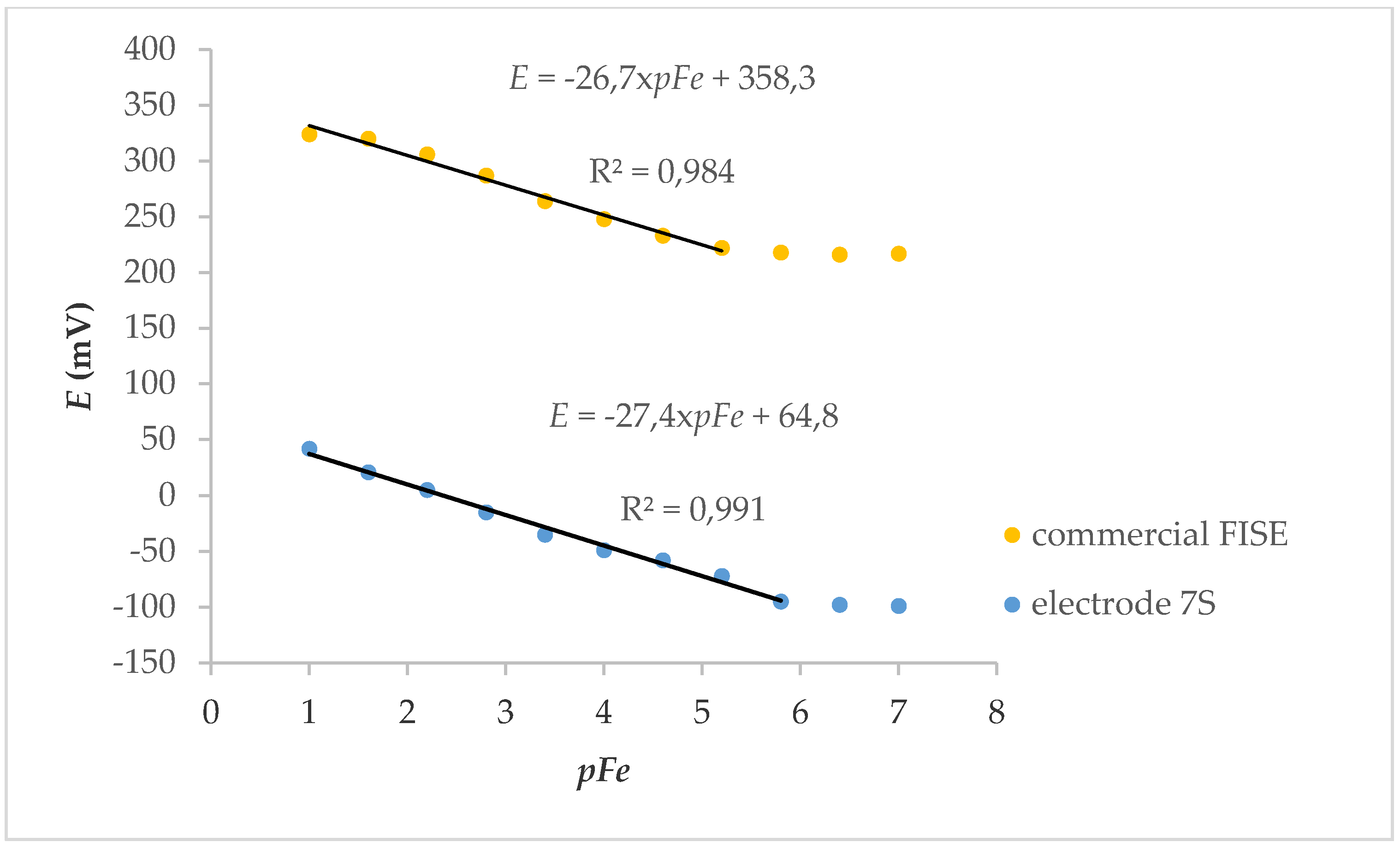
Electrode 7S was applied to the determination of iron ions, and the results are shown in Figure 6. A wider linear range with respect to the commercial electrode (and, consequently, a lower limit of detection) was observed.
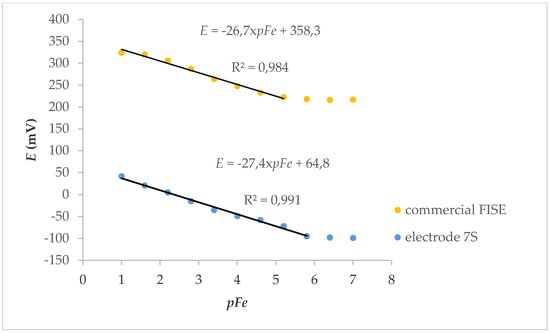
Figure 6.
