Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents
Abstract
1. Introduction
2. Results and Discussion
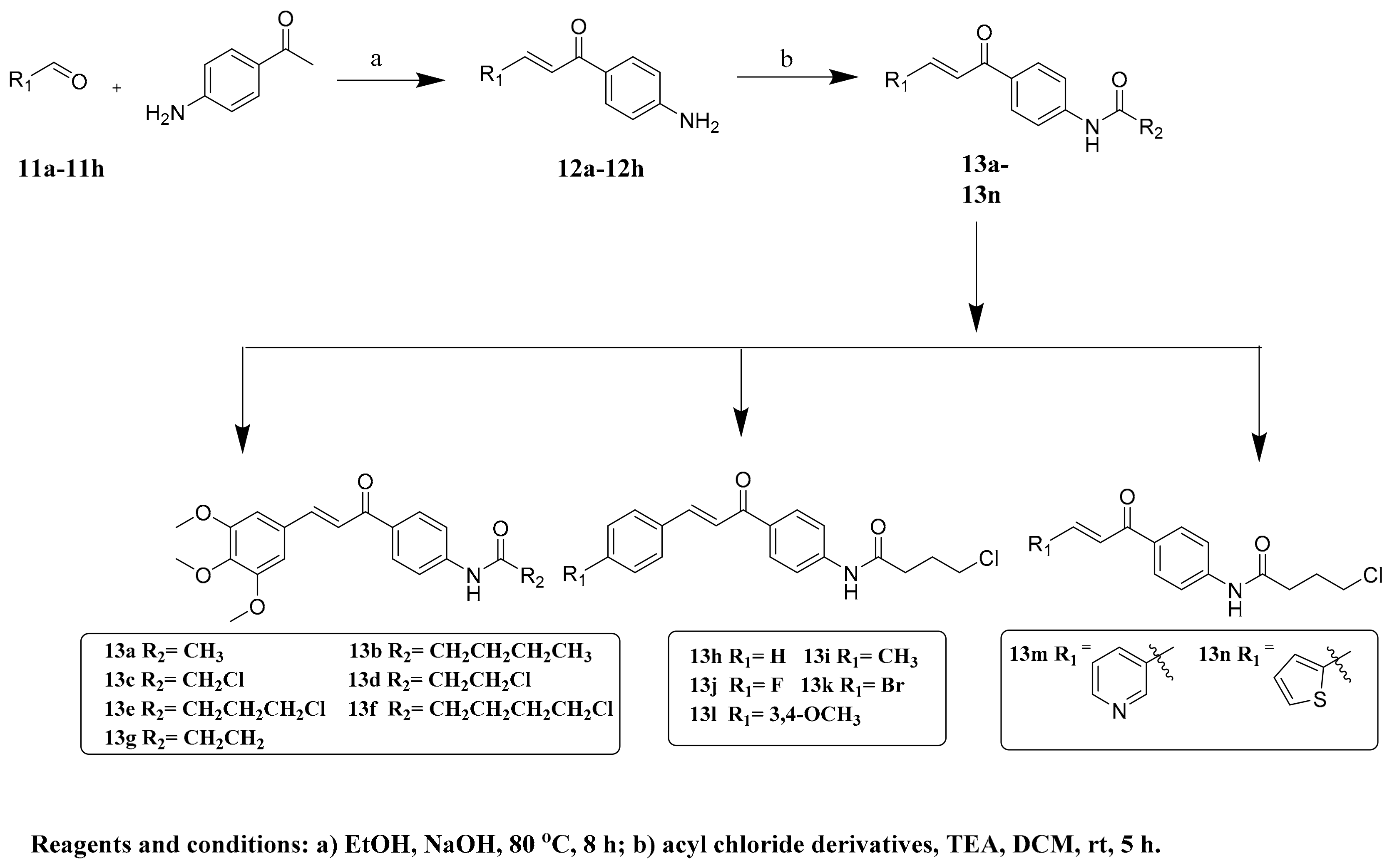
2.1. Chemistry
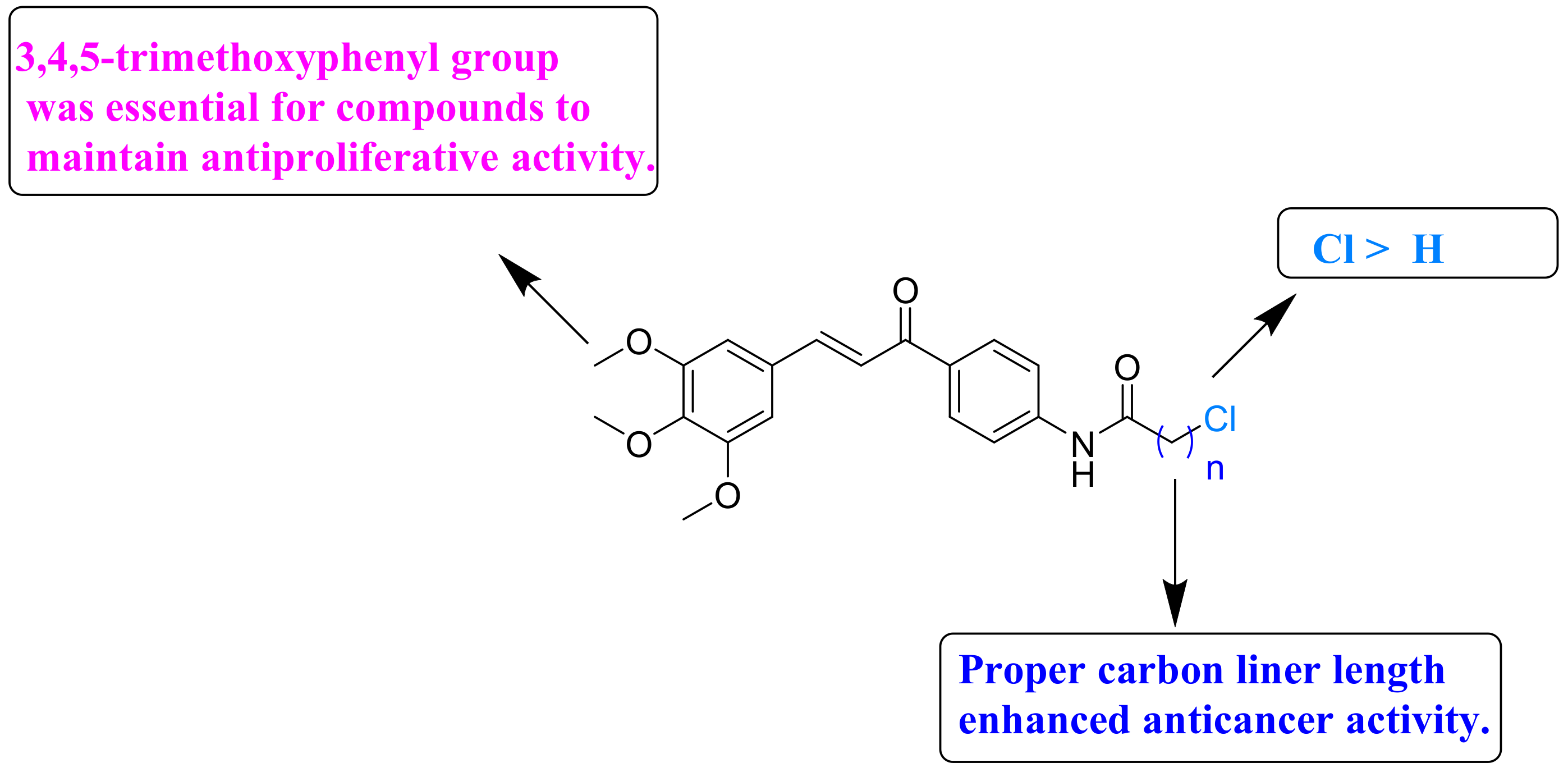
2.2. Antiproliferative Activity and Structure Activity Relationship Analysis
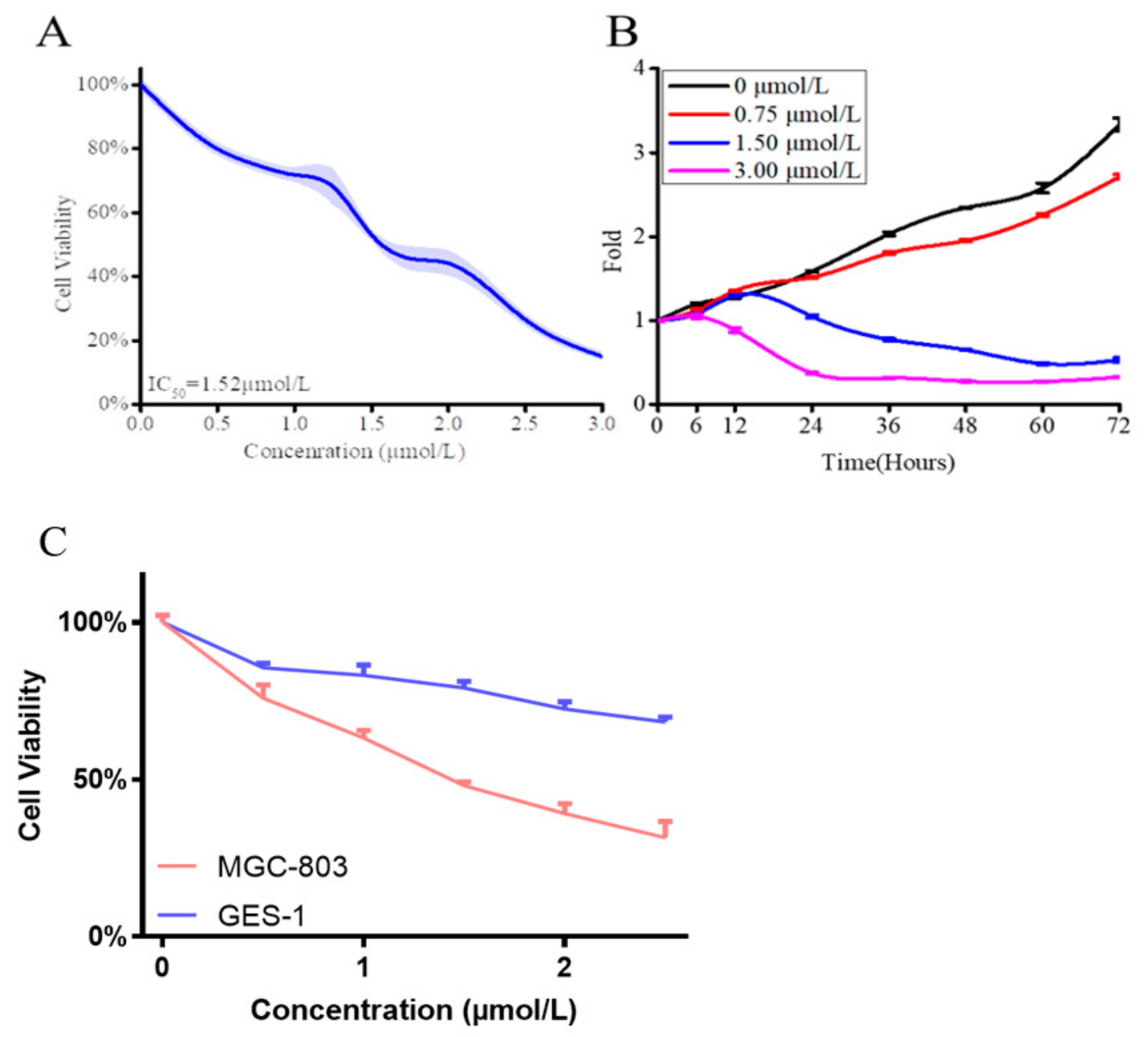
2.3. Compound 13e Inhibited Cell Viability against Gastric Cancer Cell MGC-803 Cells
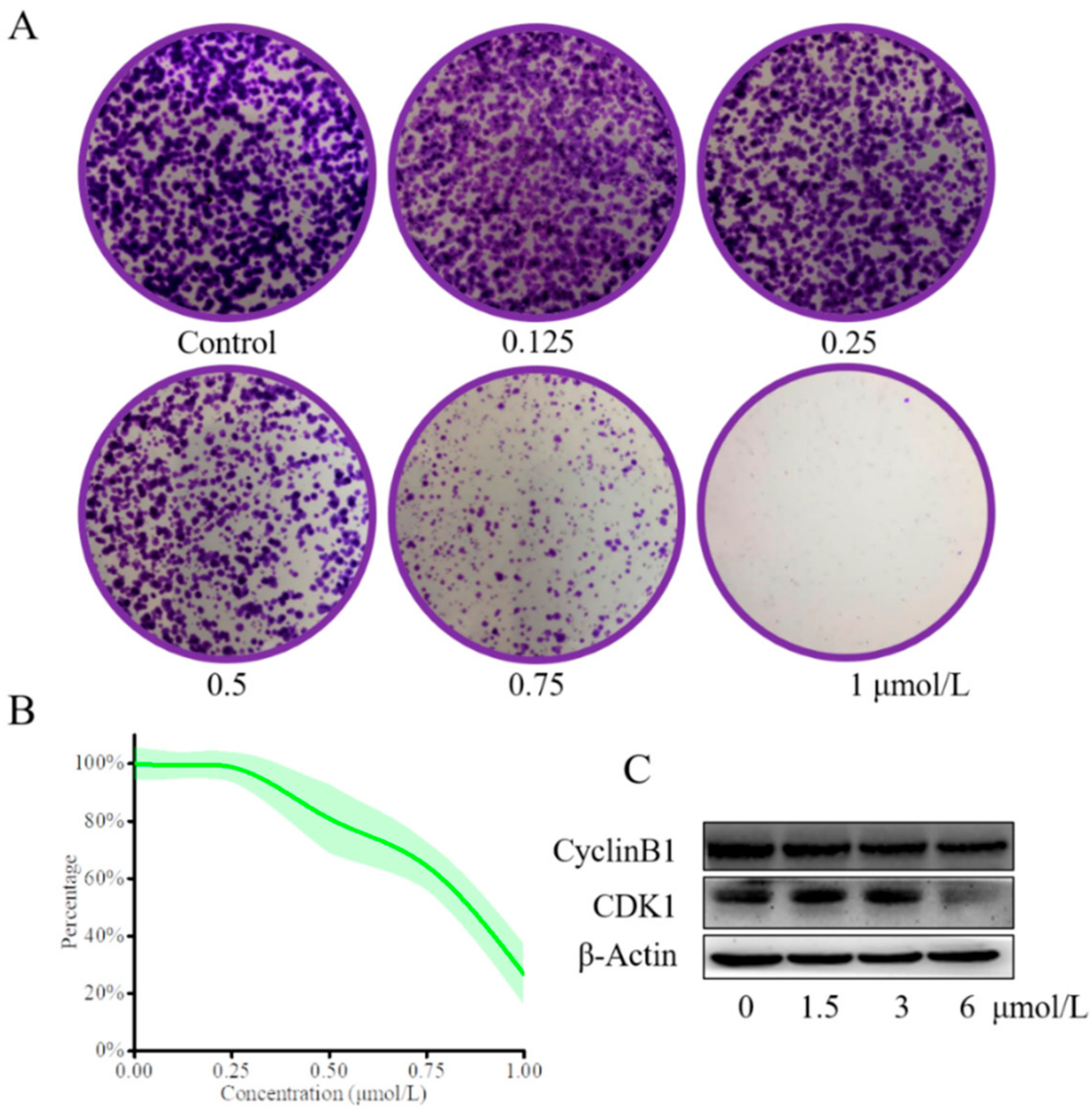
2.4. Compound 13e Inhibited Proliferation of MGC-803 Cells
2.5. Compound 13e Induced Cell Apoptosis in MGC-803 Cells
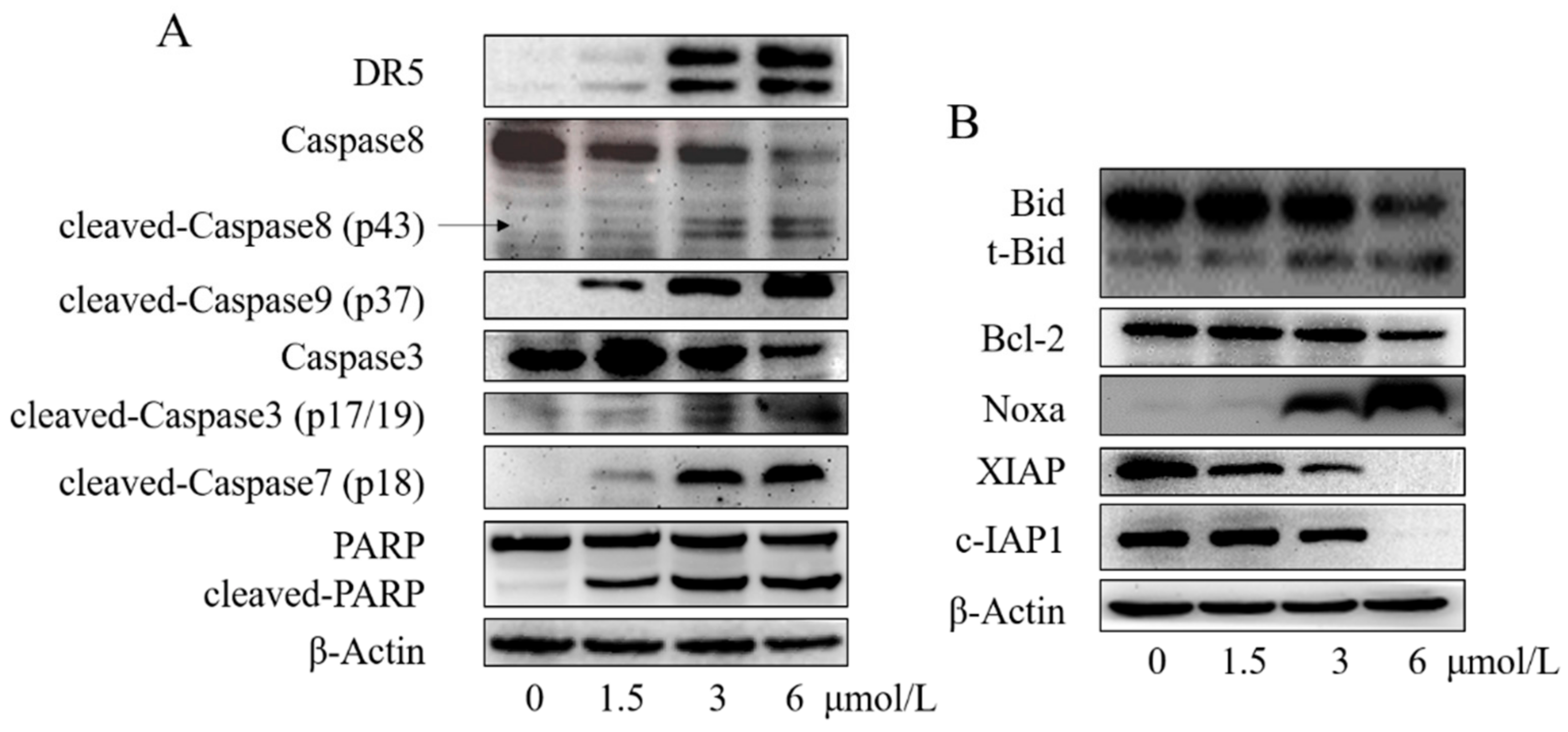
2.6. Compound 13e Induced Cell Apoptosis via the Extrinsic/Intrinsic Apoptosis Pathway
3. Materials and Methods
3.1. Synthesis of Compounds 12a–h
3.2. Synthesis of Compounds 13a–n
3.3. Cell Culture
3.4. MTT Assay
3.5. DAPI Assay
3.6. Western Blotting Analysis
3.7. General Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Feng, L.; Maddox, M.M.; Alam, Z.; Tsutsumi, L.S.; Narula, G.; Bruhn, D.F.; Wu, X.; Sandhaus, S.; Lee, R.B.; Simmons, C.J.; et al. Synthesis, Structure & ndash;Activity Relationship Studies, and Antibacterial Evaluation of 4-Chromanones and Chalcones, as Well as Olympicin A and Derivatives. J. Med. Chem. 2014, 57, 8398–8420. [Google Scholar] [PubMed]
