Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review
Abstract
1. Introduction
2. Water Treatment Processes to Remove Glyphosate from Water
2.1. Biologic Treatment Processes
2.2. Psychochemical Treatment Processes
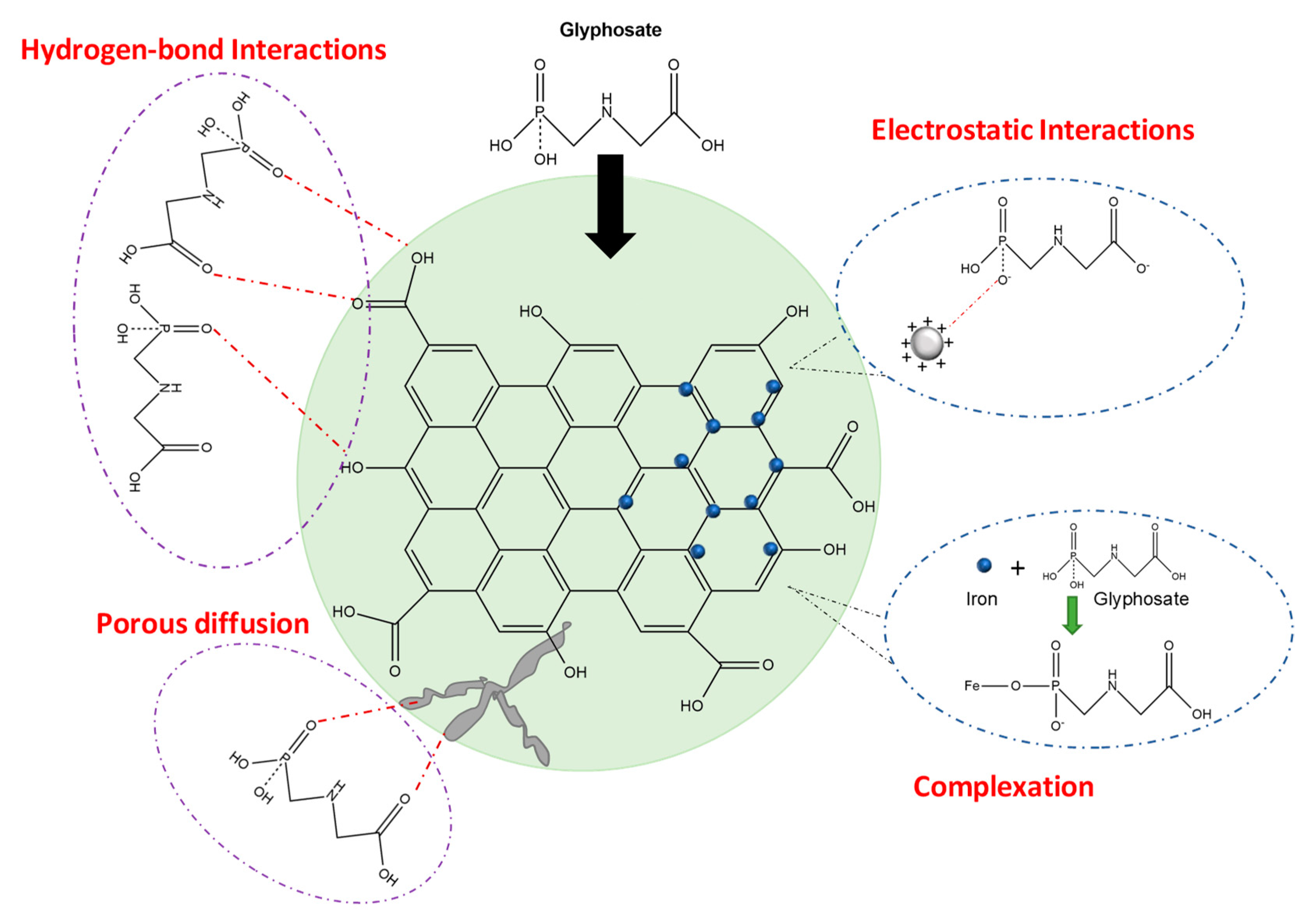
2.2.1. Adsorption
- Physical adsorption is mainly caused by the forces of molecular interactions including permanent dipole/induced dipole, Van der Waals dispersion forces as well as hydrogen-bond with acid groups like -OH and -COOH on the material surface [16], but also it is caused by diffusion through a larger number of micro-, meso- and macro-pores with high pore volume [49].
- Chemical adsorption occurs due to electrostatic attraction between the glyphosate molecule and adsorbent surface, which is strongly influenced by pH conditions. In acidic conditions, glyphosate shows a negative charge, so it is attracted to the material’s surface with a positive charge [16,49]. Additionally, onto iron-based adsorbents, the phosphonyl group of glyphosate molecule can make complexation with Fe2+ and Fe3+ ions forming stable single-tooth or bidentate complexes, thus facilitating the adsorption significantly [16].
