Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage
Abstract
:1. Introduction
2. Mechanisms of Radiation-Induced Brain Injury
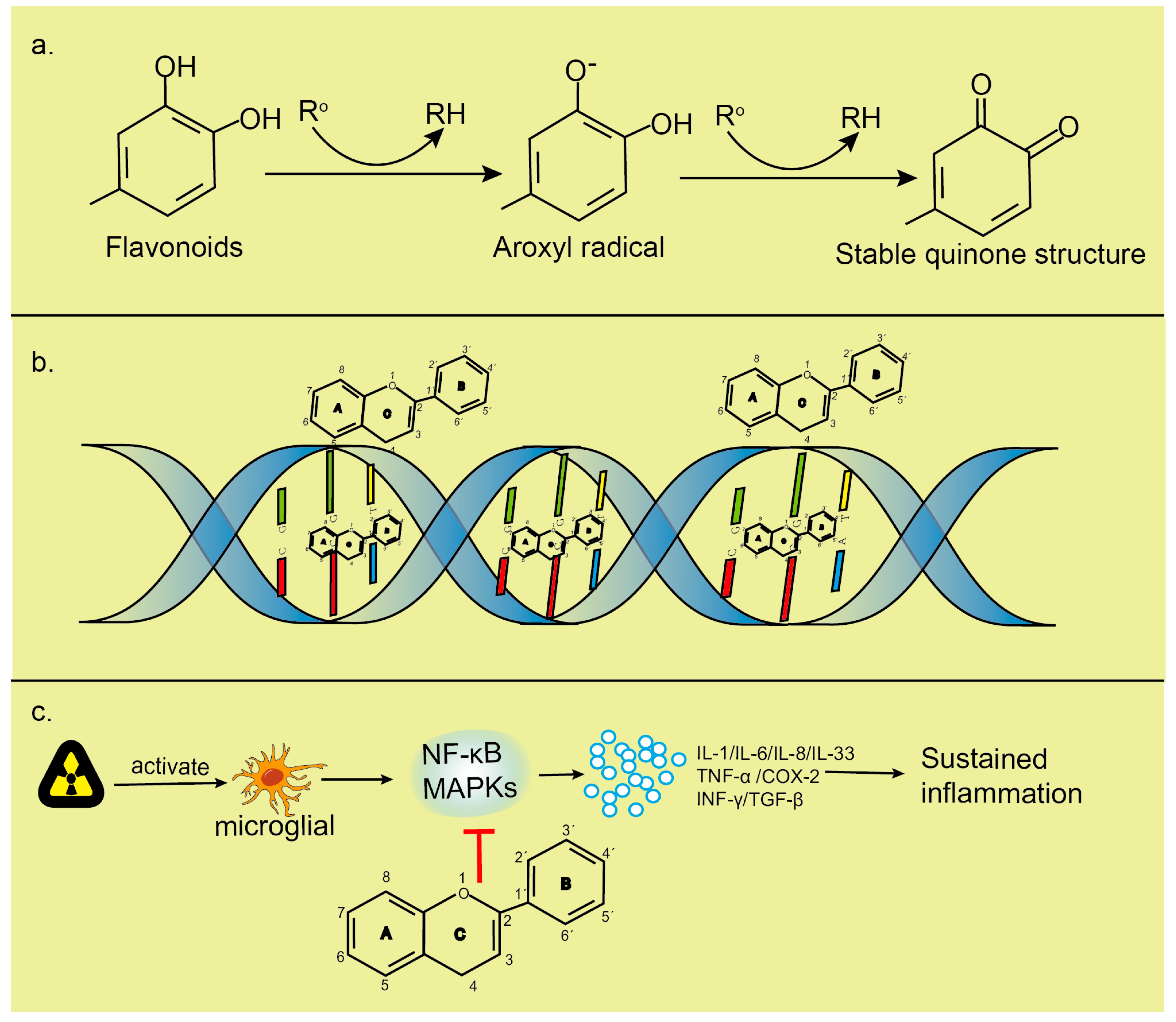
2.1. Oxidative Stress
2.2. Inflammation
2.3. DNA Damage
3. Radio-Neuro-Protective Roles of Flavonoids
4. Mechanisms of Flavonoids as Radio-Neuro-Protectants
4.1. Antioxidant Activity of Flavonoids
4.2. Flavonoids Reduce Inflammation in CNS
4.3. Flavonoids Reduce DNA Damage
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| EGCG | (−)-epigallocatechin gallate |
| BDNF | Brain-derived neurotrophic factor |
| p-CREB | Phosphor-(cyclic-AMP response element-binding protein) |
| SSBs | Single-strand breaks |
| DSBs | Double-strand breaks |
| ROS | Reactive oxygen species |
| CNS | Central nervous system |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| O2− | Superoxide anions |
| OH− | Hydroxyl radicals |
| H2O2 | Hydrogen peroxide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| SMAD | Small mother against decapentaplegic |
| IL-1 | Interleukin-1 |
| TNF-α | Tumor necrosis factor α |
| TGF-β | Transforming growth factor β |
| IFN-γ | Interferon γ |
| DAMPs | Damage-associated molecular patterns |
| PRRs | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| HMGB1 | High mobility group Box 1 |
| HSPs | Heat shock proteins |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| MAPK | Mitogen-activated protein kinases |
| NHEJ | Non-homologous end joining |
| ATM | A-T mutated |
| ATR | ATM and Rad3-related |
| DNA-PK | DNA-dependent protein kinase |
| DRG | Dorsal root ganglion |
| CHOP | C/EBP-homologous protein |
| JNK | Jun-N-terminal kinases |
| P13K | Phosphatidylinositol-3-kinase |
| AKT | Serine-threonin protein kinase |
| GSK3β | Glycogen synthase kinase 3 β |
| NRF-2 | Nuclear factor erythroid-2 related factor-2 |
| DHF | 5, 7-dihydroxyflavone |
| EGCG/AA NPs | Nanoparticles of EGCG/ascorbic acid |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| CAT | Catalase |
| HO-1 | Heme oxygenase-1 |
| XO | Xanthine oxidase |
| LOX | Lipoxygenase |
| AO | Aldehyde oxidase |
| COX-2 | Cyclooxygenase-2 |
| AP-1 | Activated protein-1 |
References
- Yang, B.; Ren, B.X.; Tang, F.R. Prenatal irradiation-induced brain neuropathology and cognitive impairment. Brain Dev. 2017, 39, 10–22. [Google Scholar] [CrossRef]
- Peng, X.C.; Huang, J.R.; Wang, S.W.; Liu, L.; Liu, Z.Z.; Sethi, G.; Ren, B.X.; Tang, F.R. Traditional Chinese Medicine in Neuroprotection after Brain Insults with Special Reference to Radioprotection. Evid. Based Complement. Alternat. Med. 2018, 2018, 2767208. [Google Scholar] [CrossRef] [Green Version]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Balcer-Kubiczek, E.K. The Role of the Apoptotic Machinery in Ionizing Radiation-Induced Carcinogenesis. Crit. Rev. Oncog. 2016, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D., Jr. Studies of atomic bomb survivors. Understanding radiation effects. JAMA 1990, 264, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Ren, B.X.; Qian, F.; Luo, X.Z.; Tang, X.; Peng, X.C.; Huang, J.R.; Tang, F.R. Radioprotective effect of epimedium on neurogenesis and cognition after acute radiation exposure. Neurosci. Res. 2019, 145, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Phan, J. Reirradiation of head and neck cancer using modern highly conformal techniques. Head Neck 2018, 40, 2078–2093. [Google Scholar] [CrossRef]
- Burns, T.C.; Awad, A.J.; Li, M.D.; Grant, G.A. Radiation-induced brain injury: Low-hanging fruit for neuroregeneration. Neurosurg. Focus 2016, 40, E3. [Google Scholar] [CrossRef]
- Fischer, N.; Seo, E.J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Seong, K.M.; Youn, B. Phenylpropanoids in radioregulation: Double edged sword. Exp. Mol. Med. 2011, 43, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadi Akbar Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Joan Abbott, N.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [Green Version]