- Dan, W.; Dai, J. Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem. 2020, 187, 111980. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-C.; Bai, P.-Y.; Yang, Z.-Q.; Cui, D.-Y.; Hua, Y.-G.; Yang, Y.; Yang, Q.; Zhang, E.; Qin, S. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. Eur. J. Med. Chem. 2018, 143, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.K.; Mishra, N.; Kumar, B.; Sharma, M.; Bhattacharya, A.; Mishra, L.C.; Bhasin, V.K. Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Med. Chem. Res. 2008, 18, 407–420. [Google Scholar] [CrossRef]
- Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and molecular docking of novel chalcone–coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 2014, 85, 65–76. [Google Scholar] [CrossRef]
- Domínguez, J.N.; León, C.; Rodrigues, J.; De Domínguez, N.G.; Gut, J.; Rosenthal, P.J. Synthesis and Evaluation of New Antimalarial Phenylurenyl Chalcone Derivatives. J. Med. Chem. 2005, 48, 3654–3658. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Lahtchev, K.; Batovska, D.; Parushev, S.; Ubiyvovk, V.; Sibirny, A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar] [CrossRef]
- Konduru, N.K.; Dey, S.; Sajid, M.; Owais, M.; Ahmed, N. Synthesis and antibacterial and antifungal evaluation of some chalcone based sulfones and bisulfones. Eur. J. Med. Chem. 2013, 59, 23–30. [Google Scholar] [CrossRef]
