The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of CP Supplementation on Blood Biochemical Makers
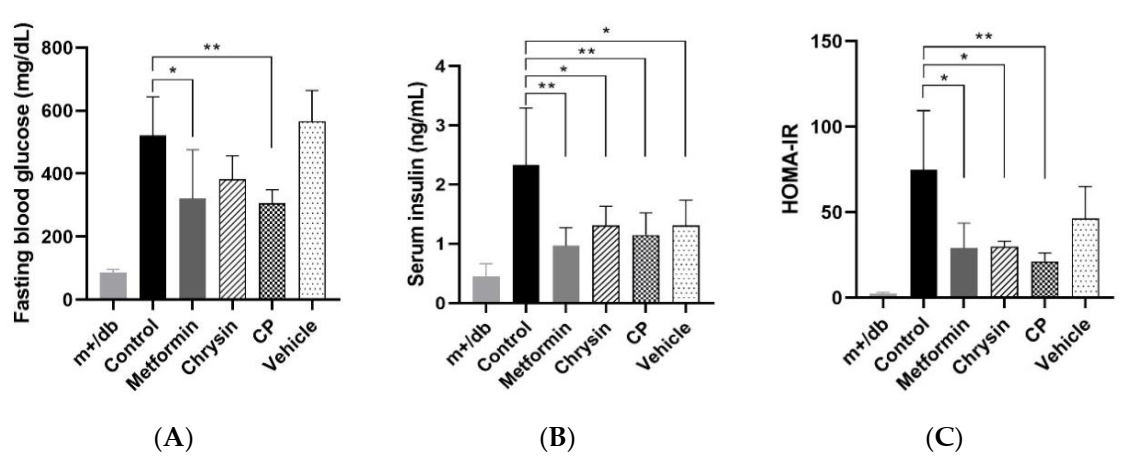
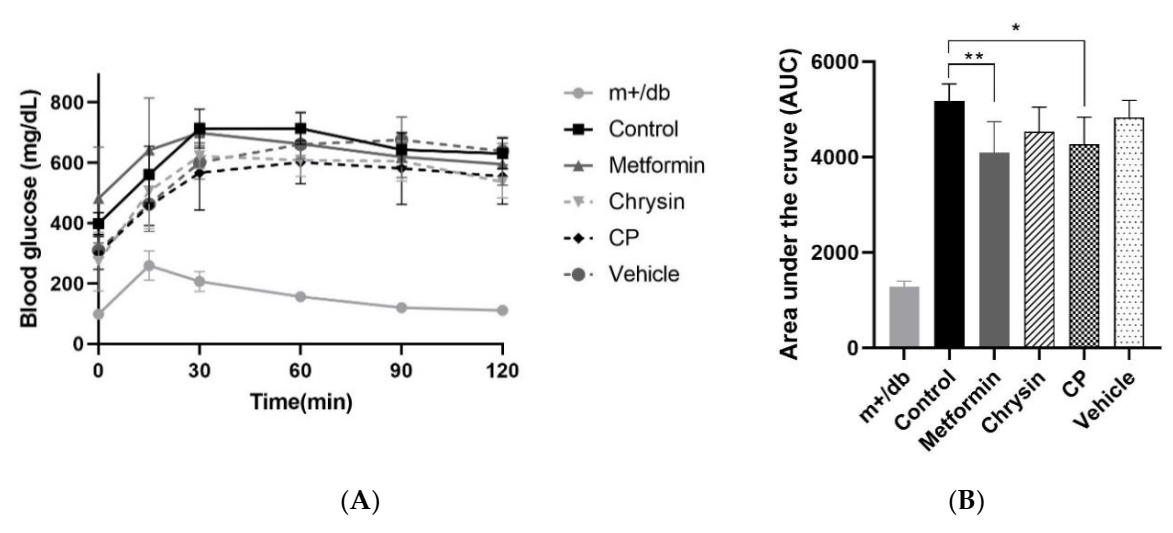
2.2. Effect of CP on Insulin Resistance
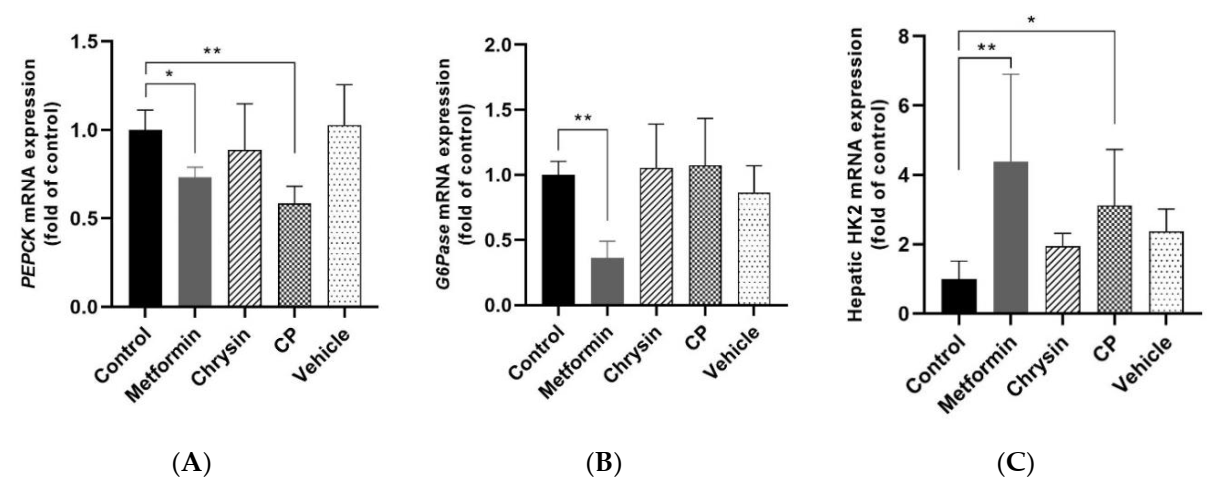
2.3. Effects of CP on the Glucose Metabolism Related Gene Expression in the Liver
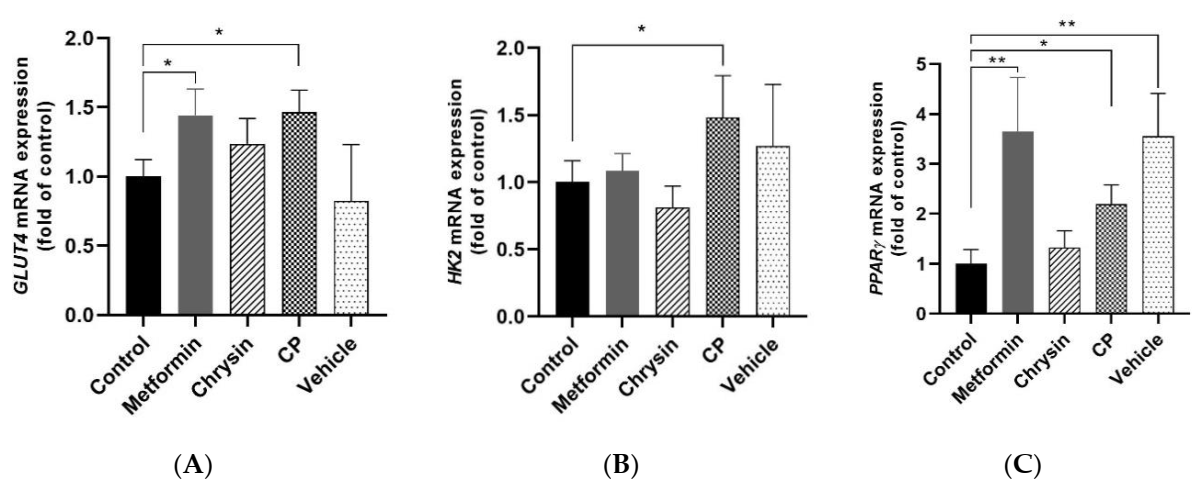
2.4. Effects of CP on Glucose Uptake Related Gene Expression in the Skeletal Muscle
2.5. Effects of CP on GLUT4 Plasma Translocation in db/db Mice
3. Materials and Methods
3.1. Materials
3.2. Preparation of CP
3.3. Animals and Diets
3.4. Blood Biochemical Analysis
3.5. Oral Glucose Tolerance Test (OGTT)
3.6. RNA Extraction and Quantitative Real-Time PCR
3.7. GLUT4 Translocation on the Plasma Membrane of Femoral Muscle
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| HbA1c | Hemoglobin A1c |
| TG | Triacylglycerol |
| TC | Total cholesterol |
| FGB | Fasting blood glucose level |
| ALT | Serum alanine aminotransferase |
| AST | Aspartate aminotransferase |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol |
| AUC | Area under the curve |
| OGTT | Oral glucose tolerance test |
| G6 Pase | Glucose-6-phosphatase |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| HK2 | Hexokinase 2 |
| GLUT4 | Glucose transporter 4 |
| PPAR γ | Peroxisome proliferator-activated receptor γ |
References
- Bavenholm, P.N.; Pigon, J.; Ostenson, C.G.; Efendic, S. Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes 2001, 50, 1449–1454. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Srivastava, A.K. Diabetes mellitus: Complications and therapeutics. Med. Sci. Monit. Basic Res. 2006, 12, RA130–RA147. [Google Scholar]
- Fang, J.-Y.; Lin, C.-H.; Huang, T.-H.; Chuang, S.-Y. In vivo rodent models of type 2 diabetes and their usefulness for evaluating flavonoid bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willet, W.; Hu, F.B.; Sun, Q.; Van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.-Y.; Bae, Y.J.; Kim, E.-Y.; Lee, E.-J. Association between flavonoid intake and diabetes risk among the Koreans. Clin. Chim. Acta 2015, 439, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7. [Google Scholar] [CrossRef]
- Li, R.; Zang, A.; Zhang, L.; Zhang, H.; Zhao, L.; Qi, Z.; Wang, H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol. Sci. 2014, 35, 1527–1532. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am. J. Chin. Med. 2014, 42, 1153–1167. [Google Scholar] [CrossRef]
