Shock Processing of Amino Acids Leading to Complex Structures—Implications to the Origin of Life
Abstract
:1. Introduction
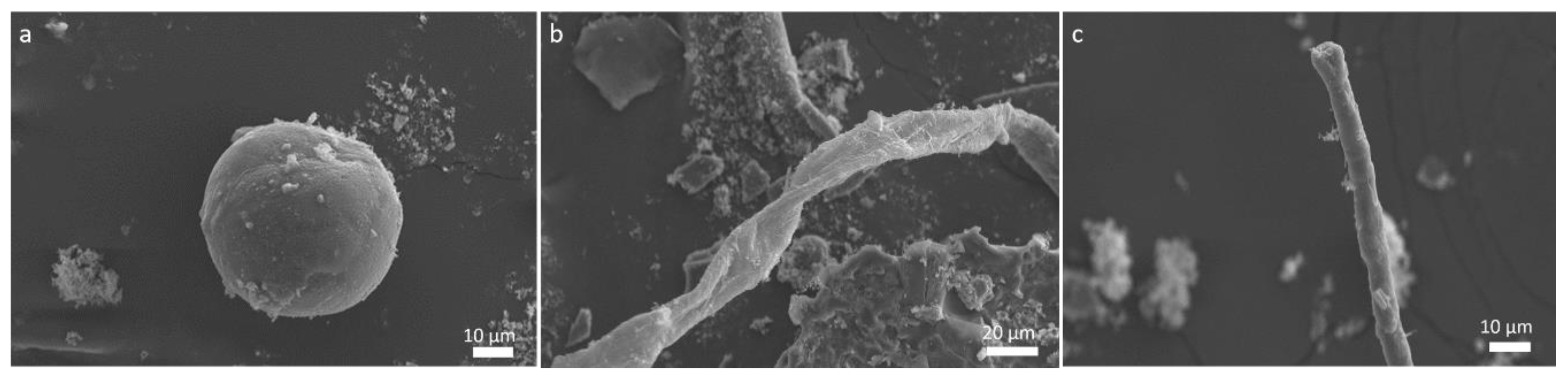
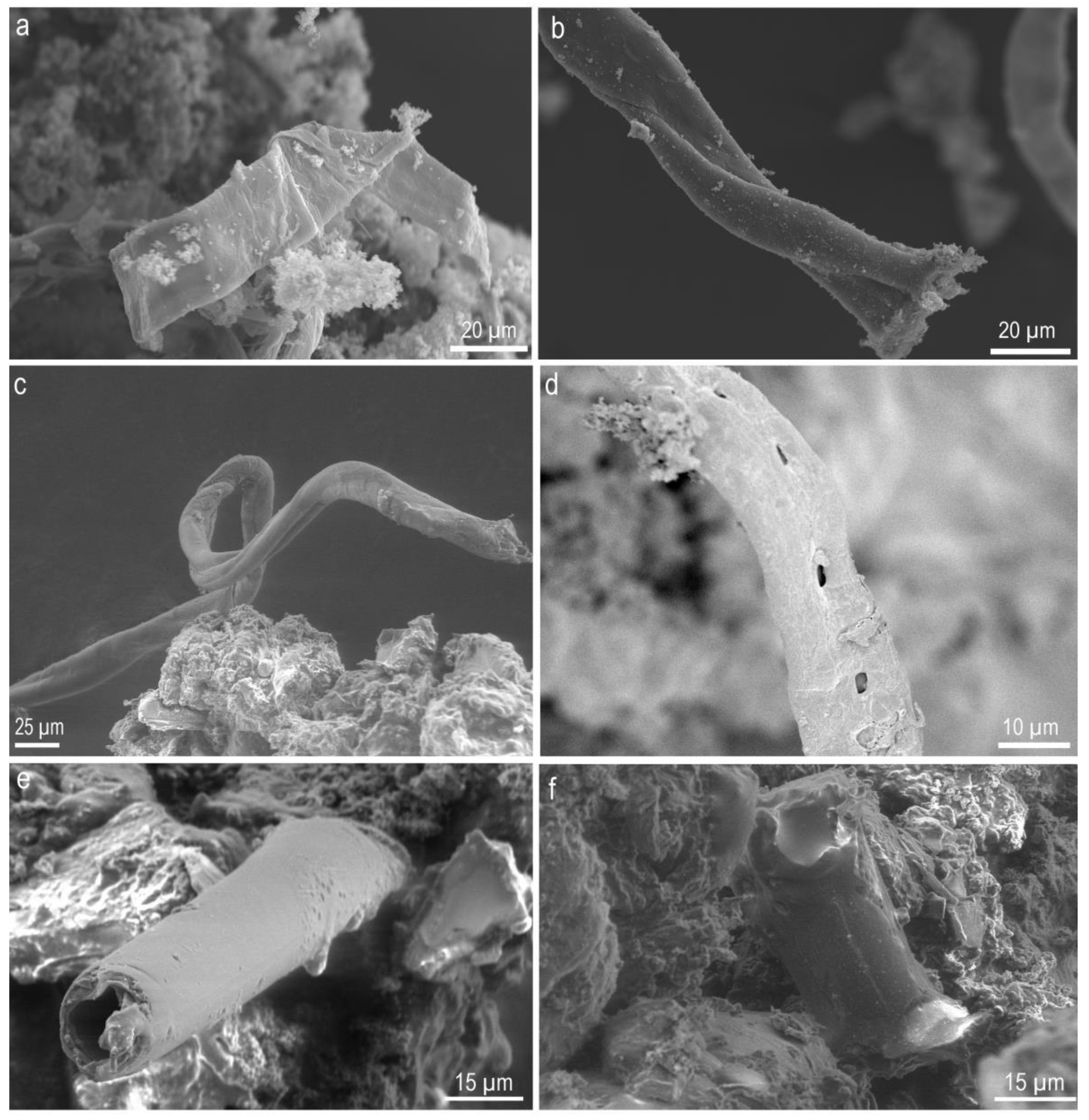
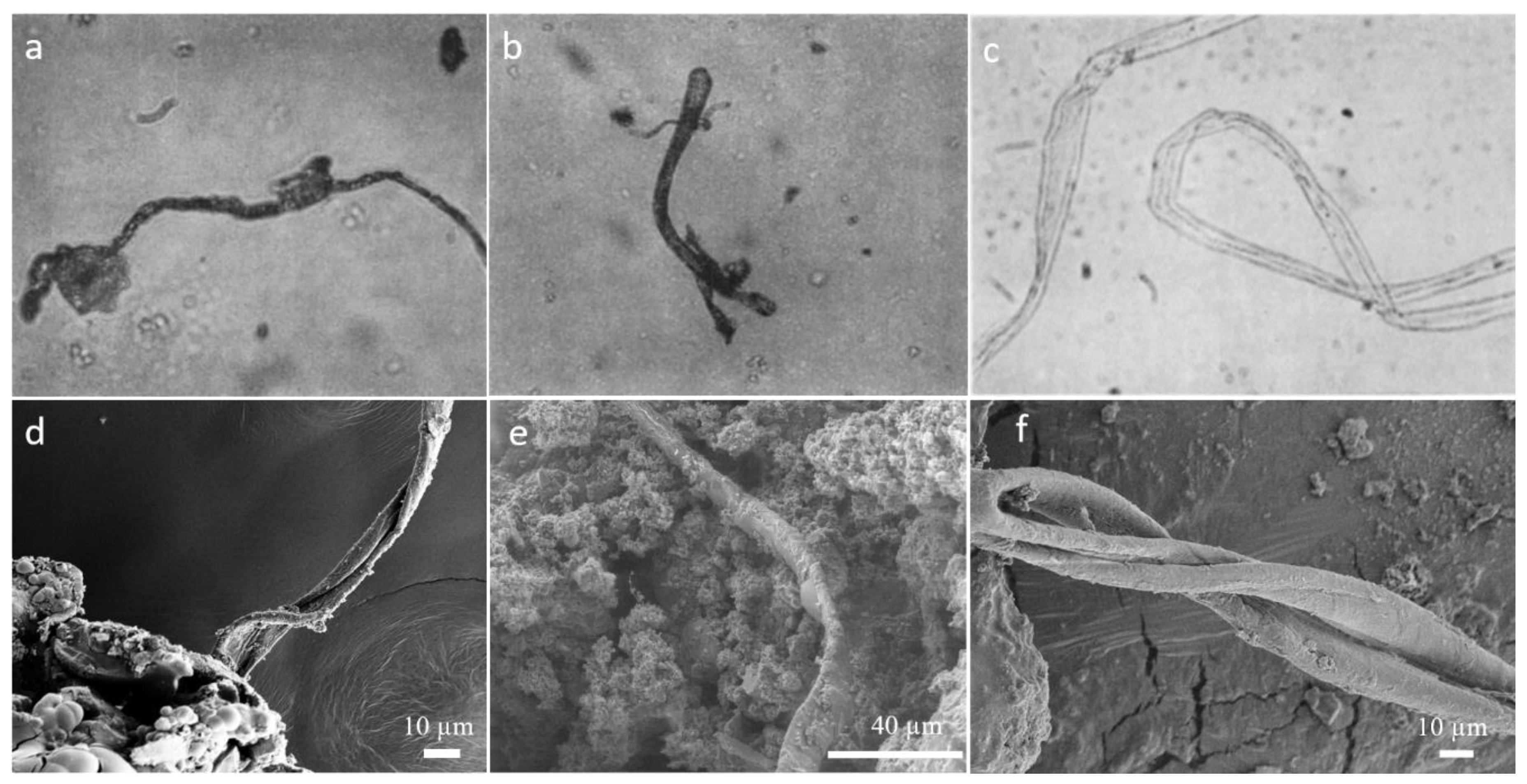
2. Results and Discussion
2.1. Complex Structure Formation and Consequences for the Studies of the Origin of Life
2.2. Implications to the Structure Observed in Meteorites
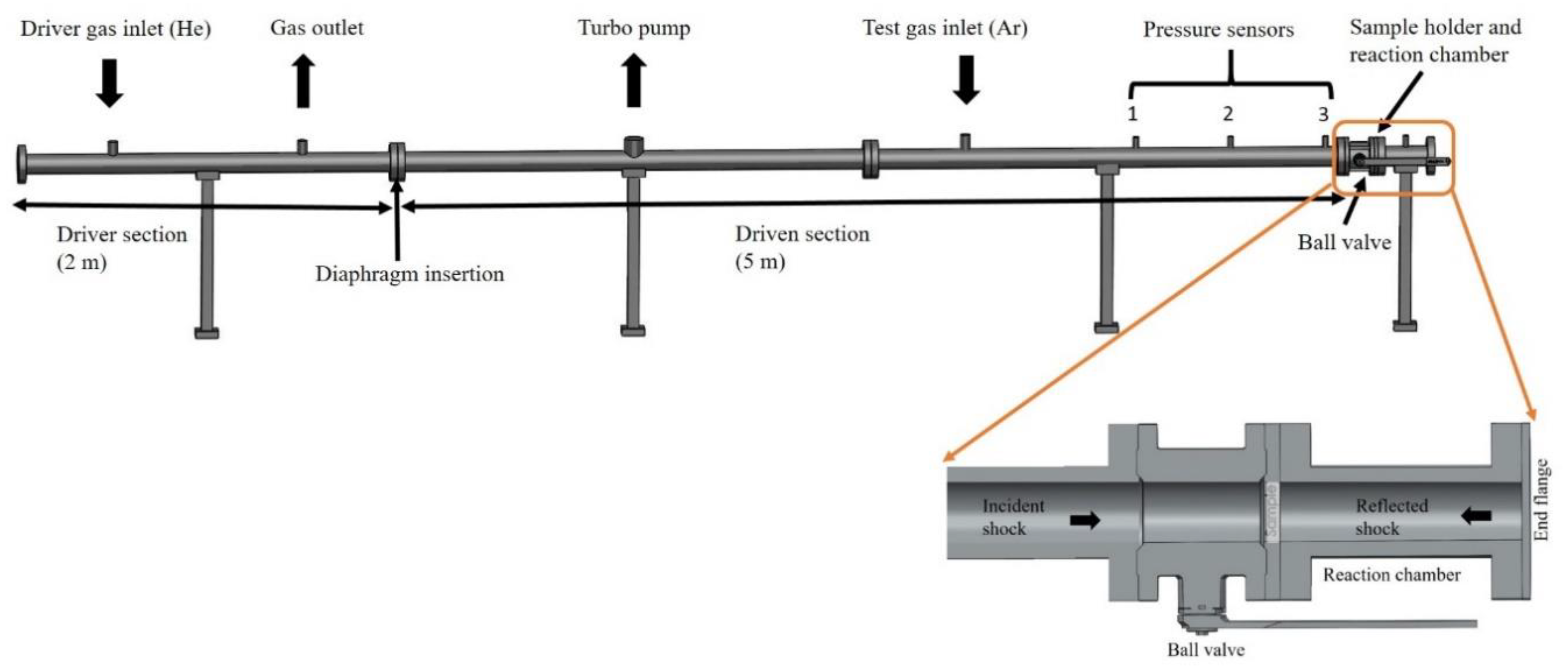
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cockell, C.S. The origin and emergence of life under impact bombardment. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1845–1856. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.L. Production of some organic compounds under possible primitive earth conditions1. J. Am. Chem. Soc. 1955, 77, 2351–2361. [Google Scholar] [CrossRef]
- Just, T.; Oparin, A.I.; Morgulis, S. The Origin of Life. Am. Midl. Nat. 1938, 20, 472. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, H.; Mimura, K. Peptide synthesis triggered by comet impacts: A possible method for peptide delivery to the early Earth and icy satellites. Icarus 2015, 257, 103–112. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Samanta, M.; Ashkenasy, G.; Leman, L.J. Prebiotic Peptides: Molecular Hubs in the Origin of Life. Chem. Rev. 2020, 120, 4707–4765. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Shirazi, A.N.; Parang, K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 2014, 12, 3544–3561. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Lemke, K.H.; Rosenbauer, R.J.; Bird, D.K. Peptide synthesis in early Earth hydrothermal systems. Astrobiology 2009, 9, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Matsui, T.; Izumi, Y.; Agui, A.; Tanaka, M.; Muro, T. Radiation-induced chemical evolution of biomolecules. Radiat. Phys. Chem. 2009, 78, 1198–1201. [Google Scholar] [CrossRef]