- Deng, J.; Sanchez, T.; Al-Mawsawi, L.Q.; Dayam, R.; Yunes, R.A.; Garofalo, A.; Bolger, M.B.; Neamati, N. Discovery of structurally diverse HIV-1 integrase inhibitors based on a chalcone pharmacophore. Bioorganic Med. Chem. 2007, 15, 4985–5002. [Google Scholar] [CrossRef]
- Monserrat, J.-P.; Al-Safi, R.I.; Tiwari, K.N.; Quentin, L.; Chabot, G.G.; Vessières, A.; Jaouen, G.; Neamati, N.; Hillard, E.A. Ferrocenyl chalcone difluoridoborates inhibit HIV-1 integrase and display low activity towards cancer and endothelial cells. Bioorganic Med. Chem. Lett. 2011, 21, 6195–6197. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Abdullah, M.I.; Ahmed, E.; Sharif, A.; Irfan, A.; Masood, S. Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential non-nucleoside reverse transcriptase inhibitors (NNRT). Bioorganic Chem. 2016, 65, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and Discovery of Novel Synthetic Chalcone Derivatives as Anti-Inflammatory Agents. J. Med. Chem. 2011, 54, 8110–8123. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, S.-N.; Gao, J.-M.; Liao, Z.-Y.; Tseng, Y.-T.; Fülöp, F.; Chang, F.-R.; Lo, Y.-C. The Antioxidant, Anti-Inflammatory, and Neuroprotective Properties of the Synthetic Chalcone Derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Shuai, W.; Sun, H.; Yao, H.; Ma, C.; Xu, S.; Yao, H.; Zhu, Z.; Yang, D.-H.; et al. Discovery of Novel Quinoline & ndash;Chalcone Derivatives as Potent Antitumor Agents with Microtubule Polymerization Inhibitory Activity. J. Med. Chem. 2019, 62, 993–1013. [Google Scholar]
