Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment
Abstract
:1. Introduction
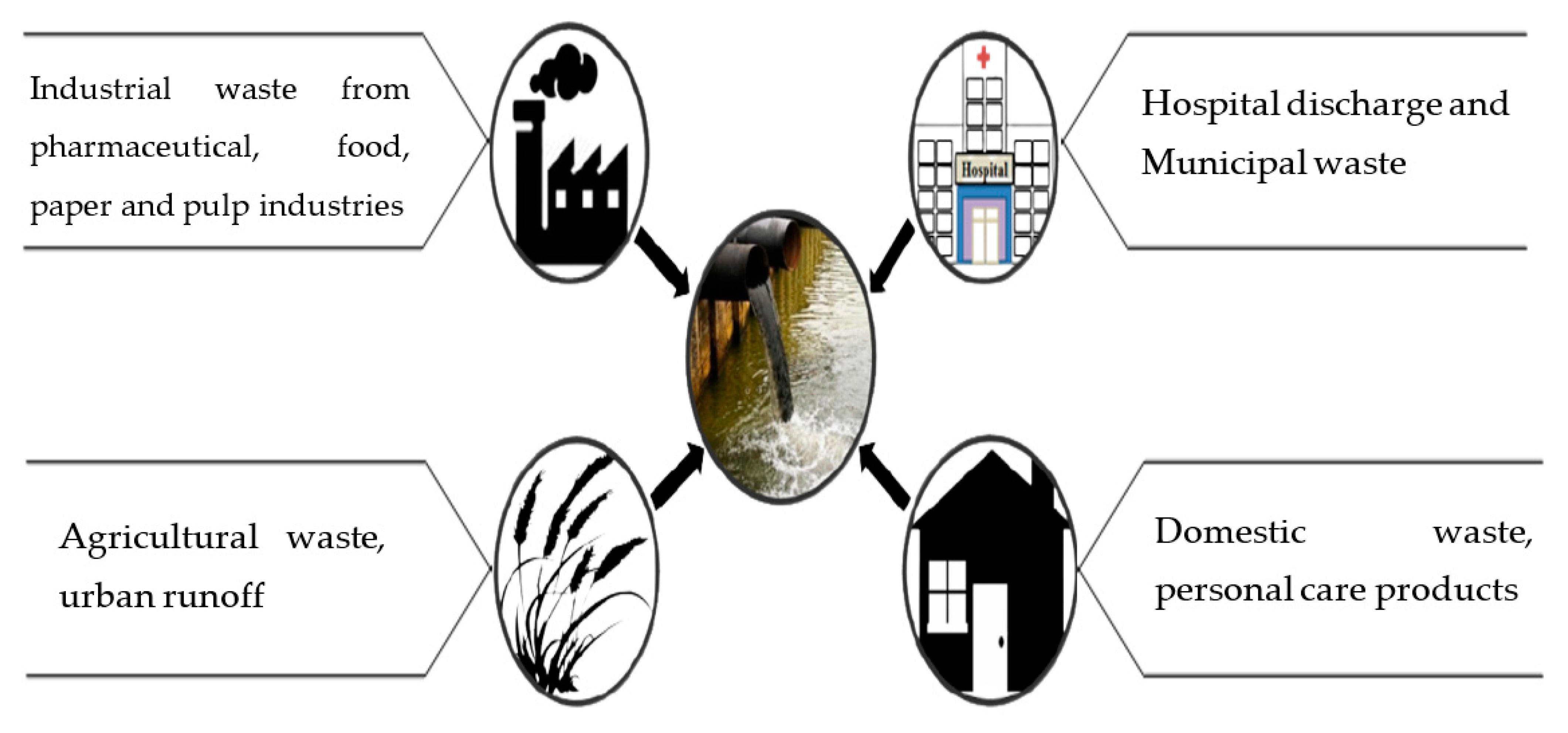
2. Wastewater: Sources and Composition
3. Common Steps in Wastewater Treatment
4. Nanotechnology in Wastewater Management
4.1. Adsorption and Biosorption
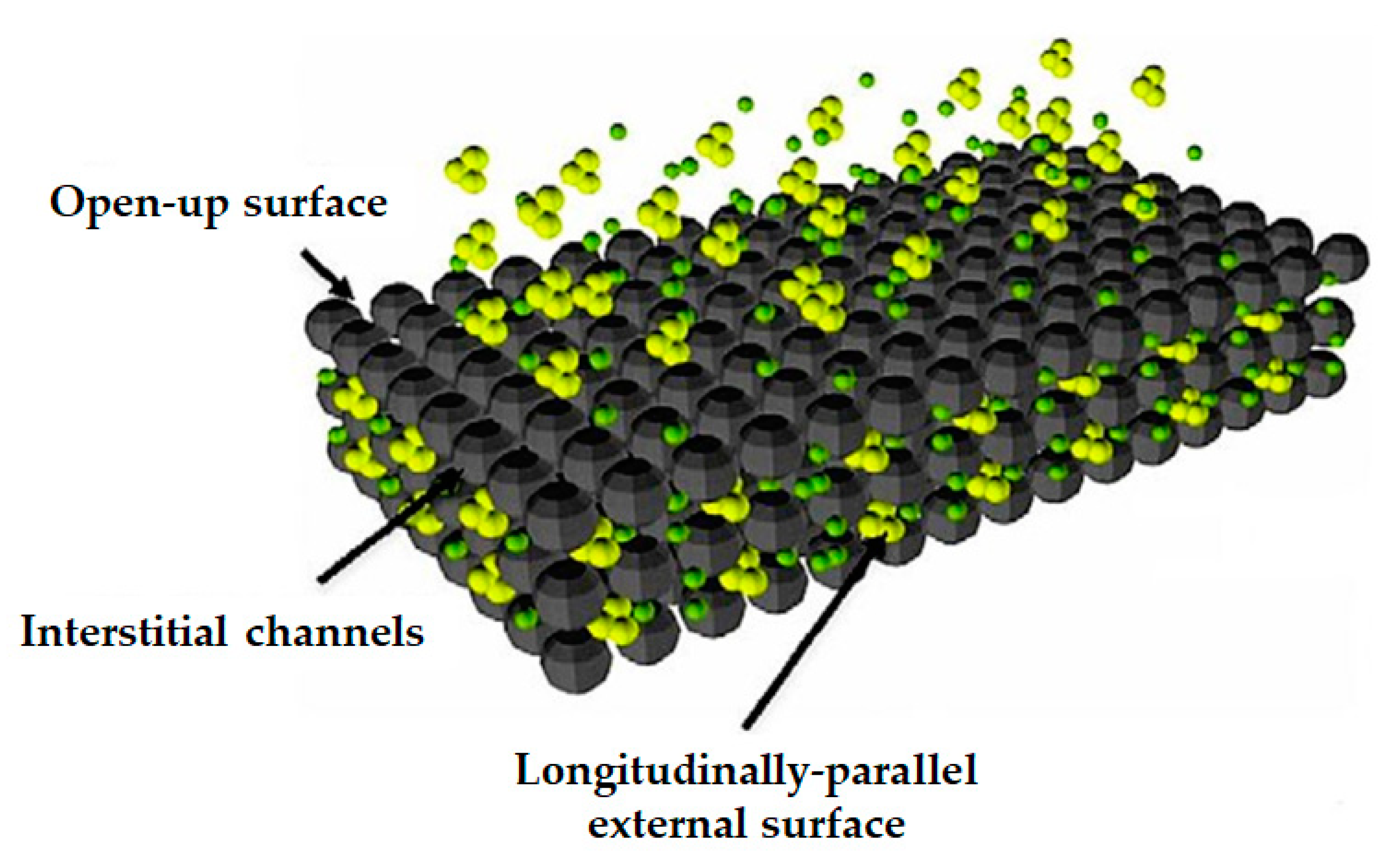
4.1.1. Carbon-based Nano-Adsorbents
4.1.2. Metal based Nanoadsorbents
4.1.3. Polymer-Based Nanoadsorbents
4.1.4. Zeolites
4.2. Nanofilters
4.3. Photocatalysis
- Diffusion of reactants/pollutants to the surface of photocatalyst
- Adsorption of reactants/pollutants on the surface of photocatalyst
- Reaction of adsorbed reactants/pollutants
- Desorption of products from the surface
- Removal/diffusion of products from interface
4.4. Disinfection and Pathological Control
4.5. Sensing and Monitoring
5. Barriers and Risks Associated with Nanotechnology
5.1. Nanomaterial Toxicity
5.2. Cost Effectiveness
5.3. Nanomaterial Transformation Risk in Water
5.4. Ecotoxicity Associated with Nanomaterials
5.5. Water Pollution
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Definition of Freshwater Resources. Available online: https://web.archive.org/web/20160411064155/http://webworld.unesco.org/water/ihp/publications/waterway/webpc/definition.html (accessed on 25 January 2021).
- Kurniawan, T.A.; Sillanpää, M.E.; Sillanpää, M. Nanoadsorbents for Remediation of Aquatic Environment: Local and Practical Solutions for Global Water Pollution Problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
- Olvera, R.C.; Silva, S.L.; Robles-Belmont, E.; Lau, E.Z. Review of nanotechnology value chain for water treatment applications in Mexico. Resour. Technol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Singh, I.; Mishra, P.K. Nano-membrane filtration a novel application of nanotechnology for waste water treatment. Mater. Today Proc. 2020, 29, 327–332. [Google Scholar] [CrossRef]
- Kamali, M.; Persson, K.M.; Costa, M.E.; Capela, I. Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: Current status and future outlook. Environ. Int. 2019, 125, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-Based Water Treatment Strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Yamamura, H.; Hashino, M.; Kubota, N. Membrane Filtration: Principle and Applications in Water Treatment. SEN-I GAKKAISHI 2011, 67, 3. [Google Scholar] [CrossRef]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse. Acc. Chem. Res. 2012, 46, 834–843. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Springer: Cham, Switzerland, 2016; pp. 17–25. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef]
- Humplik, T.; Lee, J.; O’Hern, S.C.; Fellman, B.A.; Baig, M.A.; Hassan, S.F.; Atieh, M.A.; Rahman, F.; Laoui, T.; Karnik, R.; et al. Nanostructured materials for water desalination. Nanotechnology 2011, 22, 292001. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jain, K. Dendrimers: Smart nanoengineered polymers for bioinspired applications in drug delivery. In Biopolymer-Based Composites: Drug Delivery and Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 169–220. [Google Scholar]
- Jain, K. Nanohybrids of Dendrimers and Carbon Nanotubes: A Benefaction or Forfeit in Drug Delivery? Nanosci. Nanotechnol. Asia 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Ritter, K.S.L. Sources, Pathways, and Relative Risks of Contaminants in Surface Water and Groundwater: A Perspective Prepared for the Walkerton Inquiry. J. Toxicol. Environ. Health Part A 2002, 65, 1–142. [Google Scholar] [CrossRef]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Human health risk assessment of ground water contaminated with petroleum PAHs using Monte Carlo simulations: A case study of an Indian metropolitan city. J. Environ. Manag. 2018, 205, 183–191. [Google Scholar] [CrossRef]
- Rodriguez-Proteau, R.; Grant, R.L. Toxicity Evaluation and Human Health Risk Assessment of Surface and Ground Water Contaminated by Recycled Hazardous Waste Materials. In Water Pollution: The Handbook of Environmental Chemistry; Kassim, T.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 133–189. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Aziz, T.; Zia-Ur-Rehman, M.; Sabir, M.; Khalid, H.; Hakeem, K.R.; Akhtar, J. Sources and Composition of Waste Water: Threats to Plants and Soil Health. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K., Akhtar, J., Sabir, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 349–370. [Google Scholar] [CrossRef]