Calibration curves for determination of Fe3+ with commercial and 7S fluoride ion-selective electrode (FISE).
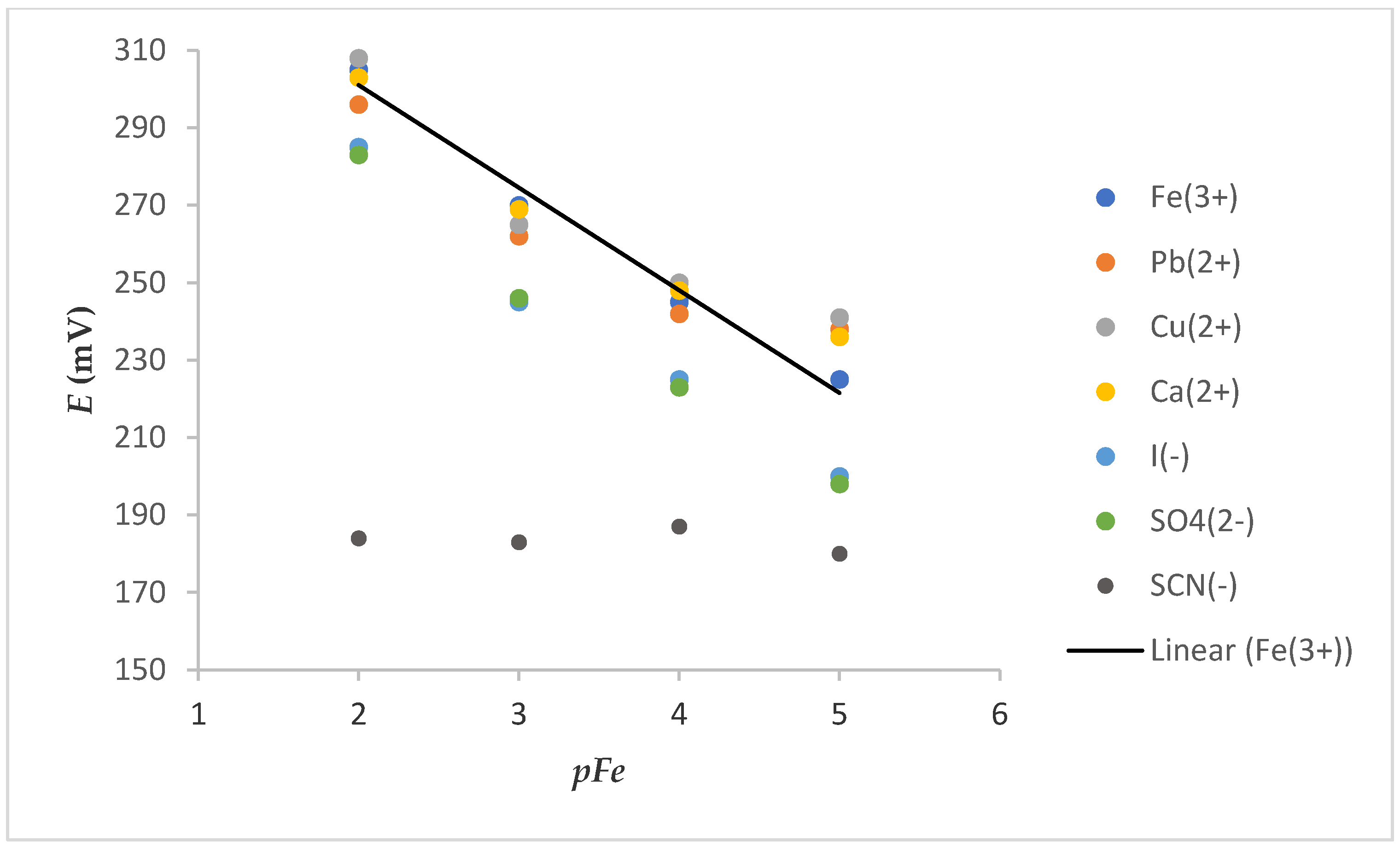
The selectivity of the ISE is one of its most important characteristics. It indicates the specificity of the sensor toward the target ion in the presence of interfering components. Slightly parallel shifts of calibration curves (Figure 7) were obtained in the presence of the tested cation (0.01 M) solution. This shift, which is more discernible at a low concentration of Fe3+, can be attributed to the change in ionic strength in the solution because of a high concentration of interfering cations. Some deviations were also observed in the presence of tested anions. These deviations were manifested as a decrease in the slope and could be explained by oxidoreduction reactions in the case of I−. The impossibility of iron determination in the presence of SCN− was probably due to strong complexation of Fe3+ with SCN− (KFe(SCN)3 = 109).