2.2.2. Membrane Filtration
2.3. Advanced Oxidation Processes (AOPs)
2.3.1. Fenton-Based Treatment Process
2.3.2. UV-Based Treatment Process
2.3.3. Electrochemical Oxidation Process
2.3.4. Photoelectrocatalytic Treatment Process
2.4. Combined Treatment Processes
3. Final Analysis and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl Radical |
| ACF | Activated Carbon Fiber |
| AMPA | Aminomethylphosphonic Acid |
| AOPs | Advanced Oxidation Processes |
| BDD | Boron-Doped Diamond |
| BZ | Buffer Zones |
| C-N | Carbon-nitrogen bond |
| COD | Chemical Organic Demand |
| C-P | Carbon-phosphorus bond |
| DSA | Dimensionally Stable Anode |
| Ea | Anodic Potential |
| eCB−/hVB+ | Electron/hole pairs |
| EO | Electrochemical Oxidation |
| EPSP | 5-enolpiruvil-shikimato-3-phosphate-synthetase enzyme |
| FAO | Food and Agriculture Organization |
| GLY | Glyphosate |
| GO/TiO2 | Graphene Oxide and Titanium Dioxide |
| IARC | International Agency for Research on Cancer |
| IBT | Immobilizing Bacterial Technology |
| Kow | Octanol-water partition coefficient |
| MO | Metal Oxide |
| NHE | Normal Hydrogen Electrode |
| PEC | Photoelectrocatalysis |
| PSf | Polysulfone |
| TMP | Trans-Membrane Pressure |
| TOC | Total Organic Carbon |
| UV | Ultraviolet light |
| WHO | World Health Organization |
References
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wen-Tien, T. Trends in the Use of Glyphosate Herbicide and Its Relevant Regulations in Taiwan: A Water Contaminant of Increasing Concern. Toxics 2019, 7, 4. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Vasquez-Medrano, R.; Espinoza-Montero, P.J. Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1476–1514. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Madrid, M.L.A. Herbicida Glifosato: Usos, Toxicidad Y Regulación. Rev. Ciencias Biol. T Salud Univ. Sonora 2011, 11, 23–28. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, K.; Schmidt, W.; Unger, C.; Hanschmann, G. Behavior of glyphosate and aminomethylphosphonic acid (AMPA) in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J. Plant Nutr. Soil Sci. 2001, 164, 65–70. [Google Scholar] [CrossRef]
- Székács, A.; Darvas, B. Forty years with glyphosate. In Herbicides-Properties, Synthesis and Control of Weeds; Nagib, M., Ed.; InTech: London, UK, 2012; pp. 247–284. [Google Scholar]
- de Brito Rodrigues, L.; Gonçalves Costa, G.; Lundgren Thá, E.; da Silva, L.R.; de Oliveira, R.; Morais Leme, D.; Cestari, M.M.; Koppe Grisolia, C.; Campos Valadares, M.; de Oliveira, G.A.R. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y. Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst. Chemosphere 2007, 67, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, C.; Wen, Y.; Wang, Y.; Yang, Y. Adsorption performance and mechanism of magnetic reduced graphene oxide in glyphosate contaminated water. Environ. Sci. Pollut. Res. 2018, 25, 21036–21048. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievskya, A.A. Microbial Degradation of Glyphosate Herbicides (Review). Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Fan, J.; Yang, G.; Zhao, H.; Shi, G.; Geng, Y.; Hou, T.; Tao, K. Isolation, identification and characterization of a glyphosate-degrading bacterium, Bacillus cereus CB4, from soil. J. Gen. Appl. Microbiol. 2012, 58, 263–271. [Google Scholar] [CrossRef]
- Wijekoon, N.; Yapa, N. Assessment of plant growth promoting rhizobacteria (PGPR) on potential biodegradation of glyphosate in contaminated soil and aquifers. Groundw. Sustain. Dev. 2018, 7, 465–469. [Google Scholar] [CrossRef]
- Fu, G.M.; Chen, Y.; Li, R.Y.; Yuan, X.Q.; Liu, C.M.; Li, B.; Wan, Y. Pathway and rate-limiting step of glyphosate degradation by Aspergillus oryzae A-F02. Prep. Biochem. Biotechnol. 2017, 47, 782–788. [Google Scholar] [CrossRef]
- Adelowo, F.E.; Olu-Arotiowa, O.A.; Amuda, O.S. Biodegradation of Glyphosate by Fungi Species Phosphorus (C-P) bond. Adv. Biosci. Bioeng. 2014, 2, 104–118. [Google Scholar]