- Cimrová, B.; Budáč, S.; Melicherová, U.; Jergelova, M.; Jagla, F. Electrophysiological evidence of the effect of natural polyphenols upon the human higher brain functions. Neuro Endocrinol. Lett. 2011, 32, 464–468. [Google Scholar]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamuara, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.R.; El-Esawy, R.O.; El-Sakaa, M.H. Troxerutin downregulates C/EBP-βgene expression via modulating the IFNγ-ERK1/2 signaling pathway to ameliorate rotenone-induced retinal neurodegeneration. J. Biochem. Mol. Toxicol. 2020, 34, e22482. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Piskin, Ö.; Bas, Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.B.; Park, H.R.; Jang, Y.J.; Choi, S.Y.; Son, T.G. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br. J. Pharmacol. 2013, 168, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béhin, A.; Delattre, J.Y. Complications of radiation therapy on the brain and spinal cord. Semin. Neurol. 2004, 24, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.J.; Kim, J.C.; Kang, S.S.; Bae, C.S.; Shin, T.; Jin, J.K.; Kim, S.H.; Wang, H.; Moon, C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J. Radiat. Res. 2008, 49, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Hladik, D.; Tapio, S. Effects of ionizing radiation on the mammalian brain. Mutat. Res. 2016, 770, 219–230. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and repair of clustered DNA lesions: What do we know so far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef]
- Shikazono, N.; Noguchi, M.; Fujii, K.; Urushibara, A.; Yokoya, A. The yield, processing, and biological consequences of clustered DNA damage induced by ionizing radiation. J. Radiat. Res. 2009, 50, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Shimura, T.; Sasatani, M.; Kawai, H.; Kamiya, K.; Kobayashi, J.; Komatsu, K.; Kunugita, N. A comparison of radiation-induced mitochondrial damage between neural progenitor stem cells and differentiated cells. Cell Cycle 2017, 16, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Wang, Y.; Wang, Y.; Zhang, X.; Zhang, H. Effects of X-irradiation on mitochondrial DNA damage and its supercoiling formation change. Mitochondrion 2011, 11, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Werbin, H. New evidence for the insertion of mitochondrial DNA into the human genome: Significance for cancer and aging. Mutat. Res. 1992, 275, 227–235. [Google Scholar] [CrossRef]
- Cheng, X.; Ivessa, A.S. The migration of mitochondrial DNA fragments to the nucleus affects the chronological aging process of Saccharomyces cerevisiae. Aging Cell 2010, 9, 919–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, B.D.; Syage, A.R.; Maroso, M.; Baddour, A.A.D.; Luong, V.; Minasyan, H.; Giedzinski, E.; West, B.L.; Soltesz, I.; Limoli, C.L.; et al. Mitigation of helium irradiation-induced brain injury by microglia depletion. J. Neuroinflamm. 2020, 17, 159. [Google Scholar] [CrossRef]
- Stein, Y.; Udasin, I.G. Electromagnetic hypersensitivity (EHS, microwave syndrome)—Review of mechanisms. Environ. Res. 2020, 186, 109445. [Google Scholar] [CrossRef]
- Ienco, E.C.; LoGerfo, A.; Carlesi, C.; Orsucci, D.; Ricci, G.; Mancuso, M.; Siciliano, G. Oxidative stress treatment for clinical trials in neurodegenerative diseases. J. Alzheimers Dis. 2011, 24, 111–126. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Connor, J.R.; Menzies, S.L. Cellular management of iron in the brain. J. Neurol Sci. 1995, 134, 33–44. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R.; Doelman, C.J. Oxidants and antioxidants: State of the art. Am. J. Med. 1991, 91, 2S–13S. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999, 9, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Tulard, A.; Hoffschir, F.; de Boisferon, F.H.; Luccioni, C.; Bravard, A. Persistent oxidative stress after ionizing radiation is involved in inherited radiosensitivity. Free Radic. Biol. Med. 2003, 35, 68–77. [Google Scholar] [CrossRef]