- Jassby, D.; Cath, T.Y.; Buisson, H. The role of nanotechnology in industrial water treatment. Nat. Nanotechnol. 2018, 13, 670–672. [Google Scholar] [CrossRef]
- Deshpande, B.; Agrawal, P.; Yenkie, M.; Dhoble, S. Prospective of nanotechnology in degradation of waste water: A new challenges. Nano-Struct. Nano-Objects 2020, 22, 100442. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Soares, A. Sensing and analysis of soluble phosphates in environmental samples: A review. Biosens. Bioelectron. 2013, 41, 1–11. [Google Scholar] [CrossRef]
- Introduction to Wastewater Treatment—Google Books. Available online: https://books.google.co.in/books?hl=en&lr=&id=yjT4w5cjrM0C&oi=fnd&pg=PA7&dq=Templeton,+M.+R.,+%26+Butler,+D.+(2011).+Introduction+to+wastewater+treatment.+London,+UK:+Bookboon&ots=dwyi24NVe1&sig=fhPmx-HoQO6j9c7YwaypWnyYyNc#v=onepage&q&f=false (accessed on 26 January 2021).
- Ahmadi, F.; Zinatizadeh, A.A.; Asadi, A.; McKay, T.; Azizi, S. Simultaneous carbon and nutrients removal and PHA pro-duction in a novel single air lift bioreactor treating an industrial wastewater. Environ. Technol. Innov. 2020, 18, 100776. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Elabedien, M.E.Z.; Ebeid, E.-Z.M. Effective treatment of industrial wastewater applying SBA-15 mesoporous silica modified with graphene oxide and hematite nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104817. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Iqbal, S.Z.; Imran, M. Implications of advanced wastewater treatment: Electrocoagulation and electrofloc-culation of effluent discharged from a wastewater treatment plant. J. Water Process Eng. 2020, 33, 101101. [Google Scholar] [CrossRef]
- Runguphan, T.; Kitpichai, J. Coaction of bio-sorption and bio-filtration for the remediation of domestic and agricultural wastewater contaminated with heavy metal. IOP Conf. Ser. Mater. Sci. Eng. 2020, 965, 012010. [Google Scholar] [CrossRef]
- Kalfa, A.; Shapira, B.; Shopin, A.; Cohen, I.; Avraham, E.; Aurbach, D. Capacitive deionization for wastewater treatment: Op-portunities and challenges. Chemosphere 2020, 241, 125003. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Bora, T.; Dutta, J. Applications of nanotechnology in wastewater treatment—A review. J. Nanosci. Nanotechnol. 2014, 14, 613–626. [Google Scholar] [CrossRef]
- Yamashita, T.; Yamamoto-Ikemoto, R. Nitrogen and phosphorus removal from wastewater treatment plant effluent via bacterial sulfate reduction in an anoxic bioreactor packed with wood and iron. Int. J. Environ. Res. Public Health 2014, 11, 9835–9853. [Google Scholar] [CrossRef] [Green Version]
- Baruah, A.; Chaudhary, V.; Malik, R.; Tomer, V.K. Nanotechnology Based Solutions for Wastewater Treatment. In Nanotechnology in Water and Wastewater Treatment: Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–368. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent Advances in Carbon Nanomaterial-Based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–157. [Google Scholar] [CrossRef]
- Refaat Alawady, A.; Ali Alshahrani, A.; Ali Aouak, T.; Mohamed Alandis, N. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 15, 124267. [Google Scholar] [CrossRef]
- Derco, J.; Vrana, B. Introductory Chapter: Biosorption. In Biosorption; IntechOpen: London, UK, 2018. [Google Scholar]
- Seung, J.K.; Jae, H.C.; Tae, Y.K.; Sung, Y.C. Biosorption of heavy metals and cyanide complexes on biomass. In New Developments and Application in Chemical Reaction Engineering; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2006; pp. 141–144. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Fard, R.F.; Azimi, A.; Bidhendi, G.N. Batch kinetics and isotherms for biosorption of cadmium onto biosolids. Desalin. Water Treat. 2011, 28, 69–74. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Yu, Y.; Shimizu, K.; Zhang, Z.; Lei, Z.; Lee, D.J. Enhanced biosorption of Cr (VI) from synthetic wastewater using algal-bacterial aerobic granular sludge: Batch experiments, kinetics and mechanisms. Sep. Purif. Technol. 2020, 251, 117323. [Google Scholar] [CrossRef]
- Ding, H.; Luo, X.; Zhang, X.; Yang, H. Alginate-immobilized Aspergillus niger: Characterization and biosorption removal of thorium ions from radioactive wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 186–195. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, E.T.; Alawadhi, H.; Elsaid, K.; Olabi, A.G.; Almakrani, M.A.; Bin Tamim, S.T.; Alafranji, G.H.M.; Abdelkareem, M.A. A Carbon-Cloth Anode Electroplated with Iron Nanostructure for Microbial Fuel Cell Operated with Real Wastewater. Sustainability 2020, 12, 6538. [Google Scholar] [CrossRef]
