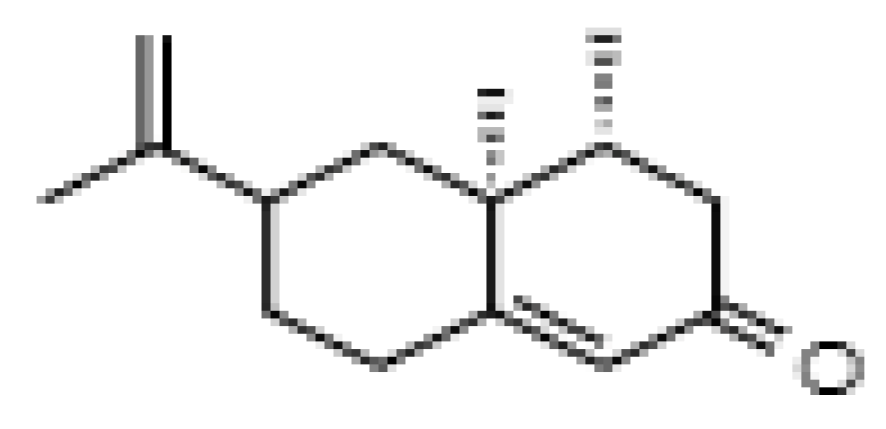
Nootkatone, a Dietary Fragrant Bioactive Compound, Attenuates Dyslipidemia and Intramyocardial Lipid Accumulation and Favorably Alters Lipid Metabolism in a Rat Model of Myocardial Injury: An In Vivo and In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Effect of NKT on Cardiac Enzyme Markers
2.2. Effect of NKT on Plasma Lipid Peroxidation and Non-Enzymatic Antioxidants
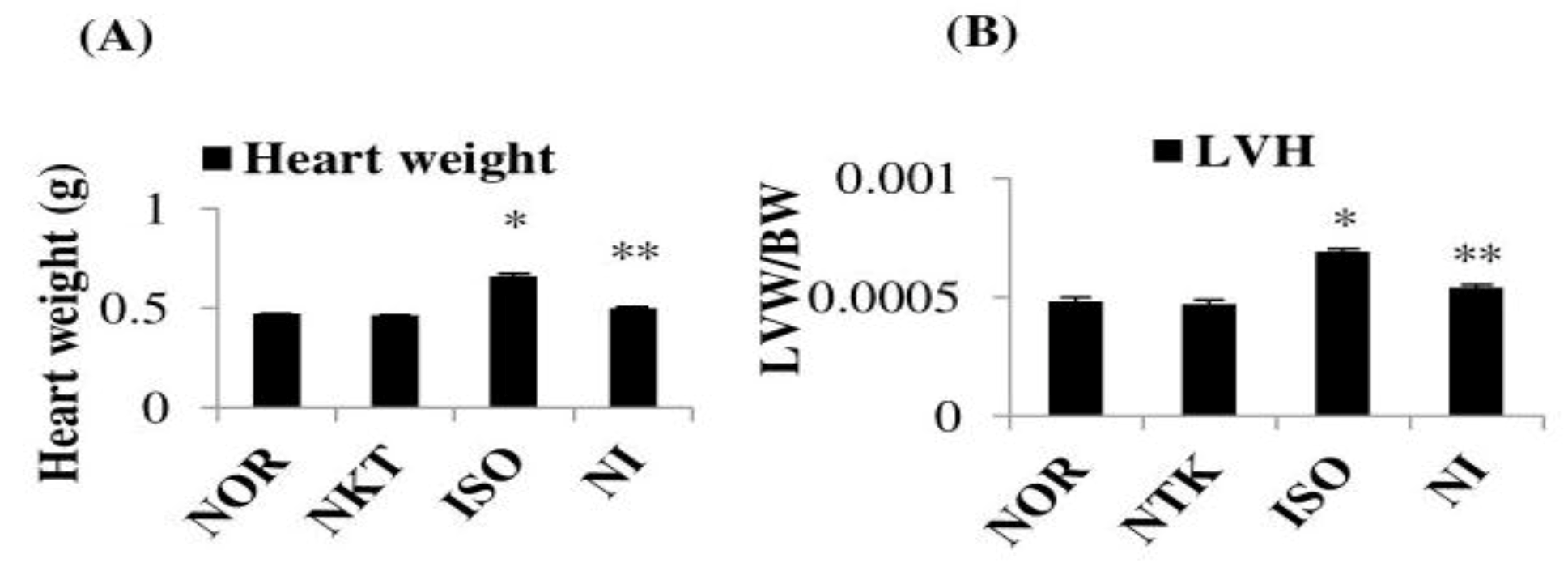
2.3. Effect of NKT on Heart Weight and Left-Ventricular Weight
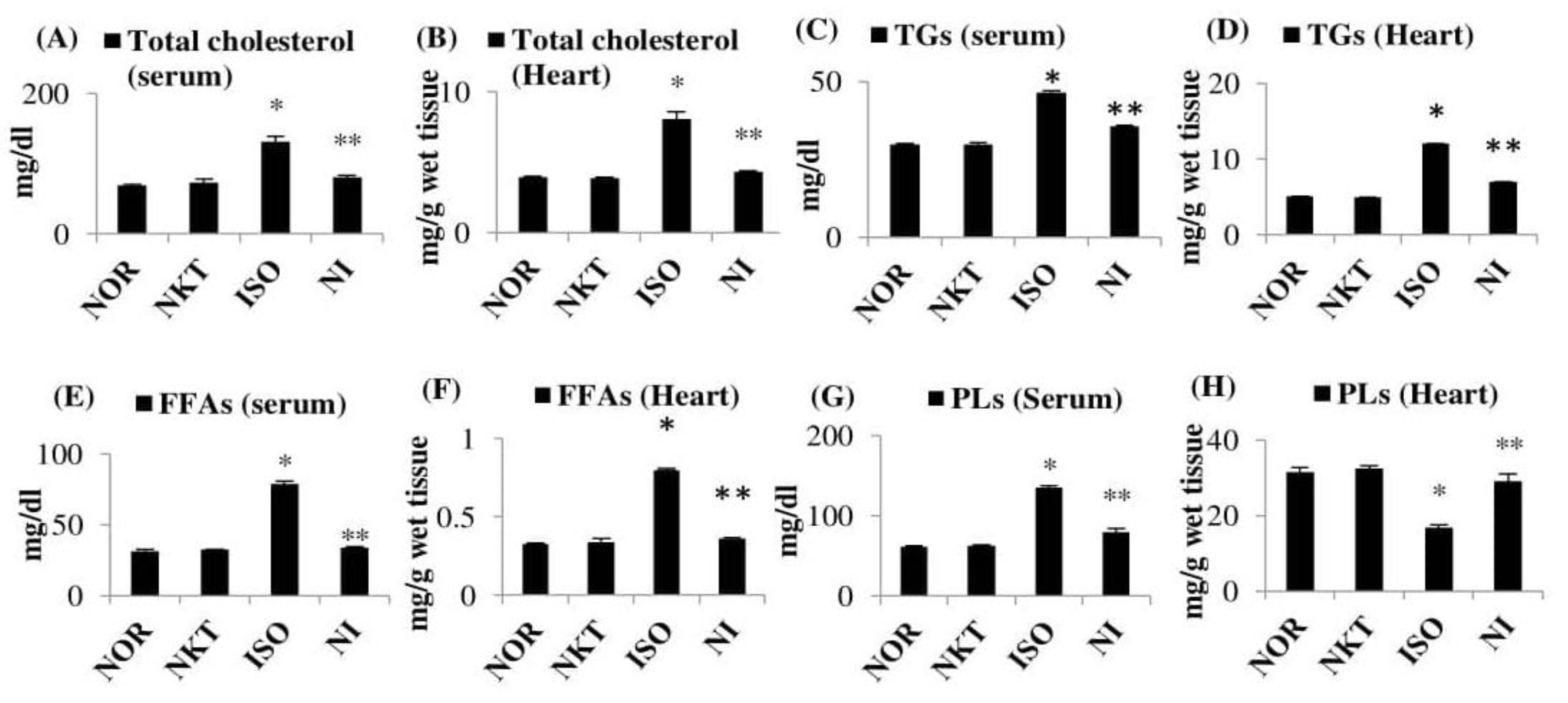
2.4. Effect of NKT on the Levels/Concentrations of Lipids in the Serum and Heart
2.5. Effect of NKT on Alteration in the Levels of Lipoproteins
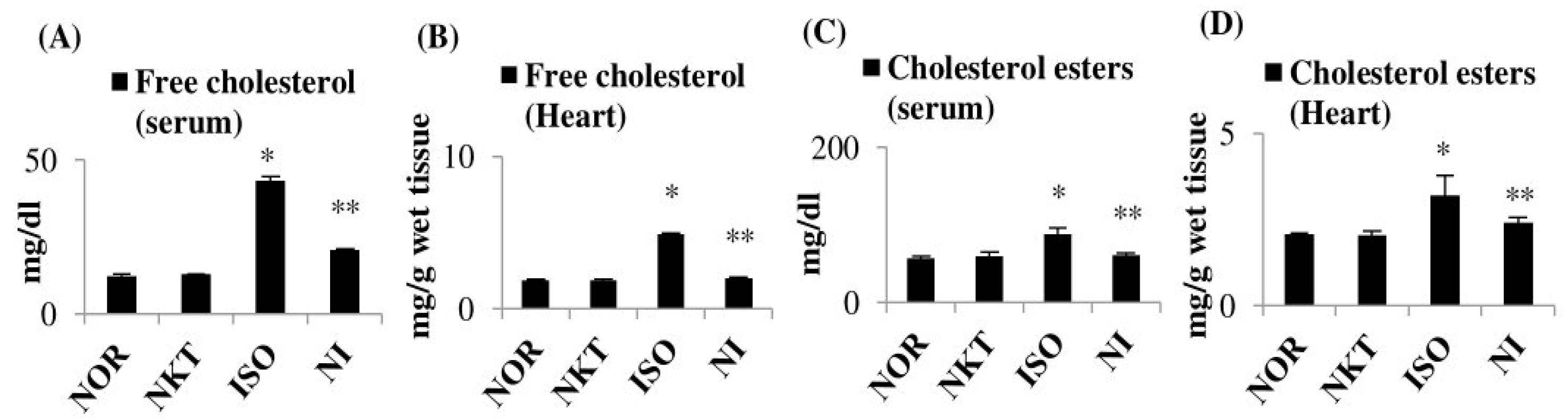
2.6. Effect of NKT on Free Cholesterol and Cholesterol Esters
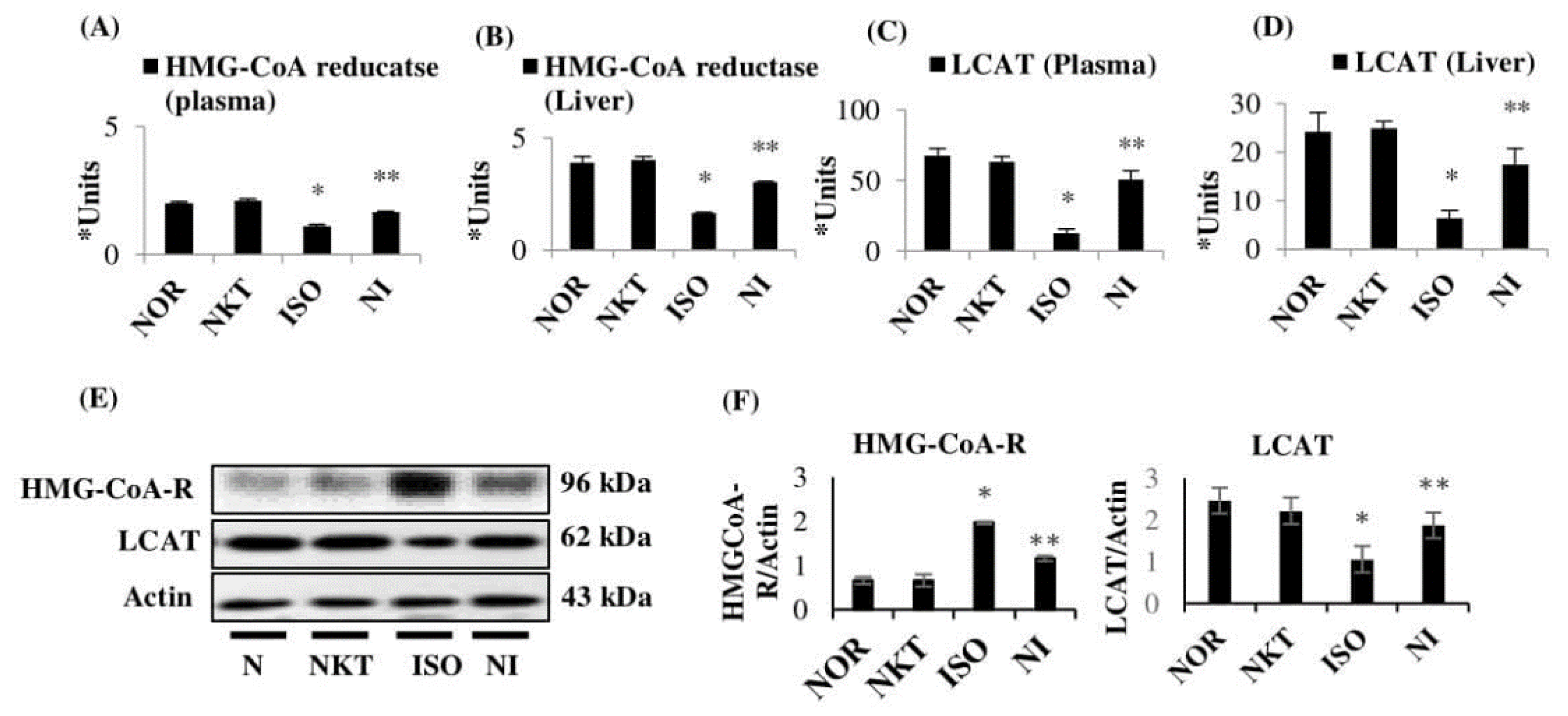
2.7. Effect of NKT on Lipid Marker Enzymes in the Plasma and Liver of Rats
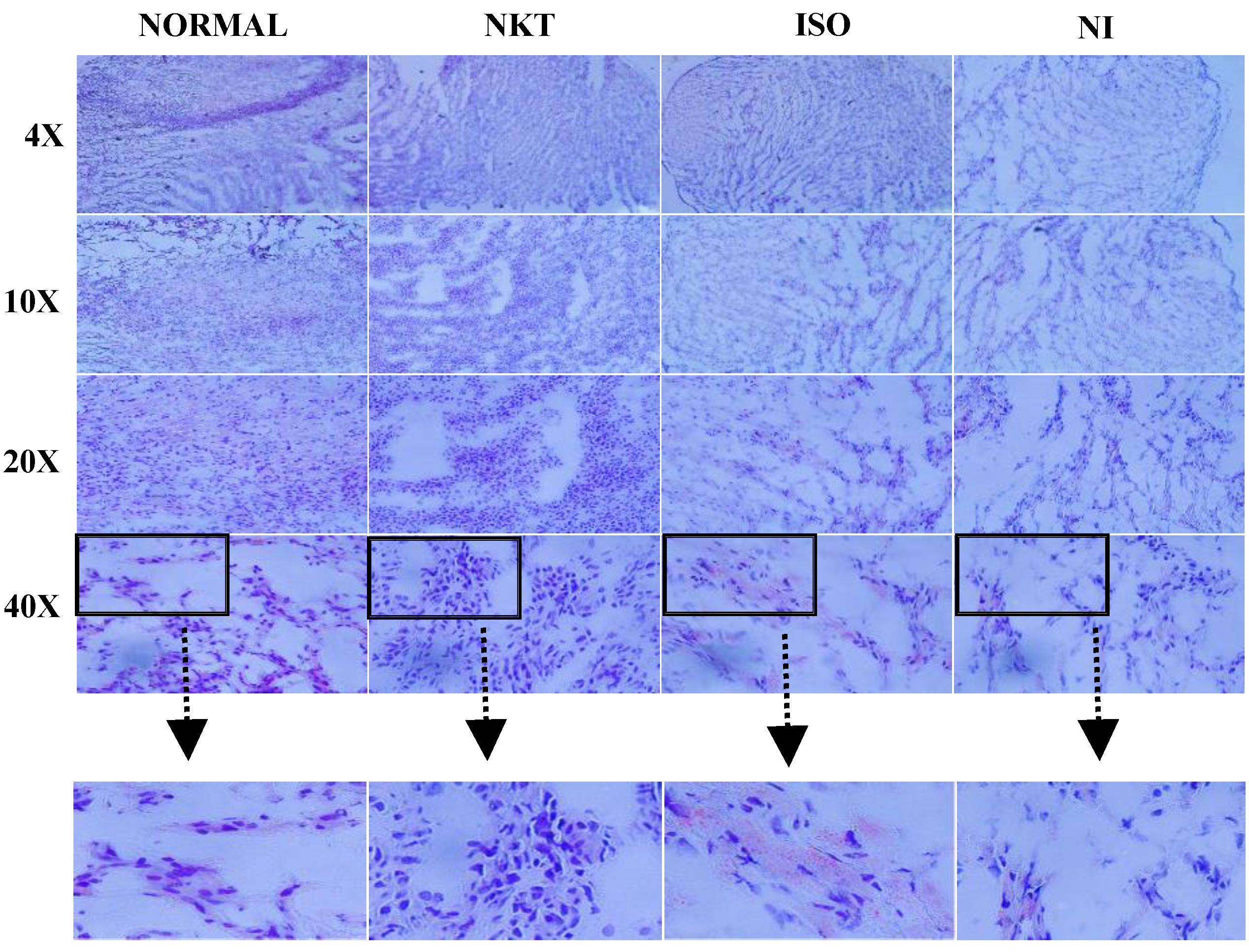
2.8. Effect of NKT on Intramyocardial Lipid Accumulation in the Myocardium
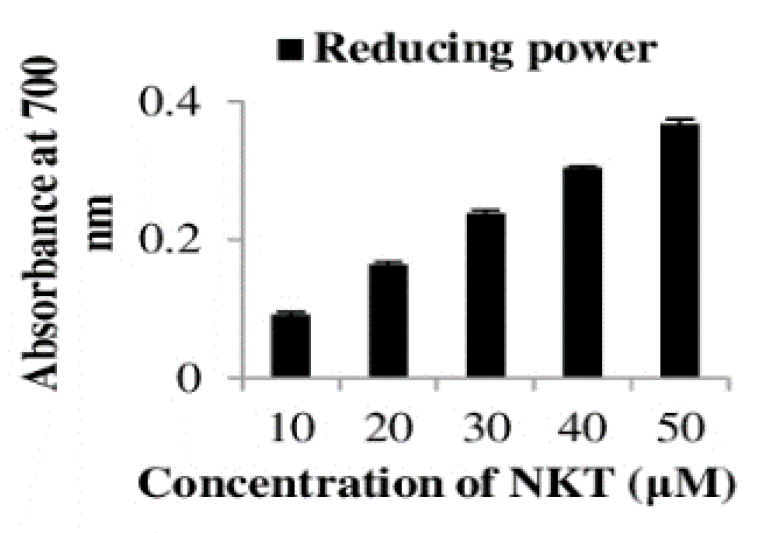
2.9. The In Vitro Reducing Power of NKT
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Experimental Animals
4.3. Induction of Experimental MI in Wistar Rats
4.4. Biochemical Estimations
4.4.1. Estimation of the Cardiomyocyte Injury Marker Enzymes
4.4.2. Determination of Plasma Thiobarbituric Acid-Reactive Substances (TBARS)
4.4.3. Estimation of Plasma Lipid Hydroperoxides (LOOH)
4.4.4. Estimation of Plasma reduced glutathione (GSH)
4.4.5. Estimation of Plasma Vitamin C
4.4.6. Estimation of Plasma Vitamin E
4.4.7. Determination of Left-Ventricular Hypertrophy (LVH)