- ElKhalifa, D.; Siddique, A.B.; Qusa, M.; Cyprian, F.S.; El Sayed, K.A.; Alali, F.; Al Moustafa, A.-E.; Khalil, A. Design, synthesis, and validation of novel nitrogen-based chalcone analogs against triple negative breast cancer. Eur. J. Med. Chem. 2020, 187, 111954. [Google Scholar] [CrossRef]
- Ocasio-Malavé, C.; Donate, M.J.; Sánchez, M.M.; Sosa-Rivera, J.M.; Mooney, J.W.; León, T.A.P.; Carballeira, N.M.; Zayas, B.; Vélez-Gerena, C.E.; Martínez-Ferrer, M.; et al. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorganic Med. Chem. Lett. 2020, 30, 126760. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef]
- Achanta, G.; Modzelewska, A.; Feng, L.; Khan, S.R.; Huang, P. A Boronic-Chalcone Derivative Exhibits Potent Anticancer Activity through Inhibition of the Proteasome. Mol. Pharmacol. 2006, 70, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorganic Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.D.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorganic Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, H.; Wan, L.; Han, X.; Wang, G.; Hu, J.; Tang, M.; Duan, X.; Fan, Y.; He, S.; et al. Millepachine, a novel chalcone, induces G 2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo. Carcinogenesis 2013, 34, 1636–1643. [Google Scholar] [CrossRef]