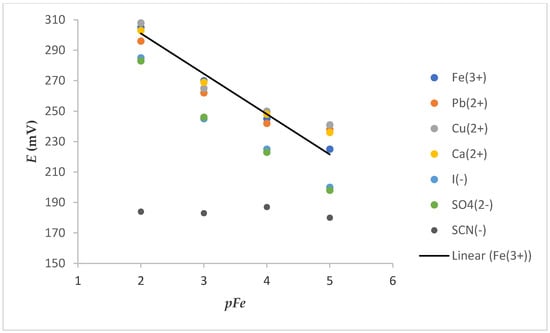
Figure 7.
Dependence of E vs. pFe on electrode 7S (after washing FexOy NPs out) in the presence of a constant concentration of various cations or anions (c = 0.01 M).
2.2. Membrane Characterization Using SEM
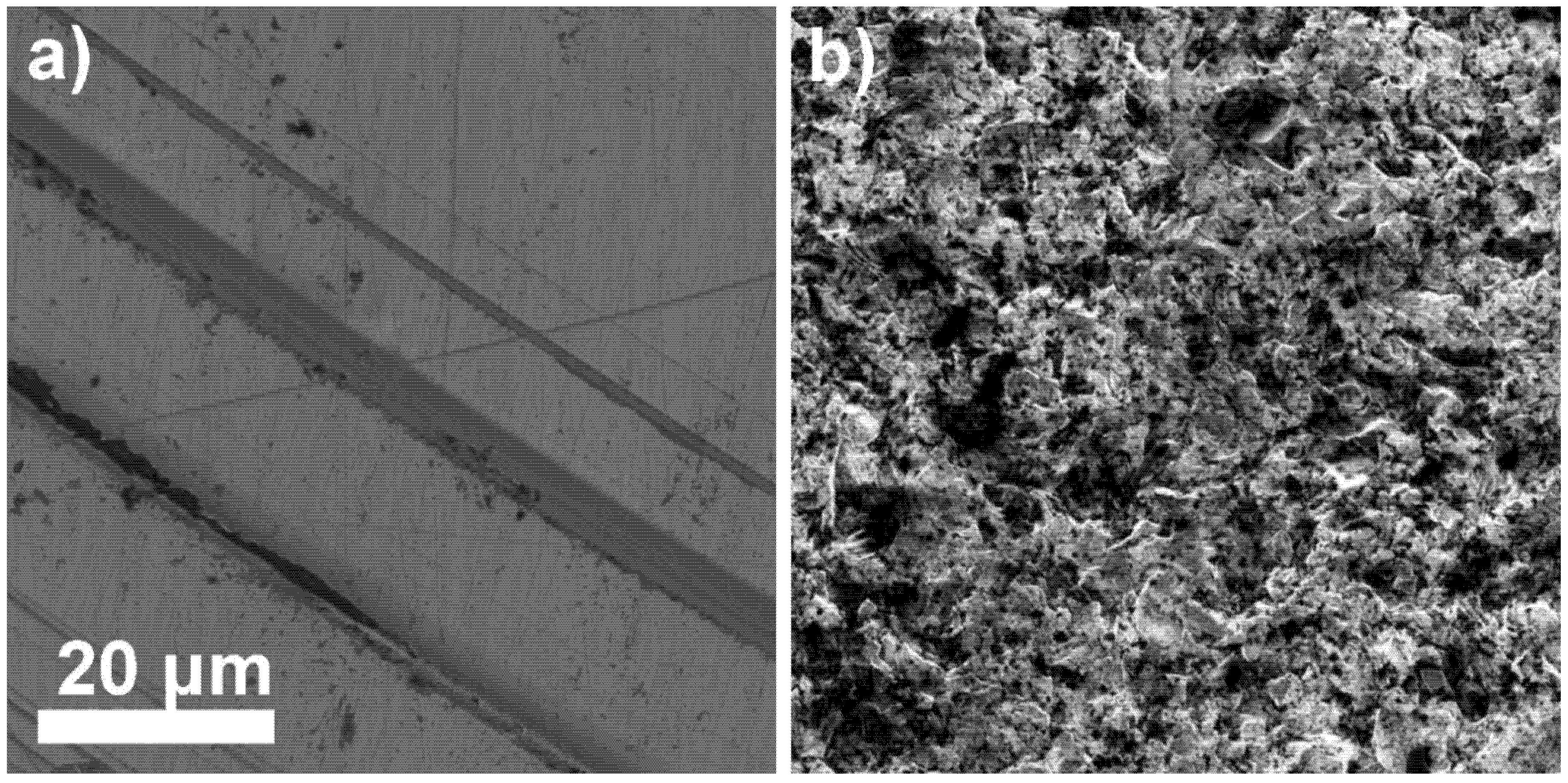
The LaF3 single-crystal membrane with diameter of 8.0 mm and thickness of 1.0 mm, doped with 1.0% Eu was morphologically analyzed (before the FexOy NP loading onto the membrane surface and after membrane acid treatment) using SEM at 3 kV. The membrane surface was smooth before the FexOy NP loading; however, tiny grooves are visible in Figure 8a, resulting from the polishing procedure. On the other hand, significant changes were observed on the FexOy NP surface-modified membrane after 24 h treatment in 1 M nitric acid solution (Figure 8b). The surface morphology in Figure 8a,b is noticeably different. The first surface is smooth with no porosity, while the second is