4.4.8. Extraction of Lipids in the Heart
4.4.9. Estimation of Total Cholesterol
4.4.10. Estimation of Triglycerides (TGs)
4.4.11. Estimation of Free Fatty Acids (FFAs)
4.4.12. Estimation of Phospholipids (PLs)
4.4.13. Estimation of Lipoproteins
4.4.14. Assay of 3-Hydroxy-3-methylglutaryl Coenzyme-A Reductase (HMG-CoA-R)
4.4.15. Assay of Lecithin Cholesterol Acyltransferase (LCAT)
4.5. Oil Red O Staining
4.6. Western Blot Analysis
4.7. Estimation of Protein in the Heart
4.8. In Vitro Reducing Power of NKT
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laslett, L.J.; Alagona, P.; Clark, B.A.; Jr, J.P.D.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The Worldwide Environment of Cardiovascular Disease: Prevalence, Diagnosis, Therapy, and Policy Issues. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Laham, F.; Azimullah, S.; Tariq, S.; Ojha, S. α-Bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol. Cell. Biochem. 2018, 453, 89–102. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Kumar, A.; Shakoor, T.; Rizwan, A. Lipid Profile of Patients with Acute Myocardial Infarction (AMI). Cureus 2019, 11, e4265. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, S.; Kapoor, N.K.; Nityanand, S. Effect of isoprenaline on lipid profile & cardiac enzymes in rats. Indian J. Exp. Biol. 1978, 16, 376–378. [Google Scholar]
- Xiang, Y.K. Compartmentalization of β-Adrenergic Signals in Cardiomyocytes. Circ. Res. 2011, 109, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Dalic, A.; Fang, L.; Kiriazis, H.; Ritchie, R.H.; Sim, K.; Gao, X.-M.; Drummond, G.R.; Sarwar, M.; Zhang, Y.-Y.; et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br. J. Pharmacol. 