- Balthazor, T.M.; Hallas, L.E. Glyphosate-degrading microorganisms from industrial activated sludge. Appl. Environ. Microbiol. 1986, 51, 432–434. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, K.S.; Hallas, L.E.; Kulpa, C.F. Glyphosate degradation by Agrobacterium radiobacter isolated from activated sludge. J. Ind. Microbiol. 1990, 6, 219–221. [Google Scholar] [CrossRef]
- Heitkamp, M.A.; Adams, W.J.; Hallas, L.E. Glyphosate degradation by immobilized bacteria: Laboratory studies showing feasibility for glyphosate removal from waste water. Can. J. Microbiol. 1992, 38, 921–928. [Google Scholar] [CrossRef]
- Roffignac, L.; Cattan, P.; Mailloux, J.; Herzog, D.; Le Bellec, F. Efficiency of a bagasse substrate in a biological bed system for the degradation of glyphosate, malathion and lambda-cyhalothrin under tropical climate conditions. Pest Manag. Sci. 2008, 64, 1303–1313. [Google Scholar] [CrossRef]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tazdaït, D.; Salah, R.; Grib, H.; Abdi, N.; Mameri, N. Kinetic study on biodegradation of glyphosate with unacclimated activated sludge. Int. J. Environ. Health Res. 2018, 28, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Singh, J. Kinetic study of the biodegradation of glyphosate by indigenous soil bacterial isolates in presence of humic acid, Fe(III) and Cu(II) ions. J. Environ. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Castro, J.V.; Peralba, M.C.R.; Ayub, M.A.Z. Biodegradation of the herbicide glyphosate by filamentous fungi in platform shaker and batch bioreactor. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2007, 42, 883–886. [Google Scholar] [CrossRef]
- Bouchiat, R.; Veignie, E.; Grizard, D.; Soebert, C.; Vigier, M.; Rafin, C. Ability of filamentous fungi to degrade four emergent water priority pollutants. Desalin. Water Treat. 2016, 57, 6740–6746. [Google Scholar] [CrossRef]
- Obojska, A.; Ternan, N.G.; Lejczak, B.; Kafarski, P.; McMullan, G. Organophosphonate Utilization by the Thermophile Geobacillus caldoxylosilyticus T20. Appl. Environ. Microbiol. 2002, 68, 2081–2084. [Google Scholar] [CrossRef]
- Carles, L.; Gardon, H.; Joseph, L.; Sanchís, J.; Farré, M.; Artigas, J. Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ. Int. 2019, 124, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, W.; Liu, F.; Yue, X.; Liu, Y.; Yang, M.; Li, Z.; Wang, J. Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide. Chem. Eng. J. 2017, 313, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, B.; Zhao, Y.; English, A.; Cannon, M. A comparison of alum sludge with peat for aqueous glyphosate removal for maximizing their value for practical use. Water Sci. Technol. 2018, 2017, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.R.T.; Andrade, M.B.; Silva, M.F.; Bergamasco, R.; Hamoudi, S. Development of α- and γ-Fe2O3 decorated graphene oxides for glyphosate removal from water. Environ. Technol. 2019, 40, 1118–1137. [Google Scholar] [CrossRef]
- Dissanayake Herath, G.A.; Poh, L.S.; Ng, W.J. Statistical optimization of glyphosate adsorption by biochar and activated carbon with response surface methodology. Chemosphere 2019, 227, 533–540. [Google Scholar] [CrossRef]
- Jia, D.; Liu, M.; Xia, J.; Li, C. Effective removal of aqueous glyphosate using CuFe2O4@biochar derived from phragmites. J. Chem. Technol. Biotechnol. 2020, 95, 196–204. [Google Scholar] [CrossRef]
- Xiao, G.; Meng, Q. D151 resin preloaded with Fe3+ as a salt resistant adsorbent for glyphosate from water in the presence 16% NaCl. Ecotoxicol. Environ. Saf. 2020, 190, 110140. [Google Scholar] [CrossRef]
- Ren, Z.; Dong, Y.; Liu, Y. Enhanced glyphosate removal by montmorillonite in the presence of Fe(III). Ind. Eng. Chem. Res. 2014, 53, 14485–14492. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, M.; Xu, J.; Fein, J.B.; Yu, Q.; Wang, Y.; Huang, Q.; Rong, X. Glyphosate adsorption onto kaolinite and kaolinite-humic acid composites: Experimental and molecular dynamics studies. Chemosphere 2020, 263, 127979. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.A.; Gehris, T.C.; Tribe, L.; Torres Sánchez, R.M.; dos Santos Afonso, M. Glyphosate adsorption on montmorillonite: An experimental and theoretical study of surface complexes. Appl. Clay Sci. 2010, 50, 167–175. [Google Scholar] [CrossRef]