rough with high surface area and visible open macropores. The reason for such a difference in surface morphology was the leaching out of some FexOy NPs from the electrode composite (LaF3/FexOy NPs).
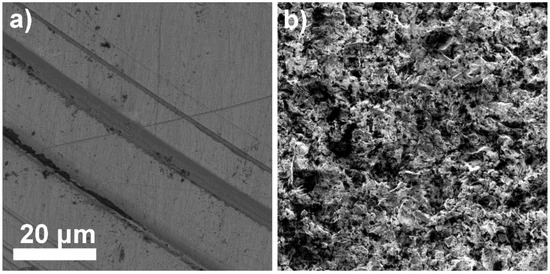
Figure 8.
LaF3 single-crystal membrane (with diameter of 8.0 mm and thickness of 1.0 mm, doped with 1.0% Eu) surface at 10,000× magnification (a) before FexOy NPs loading and (b) after leaching out some FexOy NPs.
2.3. Membrane Elemental Analysis Using EDS
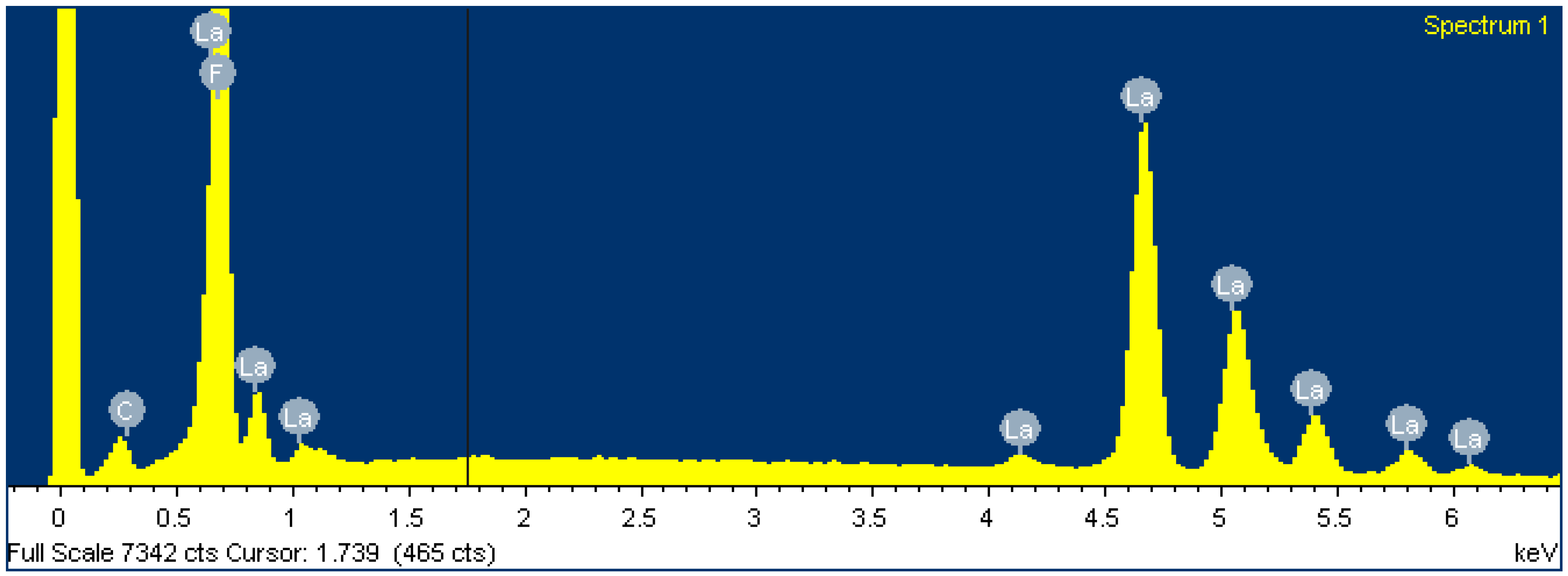
To gain insight into the chemical composition of the membrane before and after loading the FexOy NPs, an EDS analysis (within SEM) was performed. It is clear from Table 4 and Figure 9 that the membrane is chemically composed of fluorine and lanthanum in an atomic ratio of 1 to 3 and a small amount of adventitious carbon. However, this is to be expected and is consistent with the primary LaF3 composition of the membrane.