- Kim, W.; Youn, H.; Kang, C.; Youn, B. Inflammation-induced radioresistance is mediated by ROS-dependent inactivation of protein phosphatase 1 in non-small cell lung cancer cells. Apoptosis 2015, 20, 1242–1252. [Google Scholar] [CrossRef]
- Leach, J.K.; Van Tuyle, G.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Yahyapour, R.; Amini, P.; Rezapour, S.; Cheki, M.; Rezaeyan, A.; Farhood, B.; Shabeeb, D.; Musa, A.E.; Fallah, H.; Najafi, M. Radiation-induced inflammation and autoimmune diseases. Mil. Med. Res. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Rezaeyan, A.H.; Salajegheh, A.; Rezapoor, S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology 2017, 25, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Multhoff, G.; Radons, J. Radiation, inflammation, and immune responses in cancer. Front. Oncol. 2012, 2, 58. [Google Scholar] [CrossRef] [Green Version]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef]
- Verstak, B.; Nagpal, K.; Bottomley, S.P.; Golenbock, D.T.; Hertzog, P.J.; Mansell, A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J. Biol. Chem. 2009, 284, 24192–24203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Robbins, M.E. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: Therapeutic implications. Curr. Med. Chem. 2009, 16, 130–143. [Google Scholar] [CrossRef]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp. Cell Res. 2016, 340, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Nehru, B. Apocyanin, a Microglial NADPH Oxidase Inhibitor Prevents Dopaminergic Neuronal Degeneration in Lipopolysaccharide-Induced Parkinson’s Disease Model. Mol. Neurobiol. 2016, 53, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [Green Version]
- Jeggo, P.; Lobrich, M. Radiation-induced DNA damage responses. Radiat. Prot. Dosimetry 2006, 122, 124–127. [Google Scholar] [CrossRef]
- Ward, J.F. The complexity of DNA damage: Relevance to biological consequences. Int. J. Radiat. Biol. 1994, 66, 427–432. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Jeggo, P.A. The repair and signaling responses to DNA double-strand breaks. Adv. Genet. 2013, 82, 1–45. [Google Scholar] [CrossRef]
- Iyer, R.; Lehnert, B.E. Effects of ionizing radiation in targeted and nontargeted cells. Arch. Biochem. Biophys. 2000, 376, 14–25. [Google Scholar] [CrossRef]
- Ward, J.F. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: A review. Int. J. Radiat. Biol. 1990, 57, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Garrett, M.D.; Ashworth, A. Targeting the double-strand DNA break repair pathway as a therapeutic strategy. Clin. Cancer Res. 2006, 12, 4463–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Ojima, M.; Kodama, S.; Watanabe, M. Radiation-induced DNA damage and delayed induced genomic instability. Oncogene 2003, 22, 6988–6993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef]
- Kesari, S.; Advani, S.J.; Lawson, J.D.; Kahle, K.T.; Ng, K.; Carter, B.; Chen, C.C. DNA damage response and repair: Insights into strategies for radiation sensitization of gliomas. Future Oncol. 2011, 7, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM activation by oxidative stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Valentin-Vega, Y.A.; Kastan, M.B. A new role for ATM: Regulating mitochondrial function and mitophagy. Autophagy 2012, 8, 840–841. [Google Scholar] [CrossRef] [Green Version]