- Pankajakshan, A.; Sinha, M.; Ojha, A.A.; Mandal, S. Water-Stable Nanoscale Zirconium-Based Metal-Organic Frameworks for the Effective Removal of Glyphosate from Aqueous Media. ACS Omega 2018, 3, 7832–7839. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xie, M.; Ni, F.; Xu, Y.H. Nanofiltration process of glyphosate simulated wastewater. Water Sci. Technol. 2012, 65, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Duan, J.; Saint, C.P.; Mulcahy, D. Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration. Environ. Technol. 2018, 39, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Saitúa, H.; Giannini, F.; Padilla, A.P. Drinking water obtaining by nanofiltration from waters contaminated with glyphosate formulations: Process evaluation by means of toxicity tests and studies on operating parameters. J. Hazard. Mater. 2012, 227, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.; Toosi, M.R. Removal of 2,4-D, glyphosate, trifluralin, and butachlor herbicides from water by polysulfone membranes mixed by graphene oxide/TiO2 nanocomposite: Study of filtration and batch adsorption. J. Environ. Health Sci. Eng. 2019, 17, 247–258. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.A.; Vithanage, M. Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater. 2016, 225, 280–288. [Google Scholar] [CrossRef]
- Jönsson, J.; Camm, R.; Hall, T. Removal and degradation of Glyphosate in water treatment: A review. J. Water Supply Res. Technol. AQUA 2013, 62, 395–408. [Google Scholar] [CrossRef]
- Speth, T.F. Glyphosate Removal from Drinking Water. J. Environ. Eng. 1993, 119, 1139–1157. [Google Scholar] [CrossRef]
- Sheals, J.; Sjöberg, S.; Persson, P. Adsorption of Glyphosate on Goethite: Molecular Characterization of Surface Complexes. Environ. Sci. Technol. 2002, 36, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T. Membrane filtration. In Advanced Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N., Eds.; Humana Press: Totowa, NJ, USA, 2007; Volume 4, pp. 213–279. [Google Scholar]
- Andreozzi, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 8, 251–325. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Lin, Y.; Deng, N.; Bazhin, N.; Glebov, E. Photodegradation of glyphosate in the ferrioxalate system. J. Hazard. Mater. 2007, 148, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Assalin, M.R.; de Moraes, S.G.; Queiroz, S.C.N.; Ferracini, V.L.; Duran, N. Studies on degradation of glyphosate by several oxidative chemical processes: Ozonation, photolysis and heterogeneous photocatalysis. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2009, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Manassero, A.; Passalia, C.; Negro, A.C.; Cassano, A.E.; Zalazar, C.S. Glyphosate degradation in water employing the H2O2/UVC process. Water Res. 2010, 44, 3875–3882. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, G.; Xu, X.; Yang, X.; Liu, C.; Xu, Y. Preparation of titania nanotubes doped with cerium and their photocatalytic activity for glyphosate. Chem. Eng. J. 2011, 167, 397–402. [Google Scholar] [CrossRef]
- Vidal, E.; Negro, A.; Cassano, A.; Zalazar, C. Simplified reaction kinetics, models and experiments for glyphosate degradation in water by the UV/H2O2 process. Photochem. Photobiol. Sci. 2015, 14, 366–377. [Google Scholar] [CrossRef]
- López, A.; Coll, A.; Lescano, M.; Zalazar, C. Advanced oxidation of commercial herbicides mixture: Experimental design and phytotoxicity evaluation. Environ. Sci. Pollut. Res. 2018, 25, 21393–21402. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Yan, W.; Jing, C.; Zhang, Y. Comparative study of glyphosate removal on goethite and magnetite: Adsorption and photo-degradation. Chem. Eng. J. 2018, 352, 581–589. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Dachtler, W.; Altmayer, B.; Schulz, R.; Robert, D.; Seitz, F.; Rosenfeldt, R.; Keller, N. Reaction pathways, kinetics and toxicity assessment during the photocatalytic degradation of glyphosate and myclobutanil pesticides: Influence of the aqueous matrix. Chem. Eng. J. 2020, 384, 123315. [Google Scholar] [CrossRef]