2011, 162, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; You, M.-G.; Ling, J.-J.; Wei, L.-L.; Wang, K.; Li, W.-W.; Chen, T.; Du, Q.-M.; Ji, H. Regulation of antioxidant system, lipids and fatty acid β-oxidation contributes to the cardioprotective effect of sodium tanshinone IIA sulphonate in isoproterenol-induced myocardial infarction in rats. Atherosclerosis 2013, 230, 148–156. [Google Scholar] [CrossRef]
- Mohan, P.; Bloom, S. Lipolysis is an important determinant of isoproterenol-induced myocardial necrosis. Cardiovasc. Pathol. 1999, 8, 255–261. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Prince, P.S.M.; Basha, R.H. Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: An in vivo and in vitro study. Eur. J. Pharmacol. 2012, 677, 116–122. [Google Scholar] [CrossRef]
- Libby, P. Vascular biology of atherosclerosis: Overview and state of the art. Am. J. Cardiol. 2003, 91, 3–6. [Google Scholar] [CrossRef]
- Jagadeesh, G.S.; Meeran, M.F.N.; Selvaraj, P. Protective Effects of 7-Hydroxycoumarin on Dyslipidemia and Cardiac Hypertrophy in Isoproterenol-Induced Myocardial Infarction in Rats. J. Biochem. Mol. Toxicol. 2015, 30, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Messineo, F.C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ. Res. 1981, 48, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, F.; Kaur, R.; Mir, A.H.; Maqbool, I.; Lonare, M.; Singh, D.; Rampal, S.; Dar, J.A. Rosuvastatin and retinoic acid may act as ‘pleiotropic agents’ against β-adrenergic agonist-induced acute myocardial injury through modulation of multiple signalling pathways. Chem. Interact. 2020, 318, 108970. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Kurdi, A.; Sadek, B.; Kaleem, M.; Cai, L.; Kamal, M.A.; Rajesh, M. Phytochemicals as Prototypes for Pharmaceutical Leads Towards Drug Development Against Diabetic Cardiomyopathy. Curr. Pharm. Des. 2016, 22, 3058–3070. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Hassan, K.; Venkataraman, B.; Rajesh, M. Nootkatone confers hepatoprotective and anti-fibrotic actions in a murine model of liver fibrosis by suppressing oxidative stress, inflammation, and apoptosis. J. Biochem. Mol. Toxicol. 2018, 32, e22017. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Hamadi, N.; Ali, B.H. In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway. Nutrients 2018, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Murase, T.; Misawa, K.; Haramizu, S.; Minegishi, Y.; Hase, T. Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am. J. Physiol. Metab. 2010, 299, E266–E275. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Jagadeesh, G.S.; Selvaraj, P. Thymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosomal enzymes and downregulating the expressions of proinflammatory cytokines. Eur. J. Pharmacol. 2015, 754, 153–161. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Laham, F.; Al-Taee, H.; Azimullah, S.; Ojha, S. Protective effects of α-bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: In vivo and in vitro evidences. J. Biochem. Mol. Toxicol. 2018, 32, e22200. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Jagadeesh, G.S.; Selvaraj, P. Catecholamine toxicity triggers myocardial membrane destabilization in rats: Thymol and its counter action. RSC Adv. 2015, 5, 43338–43344. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Jagadeesh, G.S.; Selvaraj, P. Thymol attenuates altered lipid metabolism in β-adrenergic agonist induced myocardial infarcted rats by inhibiting tachycardia, altered electrocardiogram, apoptosis and cardiac hypertrophy. J. Funct. Foods 2015, 14, 51–62. [Google Scholar] [CrossRef]
- Yeagle, P.L. Cholesterol and the cell membrane. Biochim. Biophys. Acta 1985, 822, 267–287. [Google Scholar] [CrossRef]
- Dhivya, V.; Priya, L.B.; Chirayil, H.T.; Sathiskumar, S.; Huang, C.-Y.; Padma, V.V. Piperine modulates isoproterenol induced myocardial ischemia through antioxidant and anti-dyslipidemic effect in male Wistar rats. Biomed. Pharmacother. 2017, 87, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.M.; Quine, S.D. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism 2013, 62, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sparagna, G.C.; Hickson-Bick, D.L. Cardiac Fatty Acid Metabolism and the Induction of Apoptosis. Am. J. Med. Sci. 1999, 318, 15–21. [Google Scholar] [CrossRef]
- Franson, R.; Waite, M.; Weglicki, W. Phospholipase A activity of lysosomes of rat myocardial tissue. Biochemistry 1972, 11, 472–476. [Google Scholar] [CrossRef]
- Ashraf, M.Z.; Hussain, M.; Fahim, M. Antiatherosclerotic effects of dietary supplementations of garlic and turmeric: Restoration of endothelial function in rats. Life Sci. 2005, 77, 837–857. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.D.P.P.; Wang, Y.; Rupasinghe, H.V. Hypocholesterolemic and hypotensive effects of a fruit-based functional beverage in spontaneously hypertensive rats fed with cholesterol-rich diet. J. Funct. Foods 2013, 5, 1392–1401. [Google Scholar] [CrossRef]