- Ray, S.S.; Gusain, R.; Kumar, N. Carbon Nanomaterial-Based Adsorbents for Water Purification: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.; Nel, A.E. Autonomous in vitro anticancer drug release from meso-porous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, B.; Zheng, G.; Liu, X.; Li, T.; Yan, C.; Cheng, C.; Dai, K.; Liu, C.; Shen, C.; et al. Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C 2017, 6, 2258–2269. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Zheng, G.; Dai, K.; Liu, C.; Ma, Y.; Zhang, J.; Wang, N.; Shen, C.; Guo, Z. Continuously fabricated transparent conductive polycarbonate/carbon nanotube nanocomposite films for switchable thermochromic applications. J. Mater. Chem. C 2018, 6, 8360–8371. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; ElRouby, W.M.A.; Khedr, M.H. Decoration of multi-walled carbon nanotubes (MWCNTs) with dif-ferent ferrite nanoparticles and its use as an adsorbent. J. Nanostruct. Chem. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Verma, B.; Balomajumder, C. Surface modification of one-dimensional Carbon Nanotubes: A review for the management of heavy metals in wastewater. Environ. Technol. Innov. 2020, 17. [Google Scholar] [CrossRef]
- Moradi, O.; Fakhri, A.; Adami, S. Isotherm, thermodynamic, kinetics, and adsorption mechanism studies of Ethidium bromide by single-walled carbon nanotube and carboxylate group functionalized single-walled carbon nanotube. J. Coll. Interface Sci. 2013, 395, 224–229. [Google Scholar] [CrossRef]
- Yadav, D.K.; Srivastava, S. Carbon nanotubes as adsorbent to remove heavy metal ion (Mn +7) in wastewater treatment. Mater. Today Proc. 2017, 4, 4089–4094. [Google Scholar] [CrossRef]
- Kariim, I.; Abdulkareem, A.S.; Abubakre, O.K. Development and characterization of MWCNTs from activated carbon as ad-sorbent for metronidazole and levofloxacin sorption from pharmaceutical wastewater: Kinetics, isotherms and thermody-namic studies. Sci. Afr. 2020, 7, e00242. [Google Scholar]
- Zhao, W.; Tian, Y.; Chu, X.; Cui, L.; Zhang, H.; Li, M.; Zhao, P. Preparation and characteristics of a magnetic carbon nanotube ad-sorbent: Its efficient adsorption and recoverable performances. Sep. Purif. Technol. 2021, 257, 117917. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Yang, S.; Liao, J.; Cai, X.; Gao, Q.; Fang, Y.; Peng, F.; Zhang, S. Surface oxidized nano-cobalt wrapped by nitrogen-doped carbon nanotubes for efficient purification of organic wastewater. Sep. Purif. Technol. 2021, 259, 118098. [Google Scholar] [CrossRef]
- Bankole, M.; Abdulkareem, A.; Tijani, J.; Ochigbo, S.; Afolabi, A.; Roos, W. Chemical oxygen demand removal from electroplating wastewater by purified and polymer functionalized carbon nanotubes adsorbents. Water Resour. Ind. 2017, 18, 33–50. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Basheer, A.A.; Mbianda, X.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Ray, S.C. Application and Uses of Graphene Oxide and Reduced Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew Publishing: Norwich, NY, USA, 2015; pp. 39–55. [Google Scholar] [CrossRef]
- Jayakaran, P.; Nirmala, G.S.; Govindarajan, L. Qualitative and Quantitative Analysis of Graphene-Based Adsorbents in Wastewater Treatment. Int. J. Chem. Eng. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2013, 89, 196–205. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Meng, Y.; Cheng, Y.; Lu, J.; Wang, H. Novel graphene oxide/aminated lignin aerogels for enhanced adsorption of malachite green in wastewater. Coll. Surf. A Physicochem. Eng. Asp. 2020, 603, 125281. [Google Scholar] [CrossRef]
- Bu, J.; Yuan, L.; Zhang, N.; Liu, D.; Meng, Y.; Peng, X. High-efficiency adsorption of methylene blue dye from wastewater by a thiosemicarbazide functionalized graphene oxide composite. Diam. Relat. Mater. 2020, 101, 107604. [Google Scholar] [CrossRef]
- Lim Teik Zheng, A.; Phromsatit, T.; Boonyuen, S.; Andou, Y. Synthesis of silver nanoparticles/porphyrin/reduced graphene oxide hydrogel as dye adsorbent for wastewater treatment. FlatChem 2020, 23, 100174. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Ramkumar, K.; Meenakshi, S. Effective removal of organic pollutants by adsorption onto chitosan supported graphene oxide-hydroxyapatite composite: A novel reusable adsorbent. J. Mol. Liq. 2020, 318, 114200. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of organic pollutants by na-nomaterial-based adsorbents: An overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An Overview of Nanomaterials for Water and Wastewater Treatment. Adv. Mater. Sci. Eng. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 439–446. [Google Scholar] [CrossRef]