- Andrade, N.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardão, L.; Guimarães, J.T.; Martel, F. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur. J. Nutr. 2020, 59, 151–165. [Google Scholar] [CrossRef]
- Goes, A.T.R.; Jesse, C.R.; Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.B.L.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018, 279, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.K. Dietary flavonoids: Bioavailability, metabolic effects and safety. Ann. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [PubMed]
- Andrade, N.; Marques, C.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardao, L.; Guimaraes, J.T.; Keating, E.; Calhau, C.; Martel, F. Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats. Food Funct. 2019, 10, 4566–4576. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Mancini, S.; Nardo, L.; Gregiri, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, G. Functionalized liposomes and phytosomes loading Annona murcata L. aqueous extract: Potential nanoshuttles for brain-delivery of phenolic compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, S.; Khar, R.K. Casuarina equisetifolia extract loaded phytosomes: Optimization, characterization and in vivo evaluation of antidiabetic and antihyperlipidemic activities in Wistar rats. Drug Deliv. Lett. 2019, 9, 116–133. [Google Scholar] [CrossRef]
- Kim, S.-M.; Jung, J.-I.; Chai, C.; Imm, J.-Y. Characteristics and glucose uptake promoting effect of chrysin-loaded phytosomes prepared with different phospholipid matrices. Nutrients 2019, 11, 2549. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact. 2010, 183, 276–283. [Google Scholar] [CrossRef]
- Thapa, B.; Walia, A. Liver function test and their interpretation. Indian J. Pediatr. 2007, 74, 663–671. [Google Scholar] [CrossRef]
- Sirovina, D.; Oršolić, N.; Končić, M.Z.; Kovačević, G.; Benković, V.; Gregorović, G. Quercetin vs chrysin: Effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol. 2013, 32, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.Y.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Noh, S.K.; Koo, S.I. Egg phosphatidylcholine decreases the lymphatic absorption of cholesterol in rats. J. Nutr. 2001, 131, 2358–2363. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Uchino, H.; Shimizu, T.; Kanazawa, A.; Tamura, Y.; Sakai, K.; Tanaka, Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: Usefulness of GA for evaluation of short-term changes in glycemic control. Endocr. J. 2007, 54, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.M.; Guo, M.; Dharmage, S.C. HbA1C as a screening tool for detection of Type 2 diabetes: A systematic review. Diabet. Med. 2007, 24, 333–343. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, M.; Song, H.; Hyun, Y.; Cha, J.; Ko, J.; Cha, D. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol. Dial. Transplant. 2011, 26, 1194–1204. [Google Scholar] [CrossRef]
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosiso, S.; Allegrini, P. Improved oral absorption of quercetin phytosome®, a new delivery system based on food grade lecithin. Eur. J. Drug. Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Basu, A.; Basu, R.; Shah, P.; Vella, A.; Johnson, C.M.; Nair, K.S.; Jensen, M.D.; Schwenk, W.F.; Rizza, R.A. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: Additional evidence for a defect in hepatic glucokinase activity. Diabetes 2001, 50, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E685–E692. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, F.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dang, Y.; Li, Q.; Zhou, W.; Zuo, J.; Yao, Z.; Zhang, L.; Ji, G. Berberine alleviates hyperglycemia by targeting hepatic glucokinase in diabetic db/db mice. Sci. Rep. 2019, 9, 8003. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, M.L.; Cornish-Bowden, A.; Ureta, T. Evolution and regulatory role of the hexokinases. Biochim. Biophys. Acta Mol. Cell Res. 1998, 1401, 242–264. [Google Scholar] [CrossRef]
- Postic, C.; Shiota, M.; Niswender, K.D.; Jetton, T.L.; Chen, Y.; Moates, J.M.; Magnuson, M.A. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. 1999, 274, 305–315. [Google Scholar] [CrossRef]
- Ramnanan, C.J.; Edgerton, D.S.; Cherrington, A.D. The role of insulin in the regulation of PEPCK and gluconeogenesis in vivo. US Endocrinol. 2010, 5, 34–39. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose-and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Hwang, M.J.; Hong, C.O.; Yoo, D.S.; Kim, J.S.; Kim, D.Y.; Lee, K.W. Anti-hyperglycemic and hypolipidemic effects of black ginseng extract containing increased Rh4, Rg5, and Rk1 content in muscle and liver of type 2 diabetic db/db mice. Food. Sci. Biotechnol. 2020, 29, 1101–1112. [Google Scholar] [CrossRef]