- Azam, A.; Khan, P.; Ahmad, K.; Hassan, I.; Azam, A. Synthesis, characterization and biological evaluation of tertiary sulfonamide derivatives of pyridyl-indole based heteroaryl chalcone as potential carbonic anhydrase IX inhibitors and anticancer agents. Eur. J. Med. Chem. 2018, 155, 13–23. [Google Scholar]
- Wang, M.; Xu, S.; Wu, C.; Liu, X.; Tao, H.; Huang, Y.; Liu, Y.; Zheng, P.; Zhu, W. Design, synthesis and activity of novel sorafenib analogues bearing chalcone unit. Bioorganic Med. Chem. Lett. 2016, 26, 5450–5454. [Google Scholar] [CrossRef]
- Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Zhang, L.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Liu, H.; et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate–chalcone derivates. Bioorganic Med. Chem. Lett. 2016, 26, 3918–3922. [Google Scholar] [CrossRef]
- Fu, D.-J.; Li, J.-H.; Yang, J.-J.; Li, P.; Zhang, Y.-B.; Liu, S.; Li, Z.-R.; Zhang, S.-Y. Discovery of novel chalcone-dithiocarbamates as ROS-mediated apoptosis inducers by inhibiting catalase. Bioorganic Chem. 2019, 86, 375–385. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Fu, D.-J.; Yue, X.-X.; Liu, Y.-C.; Song, J.; Sun, H.-H.; Liu, H.-M.; Zhang, Y.