- Luo, T.; Cui, J.; Hu, S.; Huang, Y.; Jing, C. Arsenic Removal and Recovery from Copper Smelting Wastewater Using TiO2. Environ. Sci. Technol. 2010, 44, 9094–9098. [Google Scholar] [CrossRef]
- Gupta, K.; Bhattacharya, S.; Chattopadhyay, D.; Mukhopadhyay, A.; Biswas, H.; Dutta, J.; Ray, N.R.; Ghosh, U.C. Ceria associated manganese oxide nanoparticles: Synthesis, characterization and arsenic(V) sorption behavior. Chem. Eng. J. 2011, 172, 219–229. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, W.; Li, H.; Lang, L.; Xu, Z. Controllable Fabrication of Mesoporous MgO with Various Morphologies and Their Absorption Performance for Toxic Pollutants in Water. Cryst. Growth Des. 2008, 8, 3785–3790. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. J. Mater. Chem. A 2013, 1, 3325–3333. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Imani, M.; Kerdari, H. Adsorption kinetics, thermodynamic studies, and high performance of CdO cauli-flower-like nanostructure on the removal of Congo red from aqueous solution. J. Nanostruct. Chem. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- He, L.; Wang, L.; Zhu, H.; Wang, Z.; Zhang, L.; Yang, L.; Dai, Y.; Mo, H.; Zhang, J.; Shen, J. A reusable Fe3O4/GO-COOH nanoadsorbent for Ca2+ and Cu2+ removal from oilfield wastewater. Chem. Eng. Res. Des. 2021, 166, 248–258. [Google Scholar] [CrossRef]
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841. [Google Scholar] [CrossRef]
- Sadak, O.; Hackney, R.; Sundramoorthy, A.K.; Yilmaz, G.; Gunasekaran, S. Azo dye-functionalized magnetic Fe3O4/polyacrylic acid nanoadsorbent for removal of lead (II) ions. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100380. [Google Scholar] [CrossRef]
- Arshadi, M.; Soleymanzadeh, M.; Salvacion, J.; SalimiVahid, F. Nanoscale Zero-Valent Iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: Kinetics, thermodynamic and mechanism. J. Coll. Interface Sci. 2014, 426, 241–251. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S. Facile synthesis of Lead Doped Zinc-Aluminum Oxide Nanoparticles (LD-ZAO-NPs) for efficient adsorption of anionic dye: Kinetic, isotherm and thermodynamic behaviors. J. Ind. Eng. Chem. 2017, 53, 294–306. [Google Scholar] [CrossRef]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water purification by polymer nanocomposites: An overview. Nanocomposites 2017, 3, 47–66. [Google Scholar] [CrossRef]
- Berber, M.R. Current Advances of Polymer Composites for Water Treatment and Desalination. J. Chem. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Coll. Interface Sci. 2013, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672. [Google Scholar] [CrossRef]
- Abdi, J.; Abedini, H. MOF-based polymeric nanocomposite beads as an efficient adsorbent for wastewater treatment in batch and continuous systems: Modelling and experiment. Chem. Eng. J. 2020, 400, 125862. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Zhao, H.; Liu, Y.; Long, F.; Pan, X. A versatile β-cyclodextrin and polyethyleneimine bi-functionalized magnetic nanoadsorbent for simultaneous capture of methyl orange and Pb(II) from complex wastewater. Chemosphere 2019, 216, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Moharrami, P.; Motamedi, E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 2020, 313, 123661. [Google Scholar] [CrossRef] [PubMed]
- Nithya Priya, V.; Rajkumar, M.; Mobika, J.; Linto Sibi, S.P. Alginate coated layered double hydroxide/reduced graphene oxide nanocomposites for removal of toxic As (V) from wastewater. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 127, 114527. [Google Scholar] [CrossRef]
- He, L.; Yang, L.; Zhang, L.; Wang, Z.; Cheng, H.; Wang, X.; Lv, C.; Zhang, J.; Mo, H.; Shen, J. Removal of Ca2+ and Mg2+ from oilfield wastewater using re-usable PEG/Fe3O4/GO-NH2 nanoadsorbents and its efficiency for oil recovery. J. Environ. Chem. Eng. 2020, 9, 104653. [Google Scholar] [CrossRef]
- Strathmann, H.; Giorno, L.; Piacentini, E.; Drioli, E. 1.4 Basic Aspects in Polymeric Membrane Preparation. In Comprehensive Membrane Science and Engineering; Elsevier: Oxford, UK, 2017; pp. 65–84. [Google Scholar] [CrossRef]