- Lv, Y.-R.; He, R.-K.; Chen, Z.-Y.; Li, X.; Xu, Y.-H. Fabrication of hierarchical copper sulfide/bismuth tungstate p-n heterojunction with two-dimensional (2D) interfacial coupling for enhanced visible-light photocatalytic degradation of glyphosate. J. Colloid Interface Sci. 2020, 560, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.R.D.; Trovõ, A.G.; Filho, N.R.A.; Silva, M.A.A.; Machado, A.E.H. Degradation of the commercial herbicide glyphosate by photo-fenton process: Evaluation of kinetic parameters and toxicity. J. Braz. Chem. Soc. 2013, 24, 1451–1460. [Google Scholar] [CrossRef]
- Balci, B.; Oturan, M.A.; Oturan, N.; Sires, I. Decontamination of aqueous glyphosate, (aminomethyl) phosphonic acid, and glufosinate solutions by electro-fenton-iike process with Mn2+ as the catalyst. J. Agric. Food Chem. 2009, 57, 4888–4894. [Google Scholar] [CrossRef]
- Lan, H.; He, W.; Wang, A.; Liu, R.; Liu, H.; Qu, J.; Huang, C.P. An activated carbon fiber cathode for the degradation of glyphosate in aqueous solutions by the Electro-Fenton mode: Optimal operational conditions and the deposition of iron on cathode on electrode reusability. Water Res. 2016, 105, 575–582. [Google Scholar] [CrossRef]
- Aquino Neto, S.; de Andrade, A.R. Electrooxidation of glyphosate herbicide at different DSA® compositions: pH, concentration and supporting electrolyte effect. Electrochim. Acta 2009, 54, 2039–2045. [Google Scholar] [CrossRef]
- Zhang, M.D.; Wei, Y.F.; Zhao, K.; Mei, R.W.; Huang, M. Glyphosate Degradation with Industrial Wastewater Effluent by Combined Adsorption Treatment and Advanced Oxidation Processes. Adv. Mater. Res. 2011, 233–235, 369–372. [Google Scholar] [CrossRef]
- Lan, H.; Jiao, Z.; Zhao, X.; He, W.; Wang, A.; Liu, H.; Liu, R.; Qu, J. Removal of glyphosate from water by electrochemically assisted MnO2 oxidation process. Sep. Purif. Technol. 2013, 117, 30–40. [Google Scholar] [CrossRef]
- Tran, M.H.; Nguyen, H.C.; Le, T.S.; Dang, V.A.D.; Cao, T.H.; Le, C.K.; Dang, T.-D. Degradation of glyphosate herbicide by an electro-Fenton process using carbon felt cathode. Environ. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Montes, I.; Pérez, J.F.; Sáez, C.; Rodrigo, M.A.; Cañizares, P.; Aquino, J.M. Assessing the performance of electrochemical oxidation using DSA® and BDD anodes in the presence of UVC light. Chemosphere 2020, 238, 124575. [Google Scholar] [CrossRef] [PubMed]
- Rubí-Juárez, H.; Cotillas, S.; Sáez, C.; Cañizares, P.; Barrera-Díaz, C.; Rodrigo, M.A. Use of conductive diamond photo-electrochemical oxidation for the removal of pesticide glyphosate. Sep. Purif. Technol. 2016, 167, 127–135. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Serra-Clusellas, A.; De Angelis, L.; Beltramo, M.; Bava, M.; De Frankenberg, J.; Vigliarolo, J.; Di Giovanni, N.; Stripeikis, J.D.; Rengifo-Herrera, J.A.; Fidalgo De Cortalezzi, M.M. Glyphosate and AMPA removal from water by solar induced processes using low Fe(III) or Fe(II) concentrations. Environ. Sci. Water Res. Technol. 2019, 5, 1932–1942. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Doan, T.L.; Le, T.S.; Nguyen, H.C. Electrochemical degradation and mineralization of glyphosate herbicide. Environ. Technol. 2017, 38, 2939–2948. [Google Scholar] [CrossRef]
- Azrague, K.; Bonnefille, E.; Pradines, V.; Pimienta, V.; Oliveros, E.; Maurette, M.T.; Benoit-Marquié, F. Hydrogen peroxide evolution during V-UV photolysis of water. Photochem. Photobiol. Sci. 2005, 4, 406–408. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Espinoza-Montero, P.J.; Quiroz-Chávez, F.J.; Ohtani, B. Fotocatálisis: Inicio, Actualidad y Perspectivas a Través del TiO2. Avances en Química. 2017, 12, 45–49. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Comninellis, C.; Chen, G. Electrochemistry for the Environment; Springer: New York, NY, USA, 2010; ISBN 9780387369228. [Google Scholar]