- Jones, J.P.; Dohm, G.L. Regulation of glucose transporter GLUT-4 and hexokinase II gene transcription by insulin and epinephrine. Am. J. Physiol. 1997, 273, E682–E687. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Pessin, J.E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001, 56, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Crivelli, V.; Perrin, C.; Da Costa, A.; Mu, J.; Kahn, B.B.; Birnbaum, M.J.; Kahn, C.R.; Vollenweider, P.; Thorens, B. GLUT4, AMP kinase, but not the insulin receptor, are required for hepatoportal glucose sensor-stimulated muscle glucose utilization. J. Clin. Investig. 2003, 111, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Yamashita, H.; Qiao, L.; Friedman, J.E. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J. Endocrinol. 2000, 167, 107–115. [Google Scholar] [CrossRef]
- Gibbs, E.M.; Stock, J.L.; McCoid, S.C.; Stukenbrok, H.A.; Pessin, J.E.; Stevenson, R.W.; McNeish, J.D. Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J. Clin. Investig. 1995, 95, 1512–1518. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; Saldaña-Ríos, J.; García-Jiménez, S.; Villalobos-Molina, R.; Ávila-Villarreal, G.; Rodríguez-Ocampo, A.N.; Bernal-Fernández, G.; Estrada-Soto, D. Chrysin induces antidiabetic, antidyslipidemic and anti-inflammatory effects in Athymic nude diabetic mice. Molecules 2018, 23, 67. [Google Scholar] [CrossRef]
- Barroso, I.; Gurnell, M.; Crowley, V.E.F.; Agostini, M.; Schwabe, J.W.; Soos, M.A.; Maslen, G.L.; Williams, T.D.M.; Lewis, H.; Schafer, A.J.; et al. Dominant negative mutations in human PPAR gamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999, 402, 880–883. [Google Scholar] [CrossRef]
- Raskin, P.; Rappaport, E.B.; Cole, S.T.; Yan, Y.; Patwardhan, R.; Freed, M.I. Rosiglitazone short-term monotherapy lowers fasting and post-prandial glucose in patients with type 2 diabetes. Diabetologia 2000, 43, 278–284. [Google Scholar] [CrossRef]
- Way, J.M.; Harrington, W.W.; Brown, K.K.; Gottschalk, W.K.; Sundseth, S.S.; Mansfield, T.A.; Ramachandran, R.K.; Willson, T.M.; Kliewer, S.A. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor gamma activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology 2001, 142, 1269–1277. [Google Scholar] [CrossRef]