-B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules 2016, 21, 653. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Liu, Y.-C.; Sun, H.-H.; Yue, X.-X.; Zhao, R.-H. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole-chalcones. Medchemcomm 2016, 8, 1664–1671. [Google Scholar]
- Zhou, Y.; Yan, W.; Cao, D.; Shao, M.; Li, D.; Wang, F.; Yang, Z.; Chen, Y.; He, L.; Wang, T.; et al. Design, synthesis and biological evaluation of 4-anilinoquinoline derivatives as novel potent tubulin depolymerization agents. Eur. J. Med. Chem. 2017, 138, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Xiaoyan, W.; Wang, C.; Bai, P.; Wang, T.; Tang, M.; Wang, X.; Yang, Z.; Ma, B.; et al. Design, Synthesis, and Evaluation of in Vitro and in Vivo Anticancer Activity of 4-Substituted Coumarins: A Novel Class of Potent Tubulin Polymerization Inhibitors. J. Med. Chem. 2016, 59, 5721–5739. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, Q.-L.; Wu, B.-W.; Zhu, T.; Cui, X.-X.; Jin, C.-J.; Wang, S.-Y.; Wang, S.-H.; Fu, D.-J.; Liu, H.-M.; et al. Discovery of tertiary amide derivatives incorporating benzothiazole moiety as anti-gastric cancer agents in vitro via inhibiting tubulin polymerization and activating the Hippo signaling pathway. Eur. J. Med. Chem. 2020, 203, 112618. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-L.; Wu, B.