- De Battisti, A.; Martínez-Huitle, C.A. Electrochemical Waster and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Espinoza-Montero, P.J.; Vasquez-Medrano, R.; Ibanez, J.G.; Frontana-Uribe, B.A. Efficient Anodic Degradation of Phenol Paired to Improved Cathodic Production of H2O2 at BDD Electrodes. J. Electrochem. Soc. 2013, 160, G3171–G3177. [Google Scholar] [CrossRef]
- Bravo-Yumi, N.P.; Espinoza-Montero, P.; Brillas, E.; Peralta-Hernández, J.M. Electrochemical abatement of atrazine solutions using an undivided stirred tank cell with pt or BDD anode. J. Mex. Chem. Soc. 2018, 62, 295–304. [Google Scholar]
- Ochoa-Chavez, A.S.; Pieczyńska, A.; Fiszka Borzyszkowska, A.; Espinoza-Montero, P.J.; Siedlecka, E.M. Electrochemical degradation of 5-FU using a flow reactor with BDD electrode: Comparison of two electrochemical systems. Chemosphere 2018, 201, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Armijos-Alcocer, K.G.; Espinoza-Montero, P.J.; Frontana-Uribe, B.A.; Barrera-Diaz, C.E.; Nevárez-Martínez, M.C.; Fierro-Naranjo, G.C. Electrochemical Degradation of Nonylphenol Ethoxylate-7 (NP7EO) Using a DiaClean® Cell Equipped with Boron-Doped Diamond Electrodes (BDD). Water Air Soil Pollut. 2017, 228, 289. [Google Scholar] [CrossRef]
- Rubí-Juárez, H.; Cotillas, S.; Sáez, C.; Cañizares, P.; Barrera-Díaz, C.; Rodrigo, M.A. Removal of herbicide glyphosate by conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2016, 188, 305–312. [Google Scholar] [CrossRef]
- Oliveira, K.S.G.C.; Farinos, R.M.; Veroli, A.B.; Ruotolo, L.A.M. Electrochemical incineration of glyphosate wastewater using three-dimensional electrode. Environ. Technol. 2019. [Google Scholar] [CrossRef]
- Rajeshwar, K. Photoelectrochemistry and the environment. J. Appl. Electrochem. 1995, 25, 1067–1082. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Guaraldo, T.T.; de Brito, J.F.; Brugnera, M.; Zanoni, M. Achievements and Trends in Photoelectrocatalysis: From Environmental to Energy Applications. J. Photochem. Photobiol. A Chem. 2015, 6, 415–441. [Google Scholar] [CrossRef]
- Garcia-segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic technologies for environmental applications. J. Photochem. Photobiol. A Chem. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Zhang, K.; Deletic, A.; Page, D.; McCarthy, D.T. Surrogates for herbicide removal in stormwater biofilters. Water Res. 2015, 81, 64–71. [Google Scholar] [CrossRef]
- Yang, H.; Dick, W.A.; McCoy, E.L.; Phelan, P.L.; Grewal, P.S. Field evaluation of a new biphasic rain garden for stormwater flow management and pollutant removal. Ecol. Eng. 2013, 54, 22–31. [Google Scholar] [CrossRef]
- Pérez, D.J.; Okada, E.; Menone, M.L.; Costa, J.L. Can an aquatic macrophyte bioaccumulate glyphosate? Development of a new method of glyphosate extraction in Ludwigia peploides and watershed scale validation. Chemosphere 2017, 185, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Syversen, N.; Bechmann, M. Vegetative buffer zones as pesticide filters for simulated surface runoff. Ecol. Eng. 2004, 22, 175–184. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, D.; Hu, J.; Zhang, J.; Liu, Z.; Li, A.; Li, R. Glyphosate and nutrients removal from simulated agricultural runoff in a pilot pyrrhotite constructed wetland. Water Res. 2019, 168, 115154. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Chen, H.; Zhang, X. Efficient degradation of organic phosphorus in glyphosate wastewater by catalytic wet oxidation using modified activated carbon as a catalyst. Environ. Technol. 2018, 39, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Kobylanski, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2-MnO2 nanotube arrays for efficient photocatalytic degradation of toluene. Molecules 2017, 22, 564. [Google Scholar] [CrossRef]



| Microorganism (Bacteria and Fungi) | Experimental Conditions | Glyphosate Concentration (mg·a.i.·L−1) | Removal (%) | Ref. |
|---|---|---|---|---|