- Simakov, M.; Kuzicheva, E.; Dodonova, N.Y.; Antropov, A. Formation of oligopeptides on the surface of small bodies in solar system by cosmic radiation. Adv. Space Res. 1997, 19, 1063–1066. [Google Scholar] [CrossRef]
- Tanaka, M.; Kaneko, F.; Koketsu, T.; Nakagawa, K.; Yamada, T. Fragmentation and dimerization of aliphatic amino acid films induced by vacuum ultraviolet irradiation. Radiat. Phys. Chem. 2008, 77, 1164–1168. [Google Scholar] [CrossRef]
- Deming, T.J. Polypeptide and polypeptide hybrid copolymer synthesis via NCA polymerization. In Peptide Hybrid Polymers; Springer: Berlin, Germany, 2006; pp. 1–18. [Google Scholar]
- Oró, J.; Guidry, C. A novel synthesis of polypeptides. Nature 1960, 186, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P.; Borucki, W.J. Organic synthesis in experimental impact shocks. Science 1997, 276, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Bar-Nun, A.; Bar-Nun, N.; Bauer, S.; Sagan, C. Shock synthesis of amino acids in simulated primitive environments. Science 1970, 168, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Sekine, T.; Oba, M.; Kakegawa, T.; Nakazawa, H. Biomolecule formation by oceanic impacts on early Earth. Nat. Geosci. 2009, 2, 62–66. [Google Scholar] [CrossRef]
- Martins, Z.; Price, M.C.; Goldman, N.; Sephton, M.A.; Burchell, M.J. Shock synthesis of amino acids from impacting cometary and icy planet surface analogues. Nat. Geosci. 2013, 6, 1045–1049. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Furukawa, Y.; Kobayashi, T.; Sekine, T.; Terada, N.; Kakegawa, T. Impact-induced amino acid formation on Hadean Earth and Noachian Mars. Sci. Rep. 2020, 10, 9220. [Google Scholar] [CrossRef]
- Blank, J.G.; Miller, G.H.; Ahrens, M.J.; Winans, R.E. Experimental shock chemistry of aqueous amino acid solutions and the cometary delivery of prebiotic compounds. Orig. Life Evol. Biosph. 2001, 31, 15–51. [Google Scholar] [CrossRef]