-W.; Li, D.; Shi, L.; Zhu, T.; Lou, J.-F.; Jin, C.-Y.; Zhang, Y.-B.; Zhang, S.; Liu, H.-M.; et al. Novel tertiary sulfonamide derivatives containing benzimidazole moiety as potent anti-gastric cancer agents: Design, synthesis and SAR studies. Eur. J. Med. Chem. 2019, 183, 111731. [Google Scholar]
- Song, J.; Cui, X.-X.; Wu, B.-W.; Li, D.; Wang, S.-H.; Shi, L.; Zhu, T.; Zhang, Y.-B.; Zhang, S.-Y. Discovery of 1,2,4-triazine-based derivatives as novel neddylation inhibitors and anticancer activity studies against gastric cancer MGC-803 cells. Bioorganic Med. Chem. Lett. 2019, 30, 126791. [Google Scholar] [CrossRef] [PubMed]



| Compounds | IC50 (μmol/L) a | ||
|---|---|---|---|
| MGC-803 | HCT-116 | MCF-7 | |
| 13a | 3.81 ± 0.22 | 4.012 ± 0.31 | 3.56 ± 0.17 |
| 13b | 4.08 ± 0.24 | 6.72 ± 0.28 | 3.11 ± 0.34 |
| 13c | 1.88 ± 0.22 | 2.83 ± 0.03 | 3.12 ± 0.01 |
| 13d | 1.64 ± 0.18 | 2.40 ± 0.26 | 2.12 ± 0.13 |
| 13e | 1.52 ± 0.12 | 1.83 ± 0.20 | 2.54 ± 0.18 |
| 13f | 3.01 ± 0.11 | 4.28 ± 0.32 | 4.45 ± 0.11 |
| 13g | 1.83 ± 0.20 | 1.12 ± 0.11 | 2.06 ± 0.21 |
| 13h | 22.1 ± 0.75 | 13.1 ± 0.51 | 22.2 ± 0.83 |
| 13i | 16.8 ± 0.82 | 13.1 ± 0.61 | 17.6 ± 0.51 |
| 13j | >40 | >40 | >40 |
| 13k | >40 | >40 | >40 |
| 13l | 16.3 ± 0.65 | >40 | 10.1 ± 0.73 |
| 13m | 5.41 ± 0.30 | 6.12 ± 0.41 | 6.62 ± 0.48 |
| 13n | >40 | 12.5 ± 0.28 | 21.2 ± 1.12 |