- Schneider, I.; Kressel, G.; Meyer, A.; Krings, U.; Berger, R.G.; Hahn, A. Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J. Funct. Foods 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Sasikumar, C.S.; Devi, S. Effect of ‘Abana’ Pretreatment on Isoproterenol-induced Hyperlipidemia in Rats. Indian J. Pharm. Sci. 2001, 63, 101–104. [Google Scholar]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kumar, A.; Tripathi, S.; Kumar, S. Commiphora wightii down regulates HMG CoA reductase in hyperlipidemic rats. Int. J. Appl. Pharm. Sci. 2013, 4, 441–447. [Google Scholar]
- Punithavathi, V.; Prince, P.S.M. Combined effects of quercetin and α-tocopherol on lipids and glycoprotein components in isoproterenol induced myocardial infarcted Wistar rats. Chem. Interact. 2009, 181, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Jodalen, H.; Lie, R.; Rotevatn, S. Effect of isoproterenol on lipid accumulation in myocardial cells. Res. Exp. Med. 1982, 181, 239–244. [Google Scholar] [CrossRef]
- Bruneval, P.; Yunge, L.; Hüttner, I. Isoproterenol induced fatty change in rat myocardium: Freeze fracture study of the sarcolemma at sites of lipid droplet-membrane appositions. J. Submicrosc. Cytol. 1985, 17, 335–343. [Google Scholar]
- Fineschi, V.; Michalodimitrakis, M.; D’Errico, S.; Neri, M.; Pomara, C.; Riezzo, I.; Turillazzi, E. Insight into stress-induced cardiomyopathy and sudden cardiac death due to stress. A forensic cardio-pathologist point of view. Forensic Sci. Int. 2010, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, K.S.; Vasanthi, H.R.; Rajamanickam, G.V. Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol. Cell. Biochem. 