- Drisko, J.A. Chelation Therapy. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1004–1015.e3. [Google Scholar] [CrossRef]
- Esmaeili, A.; Saremnia, B. Synthesis and characterization of NaA zeolite nanoparticles from Hordeum vulgare L. husk for the separation of total petroleum hydrocarbon by an adsorption process. J. Taiwan Inst. Chem. Eng. 2016, 61, 276–286. [Google Scholar] [CrossRef]
- Ali, M.E.; Hoque, M.E.; Hossain, S.K.S.; Biswas, M.C. Nanoadsorbents for wastewater treatment: Next generation biotechnological solution. Int. J. Environ. Sci. Technol. 2020, 17, 4095–4132. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process. Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A.; Aly, A.A.; Mohamed, T.Y. A facile synthesis of mordenite zeolite nanostructures for efficient bleaching of crude soybean oil and removal of methylene blue dye from aqueous media. J. Mol. Liq. 2017, 248, 302–313. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef]
- Margeta, K.; Zabukovec, N.; Šiljeg, M.; Farkas, A. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treatment; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef] [Green Version]
- Torabian, A.; Kazemian, H.; Seifi, L.; Bidhendi, G.N.; Azimi, A.A.; Ghadiri, S.K. Removal of Petroleum Aromatic Hydrocarbons by Surfactant-modified Natural Zeolite: The Effect of Surfactant. CLEAN—Soil Air Water 2010, 38, 77–83. [Google Scholar] [CrossRef]
- Pandey, P.K.; Sharma, S.K.; Sambi, S.S. Removal of lead(II) from waste water on zeolite-NaX. J. Environ. Chem. Eng. 2015, 3, 2604–2610. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Al-Musawi, T.J.; Mohseni-Bandpi, A.; Zarrabi, M. Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J. Mol. Liq. 2015, 211, 431–441. [Google Scholar] [CrossRef]
- Gugushe, A.S.; Mpupaa, A.; Nomngongoabc, P.N. Ultrasound-assisted magnetic solid phase extraction of lead and thallium in complex environmental samples using magnetic multi-walled carbon nanotubes/zeolite nanocomposite. Microchem. J. 2019, 149, 103960. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Amedalor, R.; Yaya, A.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and Evaluation of Zeolite A/Fe3O4 Nanocomposite as a Potential Adsorbent for Removal of Organic Molecules from Wastewater. J. Chem. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mao, N. Nonwoven fabric filters. In Advances in Technical Nonwovens; Kellie, G., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 273–310. [Google Scholar] [CrossRef]
- Bowen, W.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur, R.; Turken, T.; Guclu, S.; Pasaoglu, M. Advances in water treatment by microfiltration, ultrafiltration, and nanofiltration. In Advances in Membrane Technologies for Water Treatment; Woodhead Publishing: Oxford, UK, 2015; pp. 83–128. [Google Scholar] [CrossRef]
- Charcosset, C. Some perspectives. In Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 295–321. [Google Scholar] [CrossRef]
- Nagy, E. Nanofiltration. In Basic Equations of Mass Transport Through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 417–428. [Google Scholar]
- Timmer, J.M.K. Properties of Nanofiltration Membranes: Model Development and Industrial Application. 2001. [Google Scholar]
- Wu, L.; Wang, H.; Xu, T.-W.; Xu, Z.-L. Polymeric Membranes. In Membrane-Based Separations in Metallurgy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–334. [Google Scholar] [CrossRef]
- Mulyanti, R.; Susanto, H. Wastewater treatment by nanofiltration membranes. IOP Conf. Ser. Earth Environ. Sci. 2018, 142. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Thanuttamavong, M.; Yamamoto, K.; Oh, J.I.; Choo, K.H.; Choi, S.J. Rejection characteristics of organic and inorganic pollutants by ultra low-pressure nanofiltration of surface water for drinking water treatment. Desalination 2002, 145, 257–264. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Van Der Bruggen, B.; Hoek, E.M.; Tarabara, V.V. Nanofiltration. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–23. [Google Scholar]
- Benfer, S.; Popp, U.; Richter, H.; Siewert, C.; Tomandl, G. Development and characterization of ceramic nanofiltration mem-branes. Sep. Purif. Technol. 2001, 22, 231–237. [Google Scholar] [CrossRef]
- Mostafavi, S.; Mehrnia, M.; Rashidi, A. Preparation of nanofilter from carbon nanotubes for application in virus removal from water. Desalination 2009, 238, 271–280. [Google Scholar] [CrossRef]