| 5-Fu | 6.82 ± 1.12 | 14.4 ± 1.73 | 12.1 ± 1.28 |
| Compd. | IC50 (μM) a | Fold Selectivity | |
|---|---|---|---|
| MGC-803 | GES-1 | A | |
| 13g | 1.52 | 8.22 | 5.4 |
| 5-Fu | 6.82 | 8.22 | 1.2 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-F.; Wang, S.-H.; Pang, X.-J.; Zhu, T.; Li, H.-L.; Li, Q.-R.; Li, Q.-Y.; Gu, Y.-F.; Mu, Z.-Y.; Jin, M.-J.; et al. Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents. Molecules 2020, 25, 5530. https://doi.org/10.3390/molecules25235530
Lu C-F, Wang S-H, Pang X-J, Zhu T, Li H-L, Li Q-R, Li Q-Y, Gu Y-F, Mu Z-Y, Jin M-J, et al. Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents. Molecules. 2020; 25(23):5530. https://doi.org/10.3390/molecules25235530
Chicago/Turabian StyleLu, Chao-Fan, Sheng-Hui Wang, Xiao-Jing Pang, Ting Zhu, Hong-Li Li, Qing-Rong Li, Qian-Yu Li, Yu-Fan Gu, Zhao-Yang Mu, Min-Jie Jin, and et al. 2020. "Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents" Molecules 25, no. 23: 5530. https://doi.org/10.3390/molecules25235530
APA StyleLu, C.-F., Wang, S.-H., Pang, X.-J., Zhu, T., Li, H.-L., Li, Q.-R., Li, Q.-Y., Gu, Y.-F., Mu, Z.-Y., Jin, M.-J., Li, Y.-R., Hu, Y.-Y., Zhang, Y.-B., Song, J., & Zhang, S.-Y. (2020). Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents. Molecules, 25(23), 5530. https://doi.org/10.3390/molecules25235530