- Cline, G.W.; Petersen, K.F.; Krssak, M.; Shen, J.; Hundal, R.S.; Trajanoski, Z.; Inzucchi, S.; Dresner, A.; Rothman, D.L.; Shulman, G.I. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 1999, 341, 240–246. [Google Scholar] [CrossRef]
- Eshvendar, R.K.; Lakshmi, N.B.; Chandana, C.B.; Rajaram, M.M.; Vicky, D.; Mukesh, K.B.; Ramana, R.M.; Suseela, M.; Ranadeep, G. Chemopreventive effect of chrysin, a dietary flavone against benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pharmacol. Rep. 2016, 68, 310–318. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellharmmer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Kim, M.B.; Hwang, J.K. Hypoglycemic effect of whole grain diet in C57BL/KsJ-db/db mice by activating PI3K/Akt and AMPK pathways. Food. Sci. Biotechnol. 2019, 28, 895–905. [Google Scholar] [CrossRef]
- Yoon, H.J.; Bang, M.H.; Kim, H.; Imm, J.Y. Improvement of palmitate-induced insulin resistance in C2C12 skeletal muscle cells using Platycodon grandiflorum seed extracts. Food Biosci. 2018, 25, 61–67. [Google Scholar] [CrossRef]

| Group | m+/db | Control | Metformin | Chrysin | CP | Vehicle |
|---|---|---|---|---|---|---|
| ALT (U/L) | 43 ± 14 | 120 ± 35 | 85 ± 32 | 139 ± 38 | 77 ± 23 | 88 ± 39 |
| AST (U/L) | 87 ± 27 | 183 ± 53 | 136 ± 47 | 193 ± 26 | 129 ± 26 | 140 ± 42 |
| TG (mg/dL) | 126 ± 16 | 288 ± 6 | 257 ± 93 | 392 ± 83 | 195 ± 76 | 140 ± 29 * |
| TC (mg/dL) | 103 ± 11 | 132 ± 15 | 131 ± 15 | 116 ± 14 | 113 ± 15 * | 107 ± 18 * |
| HDL-c (mg/dL) | 80 ± 9 | 96 ± 7 | 93 ± 9 | 85 ± 15 | 90 ± 14 | 82 ± 12 |
| LDL-c (mg/dL) | 49 ± 8 | 93 ± 10 | 90 ± 18 | 105 ± 18 | 62 ± 18 * | 52 ± 16 ** |
| Leptin (ng/mL) | 1.2 ± 0.9 | 22 ± 7 | 26 ± 9 | 23 ± 11 | 18 ± 5 | 21 ± 9 |
| HbA1c (%) | 4.6 ± 0.2 | 12.0 ± 2.1 | 9.0 ± 2.1 * | 10.4 ± 2.4 | 9.8 ± 0.4 | 9.4 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-m.; Imm, J.-Y. The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice. Molecules 2020, 25, 5503. https://doi.org/10.3390/molecules25235503
Kim S-m, Imm J-Y. The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice. Molecules. 2020; 25(23):5503. https://doi.org/10.3390/molecules25235503
Chicago/Turabian StyleKim, Seong-min, and Jee-Young Imm. 2020. "The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice" Molecules 25, no. 23: 5503. https://doi.org/10.3390/molecules25235503
APA StyleKim, S.-m., & Imm, J.-Y. (2020). The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice. Molecules, 25(23), 5503. https://doi.org/10.3390/molecules25235503