- Parham, H.; Bates, S.; Xia, Y.; Zhu, Y. A highly efficient and versatile carbon nanotube/ceramic composite filter. Carbon 2013, 54, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Khan, M., Pradhan, D., Sohn, Y., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–40. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Abe, R.; Ohtani, B. Preparation and Characterization of Bismuth Tungstate Polycrystalline Flake-Ball Particles for Photocatalytic Reactions. J. Phys. Chem. C 2008, 112, 9320–9326. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with solar energy at a pilot-plant scale: An overview. Appl. Catal. B Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process. Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for tox-icity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- McLain, A.A. Photocatalytic Properties of Zinc Oxide and Graphene Nanocomposites. In Photocatalytic Properties of Zinc Oxide and Graphene Nanocomposites; Public Knowledge Project: Vancouver, BC, USA, 2019. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and perspectives on the use of mag-netic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Hot, J.; Topalov, J.; Ringot, E.; Bertron, A. Investigation on Parameters Affecting the Effectiveness of Photocatalytic Functional Coatings to Degrade NO: TiO2 Amount on Surface, Illumination, and Substrate Roughness. Int. J. Photoenergy 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047–1060. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured Photocatalysts and Their Applications in the Photocatalytic Transformation of Lignocellulosic Biomass: An Overview. Materials 2009, 2, 2228–2258. [Google Scholar] [CrossRef]
- Bai, L.; Wei, M.; Hong, E.; Shan, D.; Liu, L.; Yang, W.; Tang, X.; Wang, B. Study on the controlled synthesis of Zr/TiO2/SBA-15 nanophotocatalyst and its photocatalytic performance for industrial dye reactive red X–3B. Mater. Chem. Phys. 2020, 246, 122825. [Google Scholar] [CrossRef]
- Mahmoudian-Boroujerd, L.; Karimi-Jashni, A.; Hosseini, S.N.; Paryan, M. Optimization of rDNA degradation in recombinant Hepatitis B vaccine production plant wastewater using visible light excited Ag-doped TiO2 nanophotocatalyst. Process. Saf. Environ. Prot. 2019, 122, 328–338. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Asadipour, A.; Faraji, M.; Kargar, E. A facile and green method for synthesis of ZnFe2O4@CMC as a new magnetic nanophotocatalyst for ciprofloxacin removal from aqueous media. MethodsX 2019, 6, 1575–1580. [Google Scholar] [CrossRef]
- Karimi, H.; Rajabi, H.R.; Kavoshi, L. Application of decorated magnetic nanophotocatalysts for efficient photodegradation of organic dye: A comparison study on photocatalytic activity of magnetic zinc sulfide and graphene quantum dots. J. Photochem. Photobiol. A Chem. 2020, 397, 112534. [Google Scholar] [CrossRef]
- Yosefi, L.; Haghighi, M.; Allahyari, S. Solvothermal synthesis of flowerlike p-BiOI/n-ZnFe2O4 with enhanced visible light driven nanophotocatalyst used in removal of acid orange 7 from wastewater. Sep. Purif. Technol. 2017, 178, 18–28. [Google Scholar] [CrossRef]
- Margan, P.; Haghighi, M. Sono-coprecipitation synthesis and physicochemical characterization of CdO-ZnO nanophoto-catalyst for removal of acid orange 7 from wastewater. Ultrason. Sonochem. 2018, 40, 323–332. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Yang, Y.; Zeng, G.; Zhang, C.; Zhou, Y.; Yang, J.; Huang, D.; Wang, H.; Xiong, W.; et al. Carbon nitride based photocatalysts for solar photocatalytic disinfection, can we go further? Chem. Eng. J. 2021, 404, 126540. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water And Sanitation in Developing Countries: Including Health in the Equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Zhang, W.; Wang, P.; Wang, C. Metal-free virucidal effects induced by g-C3N4 under visible light irradiation: Statistical analysis and parameter optimization. Chemosphere 2018, 195, 551–558. [Google Scholar] [CrossRef]
- Rikta, S.Y. Application of Nanoparticles for Disinfection and Microbial Control of Water and Wastewater. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–176. [Google Scholar] [CrossRef]
- Van Asselt, A.; Te Giffel, M.C. Pathogen resistance and adaptation to disinfectants and sanitisers. In Understanding Pathogen Behaviour; Elsevier: Amsterdam, The Netherlands, 2005; pp. 484–506. [Google Scholar]