- Sugahara, H.; Mimura, K. Glycine oligomerization up to triglycine by shock experiments simulating comet impacts. Geochem. J. 2014, 48, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Goldman, N.; Reed, E.J.; Fried, L.E.; Kuo, I.-F.W.; Maiti, A. Synthesis of glycine-containing complexes in impacts of comets on early Earth. Nat. Chem. 2010, 2, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Tamblyn, I. Prebiotic chemistry within a simple impacting icy mixture. J. Phys. Chem. A 2013, 117, 5124–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassone, G.; Saija, F.; Sponer, J.; Sponer, J.E.; Ferus, M.; Krus, M.; Ciaravella, A.; Jiménez-Escobar, A.; Cecchi-Pestellini, C. Dust motions in magnetized turbulence: Source of chemical complexity. Astrophys. J. Lett. 2018, 866, L23. [Google Scholar] [CrossRef]
- Pierazzo, E.; Chyba, C. Amino acid survival in large cometary impacts. Meteorit. Planet. Sci. 1999, 34, 909–918. [Google Scholar] [CrossRef]
- Umeda, Y.; Fukunaga, N.; Sekine, T.; Furukawa, Y.; Kakegawa, T.; Kobayashi, T.; Nakazawa, H. Survivability and reactivity of glycine and alanine in early oceans: Effects of meteorite impacts. J. Biol. Phys. 2016, 42, 177–198. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, M.; Van Der Gaast, S.; Vilas, F.; Hörz, F.; Haynes, G.; Chabin, A.; Brack, A.; Westall, F. The fate of amino acids during simulated meteoritic impact. Astrobiology 2009, 9, 943–951. [Google Scholar] [CrossRef]
- Furukawa, Y.; Nakazawa, H.; Sekine, T.; Kobayashi, T.; Kakegawa, T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet. Sci. Lett. 2015, 429, 216–222. [Google Scholar] [CrossRef]
- Ferus, M.; Pietrucci, F.; Saitta, A.; Ivanek, O.; Knizek, A.; Kubelík, P.; Krus, M.; Juha, L.; Dudzak, R.; Dostál, J. Prebiotic synthesis initiated in formaldehyde by laser plasma simulating high-velocity impacts. Astron. Astrophys. 2019, 626, A52. [Google Scholar] [CrossRef]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.L.; Orgel, L.E. The Origins of Life on the Earth; Concept of Modern Biology Series; Prentic-Hall: Upper Saddle River, NJ, USA, 1974; pp. 152–156. [Google Scholar]