2009, 327, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Liu, M.; Zhang, C.; Jia, L. Hepatoprotective and in vitro antioxidant effects of native depolymerised-exopolysaccharides derived from Termitomyces albuminosus. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Lipid peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 1979; Volume 62, pp. 3–11. [Google Scholar]
- Baker, H.; Frank, O.; DeAngelis, B.; Feingold, S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980, 21, 531–536. [Google Scholar]
- Gupta, R.C.; Yang, X.-P.; Mishra, S.; Sabbah, H.N. Assessment of sarcoplasmic reticulum Ca2+-uptake during the development of left ventricular hypertrophy. Biochem. Pharmacol. 2003, 65, 933–939. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Zlatkis, A.; Zak, B.; Boyle, A.J. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953, 41, 486–492. [Google Scholar]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Falholt, K.; Lund, B. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin. Chim. Acta 1973, 46, 105–111. [Google Scholar] [CrossRef]
- Zilversmit, D.B.; Davis, A.K. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J. Lab. Clin. Med. 1950, 35, 155–160. [Google Scholar]
- Rao, A.V.; Ramakrishnan, S. Indirect Assessment of Hydroxymethylglutaryl-CoA Reductase (NADPH) Activity in Liver Tissue. Clin. Chem. 1975, 21, 1523–1525. [Google Scholar] [CrossRef]
- Hitz, J.; Steinmetz, J.; Siest, G. Plasma lecithin:cholesterol acyltransferase—Reference values and effects of xenobiotics. Clin. Chim. Acta 1983, 133, 85–96. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: The samples are not available from the authors. |