- Yao, B.; Luo, Z.; Xiong, W.; Song, B.; Zeng, Z.; Zhou, Y. Disinfection techniques of human norovirus in municipal wastewater: Challenges and future perspectives. Curr. Opin. Environ. Sci. Health 2020. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Snow, B.; Santos, R.M.; Chiang, Y.W. Micro-structured copper and nickel metal foams for wastewater disinfection: Proof-of-concept and scale-up. Process. Saf. Environ. Prot. 2020, 142, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, H.; Li, J.; Song, H.; Wang, S.; Zhang, Z.; Chen, S. Green light–triggered antimicrobial cotton fabric for wastewater disinfection. Mater. Today Phys. 2020, 15, 100254. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, Z.; Zeng, G.; Lai, C.; Duan, A.; Xiao, R.; Huang, D.; Fu, Y.; Yi, H.; Li, B.; et al. Cooperative catalytic performance of bimetallic Ni-Au nanocatalyst for highly efficient hydrogenation of nitroaromatics and corresponding mechanism insight. Appl. Catal. B Environ. 2019, 259, 118035. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Zhong, H.; Wang, Z.; Liu, Z.; Cheng, M.; Liu, G.; Yang, X.; Liu, S. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction? J. Hazard. Mater. 2017, 322, 394–401. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Vervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Yu, J.C. Inorganic materials for photocatalytic water disinfection. J. Mater. Chem. 2010, 20, 4529–4536. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Jiang, X.; Sun, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Simultaneous Capture, Detection, and Inactivation of Bacteria as Enabled by a Surface-Enhanced Raman Scattering Multifunctional Chip. Angew. Chem. Int. Ed. 2015, 54, 5132–5136. [Google Scholar] [CrossRef]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Moustafa, M.T. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Alhadlaq, H.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Elmi, F.; Alinezhad, H.; Moulana, Z.; Salehian, F.; Tavakkoli, S.M.; Asgharpour, F.; Fallah, H.; Elmi, M.M. The use of antibacterial activity of ZnO nanoparticles in the treatment of municipal wastewater. Water Sci. Technol. 2014, 70, 763–770. [Google Scholar] [CrossRef]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.H.D.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Ranjana, N.; Misra, A.J.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Lundborg, C.S.; Tripathy, S.K. Disinfection of the Water Borne Pathogens Escherichia coli and Staphylococcus aureus by Solar Photocatalysis Using Sonochemically Synthesized Reusable Ag@ZnO Core-Shell Nanoparticles. Int. J. Environ. Res. Public Health 2017, 14, 747. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Wong, K.-A.; Lam, S.-M.; Sin, J.-C. Wet chemically synthesized ZnO structures for photodegradation of pre-treated palm oil mill effluent and antibacterial activity. Ceram. Int. 2019, 45, 1868–1880. [Google Scholar] [CrossRef]
- Jin, S.-E.; Jin, J.E.; Hwang, W.; Hong, S.W. Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int. J. Nanomed. 2019, 14, 1737–1751. [Google Scholar] [CrossRef] [Green Version]
- Gul, S.; Khan, S.A.; Rehan, Z.A.; Akhtar, K.; Khan, M.A.; Khan, M.I.; Rashid, M.I.; Asiri, A.M.; Khan, S.B. Antibacterial CuO-PES-CA nancomposite membranes supported Cu0 nanoparticles for water permeability and reduction of organic pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 10835–10847. [Google Scholar] [CrossRef]
- He, X.; Yang, D.P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste eggshell membrane-templated CuO-ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. Nanomaterial-based fluorescent sensors for the de-tection of lead ions. J. Hazard. Mater. 2020, 407, 124379. [Google Scholar] [CrossRef]
- Vikesland, P.J. Nanosensors for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bennett, J.; Short, L.C.; Theisen, T.S.; Wichman, M.D.; White, J.C.; Wright, S. Nanotechnology in the Water Industry, Part 1: Occurrence and Risks. J. Am. Water Work. Assoc. 2017, 109, 30–37. [Google Scholar] [CrossRef]
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Critical Issues in Sensor Science to Aid Food and Water Safety. ACS Nano 2012, 6, 4548–4556. [Google Scholar] [CrossRef]
- Graboski, A.M.; Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Nanosensors for water quality control. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–128. [Google Scholar] [CrossRef]