- Sharp, T.H.; Bruning, M.; Mantell, J.; Sessions, R.B.; Thomson, A.R.; Zaccai, N.R.; Brady, R.L.; Verkade, P.; Woolfson, D.N. Cryo-transmission electron microscopy structure of a gigadalton peptide fiber of de novo design. Proc. Natl. Acad. Sci. USA 2012, 109, 13266–13271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Yan, X.; Wang, A.; Fei, J.; Cui, Y.; He, Q.; Li, J. A peony-flower-like hierarchical mesocrystal formed by diphenylalanine. J. Mater. Chem. 2010, 20, 6734–6740. [Google Scholar] [CrossRef]
- Sawada, T.; Yamagami, M.; Akinaga, S.; Miyaji, T.; Fujita, M. Porous Peptide Complexes by a Folding-and-Assembly Strategy. Chem. Asian J. 2017, 12, 1715–1718. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhang, L.; Yan, X.; Wang, A.; Ma, G.; Li, J.; Möhwald, H.; Mann, S. Multifunctional Porous Microspheres Based on Peptide–Porphyrin Hierarchical Co-Assembly. Angew. Chem. Int. Ed. 2014, 53, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.; Chen, C.; Shen, G.; Yan, L.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Peptide-Induced Hierarchical Long-Range Order and Photocatalytic Activity of Porphyrin Assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]
- Grano-Maldonado, M.I.; de Sousa, C.B.; Rodríguez-Santiago, M.A. First insights into the ultrastructure of myosin and actin bands using transmission electron microscopy in Gyrodactylus (Monogenea). J. Microsc. Ultrastruct. 2018, 6, 177. [Google Scholar] [CrossRef]
- Jia, T.Z.; Kuruma, Y. Recent Advances in Origins of Life Research by Biophysicists in Japan. Challenges 2019, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Venkateshaiah, A.; Padil, V.V.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers 2020, 12, 512. [Google Scholar] [CrossRef] [Green Version]