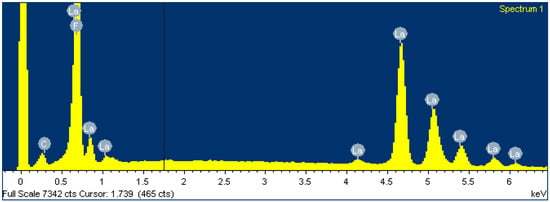
Figure 9.
Spectrum of LaF3 single-crystal membrane (with diameter of 8.0 mm and thickness of 1.0 mm, doped with 1.0% Eu) before FexOy NP loading.
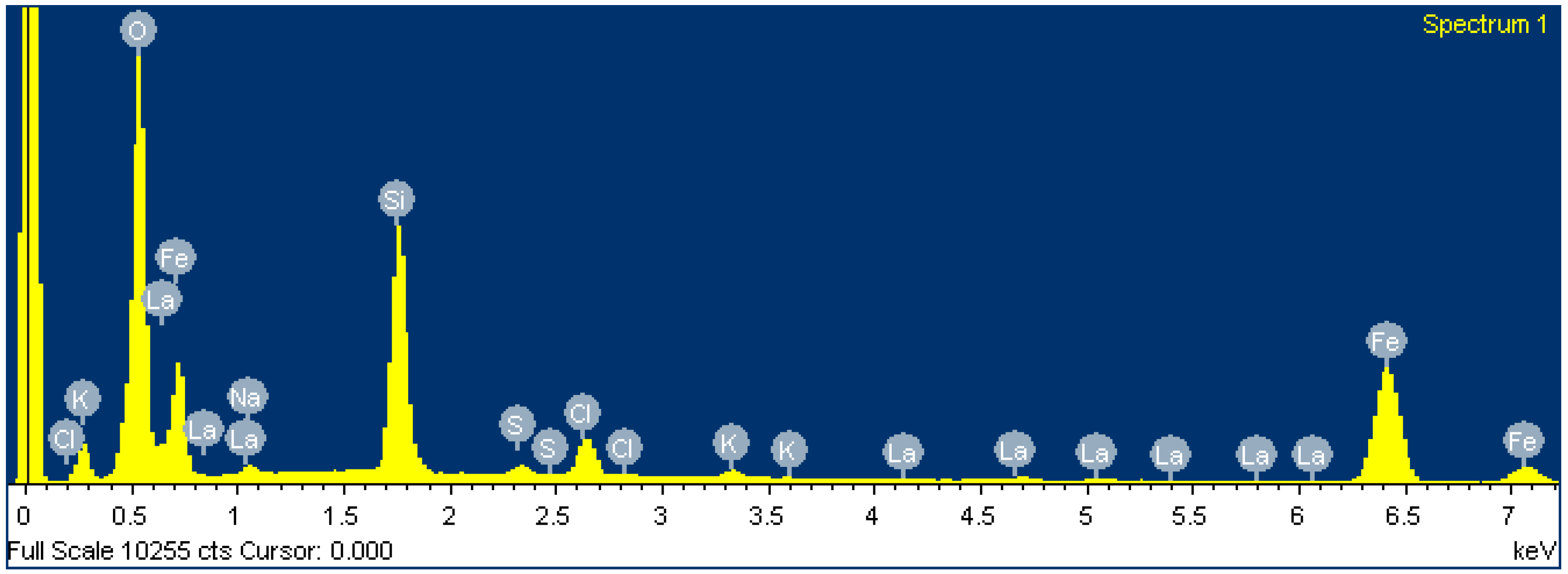
After FexOy NP membrane loading, the EDS composition showed relatively thick FexOy NP layers. The results from EDS (Table 5 and Figure 10) are consistent with the expected chemical composition of FexOy. Since the substrate LaF3 was not detected by EDS, the FexOy NPs deposits on the membrane were several µm thick. In addition, some impurities were also detected in low concentrations, which were residues from FexOy NPs synthesis [22,23] that could not be removed.