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meeran, M.F.N.; Azimullah, S.; Al Ahbabi, M.M.; Jha, N.K.; Lakshmanan, V.-K.; Goyal, S.N.; Ojha, S. Nootkatone, a Dietary Fragrant Bioactive Compound, Attenuates Dyslipidemia and Intramyocardial Lipid Accumulation and Favorably Alters Lipid Metabolism in a Rat Model of Myocardial Injury: An In Vivo and In Vitro Study. Molecules 2020, 25, 5656. https://doi.org/10.3390/molecules25235656
Meeran MFN, Azimullah S, Al Ahbabi MM, Jha NK, Lakshmanan V-K, Goyal SN, Ojha S. Nootkatone, a Dietary Fragrant Bioactive Compound, Attenuates Dyslipidemia and Intramyocardial Lipid Accumulation and Favorably Alters Lipid Metabolism in a Rat Model of Myocardial Injury: An In Vivo and In Vitro Study. Molecules. 2020; 25(23):5656. https://doi.org/10.3390/molecules25235656
Chicago/Turabian StyleMeeran, M.F. Nagoor, Sheikh Azimullah, M Marzouq Al Ahbabi, Niraj Kumar Jha, Vinoth-Kumar Lakshmanan, Sameer N. Goyal, and Shreesh Ojha. 2020. "Nootkatone, a Dietary Fragrant Bioactive Compound, Attenuates Dyslipidemia and Intramyocardial Lipid Accumulation and Favorably Alters Lipid Metabolism in a Rat Model of Myocardial Injury: An In Vivo and In Vitro Study" Molecules 25, no. 23: 5656. https://doi.org/10.3390/molecules25235656
APA StyleMeeran, M. F. N., Azimullah, S., Al Ahbabi, M. M., Jha, N. K., Lakshmanan, V.-K., Goyal, S. N., & Ojha, S. (2020). Nootkatone, a Dietary Fragrant Bioactive Compound, Attenuates Dyslipidemia and Intramyocardial Lipid Accumulation and Favorably Alters Lipid Metabolism in a Rat Model of Myocardial Injury: An In Vivo and In Vitro Study. Molecules, 25(23), 5656. https://doi.org/10.3390/molecules25235656