- Fabian, H.; Schultz, C.P. Fourier Transform Infrared Spectroscopy in Peptide and Protein Analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 5779–5803. [Google Scholar]
- Mayer, C. Life in the context of order and complexity. Life 2020, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, J.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Claus, G.; Nagy, B. A microbiological examination of some carbonaceous chondrites. Nature 1961, 192, 594–596. [Google Scholar] [CrossRef]
- Mamikunian, G.; Briggs, M.H. Some microstructures of complex morphology observed in preparations of carbonaceous chondrites made under sterile conditions. Nature 1963, 197, 1245–1248. [Google Scholar] [CrossRef]
- Nagy, B.; Fredriksson, K.; Urey, H.C.; Claus, G.; Andersen, C.A.; Percy, J. Electron probe microanalysis of organized elements in the Orgueil meteorite. Nature 1963, 198, 121–125. [Google Scholar] [CrossRef]
- Anders, E.; Fitch, F.W. Search for organized elements in carbonaceous chondrites. Science 1962, 138, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.H. Properties of the organic microstructures of some carbonaceous chondrites. Nature 1962, 195, 1076–1077. [Google Scholar] [CrossRef]
- Fitch, F.; Schwarcz, H.P.; Anders, E. ‘Organized elements’ in carbonaceous chondrites. Nature 1962, 193, 1123–1125. [Google Scholar] [CrossRef]
- Mueller, G. Interpretation of micro-structures in carbonaceous meteorites. Nature 1962, 196, 929–932. [Google Scholar] [CrossRef]
- McKay, D.S.; Gibson, E.K.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.; Maechling, C.R.; Zare, R.N. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Nagy, B.; Claus, G.; Hennessy, D.J. Organic particles embedded in minerals in the Orgueil and Ivuna carbonaceous chondrites. Nature 1962, 193, 1129–1133. [Google Scholar] [CrossRef]