Table 5.
Elemental analysis after FexOy NP loading.
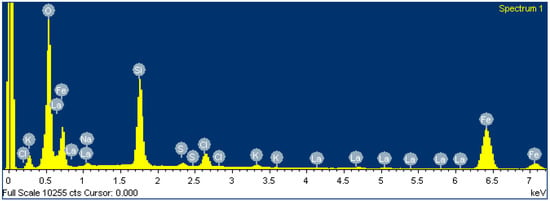
Figure 10.
Spectrum of LaF3 single-crystal membrane (with diameter of 8.0 mm and thickness of 1.0 mm, doped with 1.0% Eu) after FexOy NP loading.
3. Materials and Methods
3.1. Apparatus
The Millivoltmeter Mettler-Toledo GmbH Seven Easy was used to measure the potential of the FISE against an Ag/AgCl single-junction reference electrode in a reaction vessel at 25 °C. A lightly constructed multipurpose electrode body [8,9] was used for the assembly of the LaF3 membrane. The following LaF3 membranes were used for the measurement: with diameter of 8.0 mm and thickness of 1.0 mm, doped with 1.0% Eu (membrane 1); with diameter of 8.0 mm and thickness of 1.5 mm, doped with 0.3% Eu (membrane 2); with diameter of 8.0 mm and thickness of 5.0 mm, doped with 1.0% Eu (membrane 3). All three were manufactured by Crystran Ltd, United Kingdom. The internal contact between the Ag/AgCl reference electrode and LaF3 membrane was electrolytic or solid. An Orion fluoride ion-selective electrode Model 94-09 SC was used as a commercial electrode.
Characterization of the membrane surface and microstructure was performed with the Zeiss ULTRA plus (SEM) scanning field-emission electron microscope (Jena, Germany). Furthermore, an elemental analysis of the membranes inside SEM was performed with an EDS Oxford X-Max SDD detector (Oxford, United Kingdom) with a working area of 50 mm2, which was processed with INCA 4.14 5 software (Oxford Instruments, Oxford, United Kingdom). The SEM images were taken at 3 kV, while the EDS analysis was performed at 20 kV.
3.2. Reagents
All chemicals used were of analytical grade and were used as received without further purification. Sodium fluoride, hydrochloride acid, and silver nitrate were supplied by Sigma-Aldrich, Schnelldorf, Germany. Anhydrous sodium acetate was purchased from Gram-mol, Zagreb, Croatia. Glacial acetic acid, potassium nitrate, potassium chloride, potassium rhodanide, potassium sulfate, potassium iodide, calcium nitrate tetrahydrate, lead(II) nitrate, copper(II) nitrate trihydrate, and iron(III) nitrate nonahydrate were purchased from Kemika, Zagreb, Croatia. The solutions were prepared with double-distilled water.
Standard sodium fluoride solution (0.1000 M) was prepared in a polypropylene calibrated flask from dried (110 °C) sodium fluoride. The diluted standard solution fluoride was prepared by mixing the sodium standard solution fluoride with 0.10 M KNO3 and acetate buffer using propylene flasks and pipettes. The stock Fe3 solution (0.01 M) was prepared by weighing and dissolving an appropriate amount of Fe(NO3)3 in 0.10 M KNO3 and acetate buffer. Fe3+ was titrated using a standardized 0.01 M ethylenediaminetetraacetic acid (EDTA) solution. Other solutions of iron were prepared from the stock solution by dilution with 0.1 M KNO3 and acetate buffer. Solutions of interfering ions were prepared in the same way as the Fe3+ solution. Acetate buffer, pH 5, was prepared by diluting glacial acetic acid (6.5 mL) and sodium acetate (16.3 g) in distilled water using a 1000 mL volumetric flask.