- Guardavaccaro, D.; Pagano, M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell 2006, 22, 1–4. [Google Scholar] [CrossRef]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinimehr, S.J. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov. Today 2010, 15, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Langhnoja, J.; Pillai, P.P.; Mustak, M.S. Neuroprotective effect of quercetin against radiation-induced endoplasmic reticulum stress in neurons. J. Biochem. Mol. Toxicol. 2019, 33, e22242. [Google Scholar] [CrossRef] [PubMed]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193. [Google Scholar] [CrossRef]
- Mansour, S.Z.; Moawed, F.S.M.; Elmarkaby, S.M. Protective effect of 5,7-dihydroxyflavone on brain of rats exposed to acrylamide or γ-radiation. J. Photochem. Photobiol. 2017, 175, 149–155. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; El-Sawy, M.R.; Lebede, M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018, 94, 798–808. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, C.Y.; Sreeharsha, N.; Mishra, A.; Sreeharsha, N.; Mishra, A.; Shrotriya, V.; Sharma, A. Neuroprotective effect of Wogonin on Rat’s brain exposed to gamma irradiation. J. Photochem. Photobiol. B 2020, 204, 111775. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Nose-to-brain Delivery of Natural Compounds for the Treatment of Central Nervous System Disorders. Curr. Pharm. Des. 2020, 26, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.L.; Wang, Z.Y.; Zuo, L.L.; Tian, S.Q. Protective effect of anthocyanins from lingonberry on radiation-induced damages. Int. J. Environ. Res. Public Health 2012, 9, 4732–4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gakova, N.; Mishurova, E.; Kropachova, K. Effect of flavobion on nucleic acids in tissues of rats irradiated with gamma rays. Biull. Eksp. Biol. Med. 1992, 113, 275–277. [Google Scholar]
- Nair, C.K.; Salvi, V.P. Protection of DNA from gamma-radiation induced strand breaks by Epicatechin. Mutat. Res. 2008, 650, 48–54. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, A.; Hoshi, T.; Anzai, J.; Li, G. Electrochemical studies of (−)-epigallocatechin gallate and its interaction with DNA. Anal. Bioanal. Chem. 2006, 386, 1913–1919. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Van Hoorn, D.E.; Nijveldt, R.J.; Van Leeuwen, P.A.; Hofman, Z.; M’Rabet, L.; De Bont, D.B.; Van Norren, K. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharmacol. 2002, 451, 111–118. [Google Scholar] [CrossRef]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar]
- Tournaire, C.; Croux, S.; Maurette, M.T.; Beck, I.; Hocquaux, M.; Braun, A.M.; Oliveros, E. Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1 delta g) quenching. J. Photochem. Photobiol. B 1993, 19, 205–215. [Google Scholar] [CrossRef]
- Adhikari, M.; Arora, R.; Chawla, R.; Sharma, J.; Dhaker, A.S.; Gupta, D.; Dubey, N.; Kumar, R.; Ivanov, V.; Gadjeva, V.; et al. Evaluation of silymarin as a promising radioprotector. Z. Naturforsch. C J. Biosci. 2010, 65, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- McRobb, L.S.; McKay, M.J.; Gamble, J.R.; Grace, M.; Moutrie, V.; Santos, E.D.; Lee, V.S.; Zhao, Z.; Molloy, M.P.; Stoodley, M.A. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging 2017, 9, 1248–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanita 2007, 43, 394–405. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.L.; Huang, W.C.; Cheng, S.C.; Liou, C.J. Fisetin inhibits the generation of inflammatory mediators in interleukin-1beta-induced human lung epithelial cells by suppressing the NF-kappaB and ERK1/2 pathways. Int. Immunopharmacol. 2018, 60, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Targeting signal transduction pathways by chemopreventive agents. Mutat. Res. 2004, 555, 33–51. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Efimova, T.; Eckert, R.L. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 2002, 277, 1828–1836. [Google Scholar] [CrossRef] [Green Version]