- Scoville, S. Implications of Nanotechnology Safety of Sensors on Homeland Security Industries. In Nanotechnology Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 175–194. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A. Electrochemical carbon based nanosensors: A promising tool in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2018, 147, 439–457. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Wigginton, K.R. Nanomaterial Enabled Biosensors for Pathogen Monitoring—A Review. Environ. Sci. Technol. 2010, 44, 3656–3669. [Google Scholar] [CrossRef]
- Riquelme, M.V.; Leng, W.; Carzolio, M.; Pruden, A.; Vikesland, P. Stable oligonucleotide-functionalized gold nanosensors for environmental biocontaminant monitoring. J. Environ. Sci. 2017, 62, 49–59. [Google Scholar] [CrossRef]
- Talari, F.F.; Bozorg, A.; Faridbod, F.; Vossoughi, M. A novel sensitive aptamer-based nanosensor using rGQDs and MWCNTs for rapid detection of diazinon pesticide. J. Environ. Chem. Eng. 2021, 9, 104878. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L.; Wang, S. A sensitive molecular imprinted surface plasmon resonance nanosensor for selective determination of trace triclosan in wastewater. Sens. Actuators B Chem. 2015, 216, 638–644. [Google Scholar] [CrossRef]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, L.; Liu, J.; Wang, Q.; Jiao, L. A label-free yellow-emissive carbon dot-based nanosensor for sensitive and selective ratiometric detection of chromium (VI) in environmental water samples. Mater. Chem. Phys. 2020, 248, 122912. [Google Scholar] [CrossRef]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano. R. Soc. Chem. 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in mu-nicipal drinking water—A review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Pardhi, V.P.; Verma, T.; Flora, S.; Chandasana, H.; Shukla, R. Nanocrystals: An Overview of Fabrication, Characterization and Therapeutic Applications in Drug Delivery. Curr. Pharm. Des. 2019, 24, 5129–5146. [Google Scholar] [CrossRef]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Na-noparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.; Rajchakit, U.; Sarojini, V. Detection and removal of biological contaminants in water. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–110. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current trends in the application of nanomaterials for the removal of emerging micropollutants and pathogens from water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef]
- Westerhoff, P.K.; Kiser, M.A.; Hristovski, K. Nanomaterial Removal and Transformation During Biological Wastewater Treatment. Environ. Eng. Sci. 2013, 30, 109–117. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of upper bounds and trends in nano-TiO2 pro-duction as a basis for exposure assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kim, S.-H.; Kim, H.-C.; Lee, S.G.; Lee, S.J.; Jeong, S.W. Growth inhibition of aquatic plant caused by silver and titanium oxide nanoparticles. Toxicol. Environ. Health Sci. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov, V.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Ismail, M.; Akhtar, K.; Danish, E.Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles @ cellulose polymer paper for the resolution of waste water treatment challenges. Coll. Surf. A Physicochem. Eng. Asp. 2019, 577, 548–561. [Google Scholar] [CrossRef]
- De Matteis, V.; Rinaldi, R. Toxicity Assessment in the Nanoparticle Era. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Cimbaluk, G.V.; Ramsdorf, W.A.; Perussolo, M.C.; Santos, H.K.F.; Da Silva De Assis, H.C.; Schnitzler, M.C.; Schnitzler, D.C.; Carneiro, P.G.; Cestari, M.M. Evaluation of mul-tiwalled carbon nanotubes toxicity in two fish species. Ecotoxicol. Environ. Saf. 2018, 150, 215–223. [Google Scholar] [CrossRef]
- Khan, M.S.; Qureshi, N.A.; Jabeen, F. Assessment of toxicity in fresh water fish Labeo rohita treated with silver nanoparticles. Appl. Nanosci. 2017, 7, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules 2021, 26, 1797. https://doi.org/10.3390/molecules26061797
Jain K, Patel AS, Pardhi VP, Flora SJS. Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules. 2021; 26(6):1797. https://doi.org/10.3390/molecules26061797
Chicago/Turabian StyleJain, Keerti, Anand S. Patel, Vishwas P. Pardhi, and Swaran Jeet Singh Flora. 2021. "Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment" Molecules 26, no. 6: 1797. https://doi.org/10.3390/molecules26061797
APA StyleJain, K., Patel, A. S., Pardhi, V. P., & Flora, S. J. S. (2021). Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules, 26(6), 1797. https://doi.org/10.3390/molecules26061797