The electrode inner electrolyte solution was prepared by mixing 10 mL of saturated KCl, 1 mL of concentrated HCl, and one or two drops of 0.10 M AgNO3. The preparation and characterization of FexOy NPs have already been described in the literature [22,23].
3.3. Potentiometric Measurements
To measure the potential reaction, 50.0 mL of 0.1000 M NaF, prepared in 0.10 M KNO3 in acetate buffer solution, was added to the reaction vessel at 25 °C. The potential response of the FISE was measured by serial dilution (to 10−7 M) of the cell solution. During the measurements, the solution was stirred with a polytetrafluoroethylene (PTFE)-coated magnetic rod. The potential–time behavior of the electrode was measured using a regular analysis set-up and recorded on a computer.
3.4. Elemental Analysis
Membranes were adhered to the aluminum SEM holder with conductive carbon tape and introduced to the SEM. The membranes were then analyzed using an Oxford X-Max SDD detector (calibrated by Co-Standard, Haarlem, The Netherlands) inside the SEM at 20 kV using point analysis.
4. Conclusions
For the first time, an FISE with an LaF3 membrane coated with FexOy NPs was prepared. The membranes of LaF3 single crystals were of different thicknesses and had different Eu ratios. The Eu ratio and the membrane thickness influenced the response of the electrodes. Without FexOy NP loading, the electrodes showed non-Nernstian behavior. After treatment of the electrode with FexOy NPs, the potential change per concentration decade increased and ranged between 44.1 and 62.4 mV. A detection fluoride limit of 7.41 × 10−8 M was calculated. Loss in electrode sensitivity on fluoride determination was not observed over 2 years.
Iron ions are able to form complexes with fluoride ions; thus, the prepared electrode is selective for iron ions. A detection limit for iron below a concentration of 10−5 M was observed. No significant influence of cations as an interfering species was observed, while the pronounced interfering species constituted SCN− anions. An elemental analysis after FexOy NP loading showed that they were mainly present in a thin film on the membrane surface. The advantage of this electrode is preparation simplicity, as well as a solid-state contact, which allows the electrode to be used in different positions and at higher temperatures.
Supplementary Materials
Figure S1. Potentiometric response of internal solid contact LaF3 electrodes before FexOy NP loading; Figure S2. Potentiometric response of internal electrolyte contact LaF3 electrodes before FexOy NP loading, Figure S3. Potentiometric response of internal solid contact LaF3 electrodes after washing FexOy NPs out; Figure S4. Potentiometric response of internal electrolyte contact LaF3 electrodes after washing FexOy NPs out; Figure S5. Calibration curves for fluoride determination with 7E and commercial FISE.
Author Contributions
Conceptualization, J.R., M.B.; methodology, J.R., M.B., formal analysis, J.R.; investigation, J.R., M.B.; resources, M.B., B.G., A.P., I.M.; data curation, J.R.; writing—original draft preparation, J.R., M.B.; writing—review and editing, J.R., M.B., M.K.; supervision, J.R., M.B.; funding acquisition, M.B., A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by Croatian Science Foundation, project number UIP-2017-05-6282.