| Pseudomonas sp.; Bacillus sp. | Batch culture | 50,000–150,000 | - | [19] |
| Flavobacterium sp. | Batch culture isolated from activated sludge | - | 25.0 | [22] |
| Agrobacterium radiobacter | Batch culture isolated from a bench scale sequencing batch reactor | 0.001 | 99.0 | [23] |
| Pseudomonas spp. | Biofilter | 10–50 | 90.0–95.0 | [24] |
| Microorganisms attached to bagasse | Biofilter (biomix) | - | 99.0 | [25] |
| Native bacteria from seawater | Batch culture | 0.01 | 48.0 | [26] |
| Activated sludge of wastewater treatment plant | Batch culture | 100−1000 | - | [27] |
| Streptomyces sp., Bacillus subtilis and Rhizobium leguminosarum | Batch culture | 250 | 89.7 87.6 86.2 | [28] |
| Geobacillus caldoxylosilyticus | Batch culture isolated from central heating system water | 169.07 | - | [31] |
| Biofilm | Laboratory aquarium | 0.01–0.1 | Complete dissipation | [32] |
| Aspegillus oryzae A-F02 | Batch culture, isolated from an aeration tank of a pesticide factory | 1000 | - | [20] |
| Fusarium oxysporum | Platform shaker and Batch bioreactor | 50 | 42.0 | [29] |
| Trichoderma harzianum | Batch culture | 0.01 | 69.0 | [30] |
| Experimental Conditions | Removal (%) | Ref. | ||
|---|---|---|---|---|
| Adsorbent | Operating Conditions | Glyphosate Concentration (mg·a.i.·L−1) | ||
| 10 mg (RGO/Fe3O4) | Batch scale, pH solutions: 4; Solid/solution ratio: 1 g·L−1 | 40–40 | 73.0 | [16] |
| Residual sludge from industrial water | - | 50–100 200–500 | 91.6 97.4 | [33] |
| Metal organic framework/grapheme oxide hybrid nanocomposite (UiO-67/GO) | pH solutions: 4; Treatment time: 3 h | 2.560 | - | [34] |
| Alum sludge | Filter: Pot test filled with adsorbents | 50 | 99.8 | [35] |
| Cu-zeolite 4A | Batch scale; Solid/solution ratio: 2 g·L−1 | 50–150 | - | [36] |
| GO-α-γ-Fe2O3 | Batch scale; Solid/solution ratio: 0.5–3.0 g·L−1 | 1–80 | 92.0 | [37] |
| Coconut shell activated carbon and wood biochar | Batch scale; Solid/solution ratio: 11.4 g·L−1 and 12.3 g·L−1 | 0.2–20 | 98.45 100.0 | [38] |
| Nano-CuFe2O4 modified | Temperature: 25 °C; Treatment time: 4 h; pH solution: 4 | 600 | 98.9 | [39] |
| D151 resin preloaded with Fe3+ | Temperature: 10–40 °C; Treatment time: 24 h; pH solution: 3.35; NaCl Concentration: 16% | 500–1100 | - | [40] |
| Montmorillonite- Fe(III) | Batch scale: Fe(III)-glyphosate 1:1 molar ratio; pH > 5.9; Treatment time: 3 h; Agitation speed: 150 rpm | 350.0 | 98.05 | [41] |
| Kaolinite and Kaolinite-humic acid composite | Batch scale; 10 g of sorbent; Agitation speed: 150 rpm; Treatment time: 6 h; Temperature: 28 °C | 40.0 | - | [42] |
| Montmorillonite | Ionic strengths of NaCl 0–0.7; pH solution: 2.0–9.0 | 0–169.07 | - | [43] |
| Zr-based MOFs (NU-1000, UiO-67) | Batch scale; 3 mg of activated MOFs; Treatment Time: 5 h; mechanical shaker: 180 rpm | 1.7 | - | [44] |
| Experimental Conditions | Removal (%) | Ref. | ||
|---|---|---|---|---|
| Membrane Filtration | Operating Conditions | Glyphosate Concentration (mg·a.i.·L−1) | ||
| Organic GK NF membranes | Cross–flow mode system; Temperature: 20 °C; pH solution: 2.96, TMP: 2.5 MPa | 500 | 94.8 | [45] |
| Polyamide membranes: NFX and NFY | Temperature: 25 °C; TMP: 2.5 MPa | 0.05 0.049 ** | 82.8 73.5 | [46] |
| (TFC) Polyamide membrane | Transversal-flow mode system; pH solution: 8.5; TMP: 4–10 bar | 48.0 | 80.0 | [47] |
| GO/TiO2/PSf membranes | Dead-end flow mode system; 25 °C, TMP 1 bar | 20.0 | 53.0 | [48] |
| AOPs | Operating Conditions | Glyphosate Concentration (mg a.i. L−1) | Removal (%) | Ref. |
|---|---|---|---|---|
| UV/Ferrioxalate | V = 80 mL (eight quartz tubes/10 mL); pH = 3.5–6.0; UV-vis Lamp 250 W (λ ≥ 365 nm); t = 180 min | 1.0–5.0 | - | [57] |
| UV/TiO2 | V = 400 mL (cylindrical annular-type reactor); pH from 2.0 to 12.0; UV Lamp = 365 nm; illumination time = 1 h | 42.25 | 9.8–50.2 | [15] |
| Photocatalytic degradation(UV-TiO2) | V = 200 mL; high-pressure mercury lamp (125 W, λ > 290 nm); amount of catalyst = 0.1 g·L−1 of TiO2; t = 30 min. | 42.3 | 99.9 | [58] |