- Hoover, R.B.; Jerman, G.; Rozanov, A.Y.; Sipiera, P.P. Indigenous Microfossils in Carbonaceous Meteorites; Instruments, Methods, and Missions for Astrobiology VIII; International Society for Optics and Photonics: Washington, DC, USA, 2004; pp. 1–17. [Google Scholar]
- Hoover, R.B. Fossils of cyanobacteria in CI1 carbonaceous meteorites. J. Cosmol. 2011, 13, 3811–3848. [Google Scholar]
- Biennier, L.; Jayaram, V.; Suas-David, N.; Georges, R.; Singh, M.K.; Arunan, E.; Kassi, S.; Dartois, E.; Reddy, K. Shock-wave processing of C60 in hydrogen. Astron. Astrophys. 2017, 599, A42. [Google Scholar] [CrossRef] [Green Version]
- Gaydon, A.G.; Hurle, I.R. The Shock Tube in High-Temperature Chemical Physics; Chapman and Hall: London, UK, 1963. [Google Scholar]
Sample Availability: Samples of the compounds that support the findings of the study are available from the corresponding authors upon request. |



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.V.; Vishakantaiah, J.; Meka, J.K.; Sivaprahasam, V.; Chandrasekaran, V.; Thombre, R.; Thiruvenkatam, V.; Mallya, A.; Rajasekhar, B.N.; Muruganantham, M.; et al. Shock Processing of Amino Acids Leading to Complex Structures—Implications to the Origin of Life. Molecules 2020, 25, 5634. https://doi.org/10.3390/molecules25235634
Singh SV, Vishakantaiah J, Meka JK, Sivaprahasam V, Chandrasekaran V, Thombre R, Thiruvenkatam V, Mallya A, Rajasekhar BN, Muruganantham M, et al. Shock Processing of Amino Acids Leading to Complex Structures—Implications to the Origin of Life. Molecules. 2020; 25(23):5634. https://doi.org/10.3390/molecules25235634
Chicago/Turabian StyleSingh, Surendra V., Jayaram Vishakantaiah, Jaya K. Meka, Vijayan Sivaprahasam, Vijayanand Chandrasekaran, Rebecca Thombre, Vijay Thiruvenkatam, Ambresh Mallya, Balabhadrapatruni N. Rajasekhar, Mariyappan Muruganantham, and et al. 2020. "Shock Processing of Amino Acids Leading to Complex Structures—Implications to the Origin of Life" Molecules 25, no. 23: 5634. https://doi.org/10.3390/molecules25235634