Acknowledgments
The authors are grateful to Crystran Ltd, UK, for free samples of LaF3 monocrystals and acknowledged the financial support received from the program P1-0153 of the Slovenian Research Agency (ARRS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhillon, A.; Nair, M.; Kumar, D. Analytical methods for determination and sensing of fluoride in biotic and abiotic sources: A review. Anal. Methods 2016, 8, 5338–5352. [Google Scholar] [CrossRef]
- Štepec, D.; Tavčar, G.; Ponikvar-Svet, M. Surprisingly high fluorine content in some exotic superfoods. J. Fluor. Chem. 2020, 234, 109525. [Google Scholar] [CrossRef]
- Goschorska, M.; Gutowska, I.; Baranowska-Bosiacka, I.; Rać, M.E.; Chlubek, D. Fluoride Content in Alcoholic Drinks. Biol. Trace Elem. Res. 2016, 171, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Guccini, V.; Ciulu, M.; Floris, I.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Sanna, G. Free fluoride determination in honey by ion-specific electrode potentiometry: Method assessment, validation and application to real unifloral samples. Arab. J. Chem. 2018, 11, 492–500. [Google Scholar] [CrossRef]
- Frant, M.S.; Ross, J.W. Electrode for sensing fluoride ion activity in solution. Science 1966, 154, 1553–1555. [Google Scholar] [CrossRef]
- Bralić, M.; Radić, N.; Brinić, S.; Generalić, E. Fluoride electrode with LaF3 membrane and simple disjoining solid-state internal contact. Talanta 2001, 55, 581–586. [Google Scholar] [CrossRef]
- Komljenović, J.; Krka, S.; Radić, N. All-solid-state fluoride electrode. Anal. Chem. 1986, 58, 2893–2895. [Google Scholar] [CrossRef]
- Radić, N.; Bralić, M. Fluoride-selective electrode with internal redox reference electrode. Microchim. Acta 1995, 118, 221–227. [Google Scholar] [CrossRef]
- Radić, N.; Bralić, M. Design of Fluoride-selective Electrode with Internal Contact Based on an Cu(II) Ion-selective Electrode. Microchem. J. 1993, 48, 377–382. [Google Scholar] [CrossRef]
- Somer, G.; Kalaycı, Ş.; Başak, I. Preparation of a new solid state fluoride ion selective electrode and application. Talanta 2010, 80, 1129–1132. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rahdar, S.; Igwegbe, C.A.; Rahdar, A.; Shafighi, N.; Sadeghfar, F. Data on the removal of fluoride from aqueous solutions using synthesized P/γ-Fe2O3 nanoparticles: A novel adsorbent. MethodsX 2019, 6, 98–106. [Google Scholar] [CrossRef]
- Gayathri, V.; Maheswaria, J. Removal of fluoride ions in drinking water using Fe2O3-ZnO nanocomposite embedded with grass fibre. JOAASR 2017, 1, 102–106. [Google Scholar]
- Jayarathna, L.; Bandara, A.; Ng, W.J.; Weerasooriya, R. Fluoride adsorption on γ–Fe2O3 nanoparticles. J. Environ. Health Sci. Eng. 2015, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M. Development of ionic-sensor based on sono-chemically prepared 4 low-dimensional β-Fe2O3 nanoparticles onto flat-gold electrodes 5 by an electrochemical approach. Sens. Biosens. Res. 2015, 4, 109–117. [Google Scholar] [CrossRef]
- Moritz, W.; Müller, L. Mechanistic study of fluoride ion sensors. Analyst 1991, 116, 589–593. [Google Scholar] [CrossRef]
- Faridbod, F.; Ganjali, M.R.; Pirali-Hamedani, M.; Norouzi, P. MWCNTs-Ionic Liquids-Ionophore-Graphite Nanocomposite Based Sensor for Selective Determination of Ytterbium(III) Ion. Int. J. Electrochem. Sci. 2010, 5, 1103–1112. [Google Scholar]
- Biyareh, M.N.; Rezvani, A.R.; Dashtian, K.; Montazerozohori, M.; Ghaedi, M.; Asl, A.M.; White, J. Potentiometric Ion-Selective Electrode Based on a New Single Crystal Cadmium(II) Schiff Base Complex for Detection of Fluoride Ion: Central Composite Design Optimization. IEEE Sens. J. 2019, 19, 413–425. [Google Scholar] [CrossRef]
- Bralić, M.; Radić, N. Flow injection potentiometric determination of Fe(III) using a fluoride-selective electrode as detector. Analusis 1999, 27, 57–60. [Google Scholar] [CrossRef]
- Poursaberi, T.; Ganjali, M.R.; Hassanisadi, M. A novel fluoride-selective electrode based on metalloporphyrin grafted-grapheneoxide. Talanta 2012, 101, 128–134. [Google Scholar] [CrossRef]
- Rajković, M.B.; Novaković, I.D. Determination of fluoride content in drinking water and tea infusions using fluoride ion selective electrode. J. Agric. Sci. 2007, 52, 155–168. [Google Scholar] [CrossRef]
- Prkić, A.; Vukušić, T.; Mitar, I.; Giljanović, J.; Sokol, V.; Bosković, P.; Jakić, M.; Sedlar, A. New sensor based on AgCl containing Iron Oxide or Zinc Oxide Nanoparticles for Chloride Determination. Int. J. Electrochem. Sci. 2019, 14, 861–874. [Google Scholar] [CrossRef]
- Ristić, M.; Kuzmann, E.; Homonnay, Z.; Mitar, I.; Musić, S. Hydrolysis of Fe(III) in the presence of mixed anions and promoters. J. Radioanal. Nucl. Chem. 2020, 324, 1293–1302. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).