| H2O2/UV | Vreactor = 110 cm3; [H2O2] = 75–200 mg·L−1; t = 5 h; 2 UV lamp of 40 W | 50.0 | 70.0 | [59] |
| Photocatalysis Ce-TiO2 | 0.15% Ce-TiO2 nanotubes annealed at 400 °C; V = 500 mL; t = 1 h; pH = 7; 125 high-pressure mercury lamps. | 22.8 | 76.0 | [60] |
| UV/H2O2 experimental and mathematical model | V = 2000 mL (quartz cylindrical reactor, 110 mL, with recirculation); flow rate = 5 × 10−2 cm3·s−1; UV Lamp = 253.7 nm; pH = 5.2; [H2O2] = 0 to 403 mg·L−1; t = 12 h | 140.0 | 80.0 GLY 70.0 TOC | [61] |
| UV/H2O2 | V = 1000 cm3; two low-pressure mercury vapor lamps with one emission wavelength at λ = 253.7 nm; Q = 2 L·s−1; t = 8 h | 30.0 | - | [62] |
| UV/Goethite | incident light intensity 500–2000 W/m2; T = 20 °C, pH 3–9 | 10.0 | 92.0 99.3 | [63] |
| Aeroxide TiO2 -P25 | Volume 250 mL, stirring 600 rpm, UV-A light 60 W/m2 wavelength at λ = 365 nm, Time = 240 min | 25.0 | 100 | [64] |
| Photochemical degradation over CuS/Bi2WO6 | Hierarchical CuS/Bi2WO6 p-n junction photocatalyst; illumination time: 180 min; 44 W light-emitting diode (LED) light irradiation (λ > 400 nm) | 16.9 | 85.9 | [65] |
| Photo-Fenton | V = 50 L; closed recirculating system at a flow rate of 2.37 L·min−1; [Fe2+] or [Fe2+/Fe 3+] = 0.27 mmol·L−1; [H2O2] = 10.3 mmol·L−1; pH 2.8 ± 0.2 | 100.0 100.0 | - - | [66] |
| Electro-Fenton Mn2+ | V = 200 mL; 100 mA constant current; catalyst = 0.1 mM Mn2+ | 22.8 | 92.0–100.0 | [67] |
| Electro–Fenton | t = 360 min; pH = 3; current intensity = 0.36 A; 1 mM of Fe2+; pure O2 flow rate = 100 mL·min−1 | 22.8 | - | [68] |
| Electrochemical oxidation with RuO2/IrO2 electrodes | i = 50 mA·cm−2; t = 4 h; electrode composition = Ti/Ir0.30Sn0.70 O2; | 1000.0 | 24.0 | [69] |
| Adsorption and POA’s (H2O2) | V = 150 mL of glyphosate residue solution; pH = 2–4; adsorbent = nano-tungsten/D201 resin + H2O2 | 258.0 | 60.5 | [70] |
| Electrochemical degradation with MnO2 | V = 400 mL; acidic pH; i = 10 mA·cm−2; t = 120 min | 22.8 | 80.0 | [71] |
| Electrochemical degradation | Anode: Ti/PbO2; pH: 3–10; current intensity: 4.77 A; reaction time: 173 min; electrolyte: Na2SO4 | 4–16 | 95.16 | [72] |
| Electrochemical oxidation BDD | Electric charge = 6.0 Ah·dm−3; glyphosate pure; t = about 150 min; Chloride media | 100.0 | - - | [73] |
| Photochemical Oxidation with BDD | UV lamp (λ = 254 nm); i = 100 mA·cm−2; t = about 200 min; supporting electrolyte = NaCl | 100.0 | - | [74] |
| Treatment Technology | Treatment Process Associated | Glyphosate Concentration (mg·a.i.·L−1) | Removal (%) | Ref. |
|---|---|---|---|---|
| Vegetated buffer zones | Adsorption in organic components and clays | 0.015–0.030 | 39 | [96] |
| Biphasic rain garden | Adsorption and microbial degradation | 35–1500 | 99 | [94] |
| Biofilters with plants | Adsorption mixed with microbial degradation | 0.0001–0.25 | 90 | [93] |
| Constructed wetlands | Adsorption and microbial activity | - | 90.3 | [97] |
| Adsorption and POA’s (H2O2) | V = 150 mL of glyphosate residue solution; pH = 2–4; adsorbent = nano-tungsten/D201 resin + H2O2 | 258.0 | 60.5 | [70] |
| Adsorption with AOPs | Catalytic wet oxidation using modified activated carbon as a catalyst in a co-current up flow fixed bed reactor; | 200–300 mg·L−1 | 100.0 93.0 | [98] |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules 2020, 25, 5550. https://doi.org/10.3390/molecules25235550
Espinoza-Montero PJ, Vega-Verduga C, Alulema-Pullupaxi P, Fernández L, Paz JL. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules. 2020; 25(23):5550. https://doi.org/10.3390/molecules25235550
Chicago/Turabian StyleEspinoza-Montero, Patricio J., Carolina Vega-Verduga, Paulina Alulema-Pullupaxi, Lenys Fernández, and Jose L. Paz. 2020. "Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review" Molecules 25, no. 23: 5550. https://doi.org/10.3390/molecules25235550
APA StyleEspinoza-Montero, P. J., Vega-Verduga, C., Alulema-Pullupaxi, P., Fernández, L., & Paz, J. L. (2020). Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules, 25(23), 5550. https://doi.org/10.3390/molecules25235550