- Masella, R.; Di Benedetto, R.; Vari, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Mann, G.E.; Rowlands, D.J.; Li, F.Y.; de Winter, P.; Siow, R.C. Activation of endothelial nitric oxide synthase by dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 2007, 75, 261–274. [Google Scholar] [CrossRef]
- Gong, J.H.; Shin, D.; Han, S.Y.; Kim, J.L.; Kang, Y.H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 2012, 142, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, M.; Wei, J.; Bhatt, S.; Paul, M.; Yakir, S.; Sampson, H.A. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J. Allergy Clin. Immunol. 2011, 128, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Greenstein, J.P.; Hollaender, A. Effects of X-radiation on sodium thymus nucleate. Arch. Biochem. 1948, 16, 19–31. [Google Scholar] [PubMed]
- Olive, P.L. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiat. Res. 1998, 150, S42–S51. [Google Scholar] [CrossRef]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Cox, R. Molecular mechanisms of radiation oncogenesis. Int. J. Radiat. Biol. 1994, 65, 57–64. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hosseinimehr, S.J.; Naghshvar, F.; Hajir, E.; Ghahremani, M. Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch. Pharm. Res. 2008, 31, 794–797. [Google Scholar] [CrossRef]
- Kyle, E.; Neckers, L.; Takimoto, C.; Curt, G.; Bergan, R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol. Pharmacol. 1997, 51, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.H.; Yan, H.L.; Cai, D.L. Protective effects of soybean isoflavone against gamma-irradiation induced damages in mice. J. Radiat. Res. 2006, 47, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.A.; Clarke, T.K.; Mog, S.R.; Landauer, M.R. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int. J. Radiat. Biol. 2007, 83, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, V.; Orsolic, N.; Knezevic, A.H.; Ramic, S.; Dikic, D.; Basic, I.; Kopjar, N. Evaluation of the radioprotective effects of propolis and flavonoids in gamma-irradiated mice: The alkaline comet assay study. Biol. Pharm. Bull. 2008, 31, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, D.K.; Balakrishnan, S.; Salvi, V.P.; Nair, C.K. Protection of cellular DNA from gamma-radiation-induced damages and enhancement in DNA repair by troxerutin. Mol. Cell. Biochem. 2005, 280, 57–68. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K. The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: A micronucleus study. Mutat. Res. 2002, 519, 37–48. [Google Scholar] [CrossRef]
- Janjua, N.K.; Siddiqu, A.; Sabahat, S.; Qureshi, R.; Haque, S. Spectrophotometric analysis of flavonoid-DNA binding interaction at physiological conditions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 1135–1137. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.F.; Wang, J.Y.; Tang, N. Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth(III) complexes. J. Inorg. Biochem 2001, 83, 41–48. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Interaction of antioxidant flavonoids with tRNA: Intercalation or external binding and comparison with flavonoid-DNA adducts. DNA Cell Biol. 2006, 25, 116–123. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Diamantoglou, S.; Tajmir-Riahi, H.A. An overview of DNA and RNA bindings to antioxidant flavonoids. Cell Biochem. Biophys. 2007, 49, 29–36. [Google Scholar] [CrossRef]
- Ragazzon, P.A.; Iley, J.; Missailidis, S. Structure-activity studies of the binding of the flavonoid scaffold to DNA. Anticancer Res. 2009, 29, 2285–2293. [Google Scholar] [PubMed]


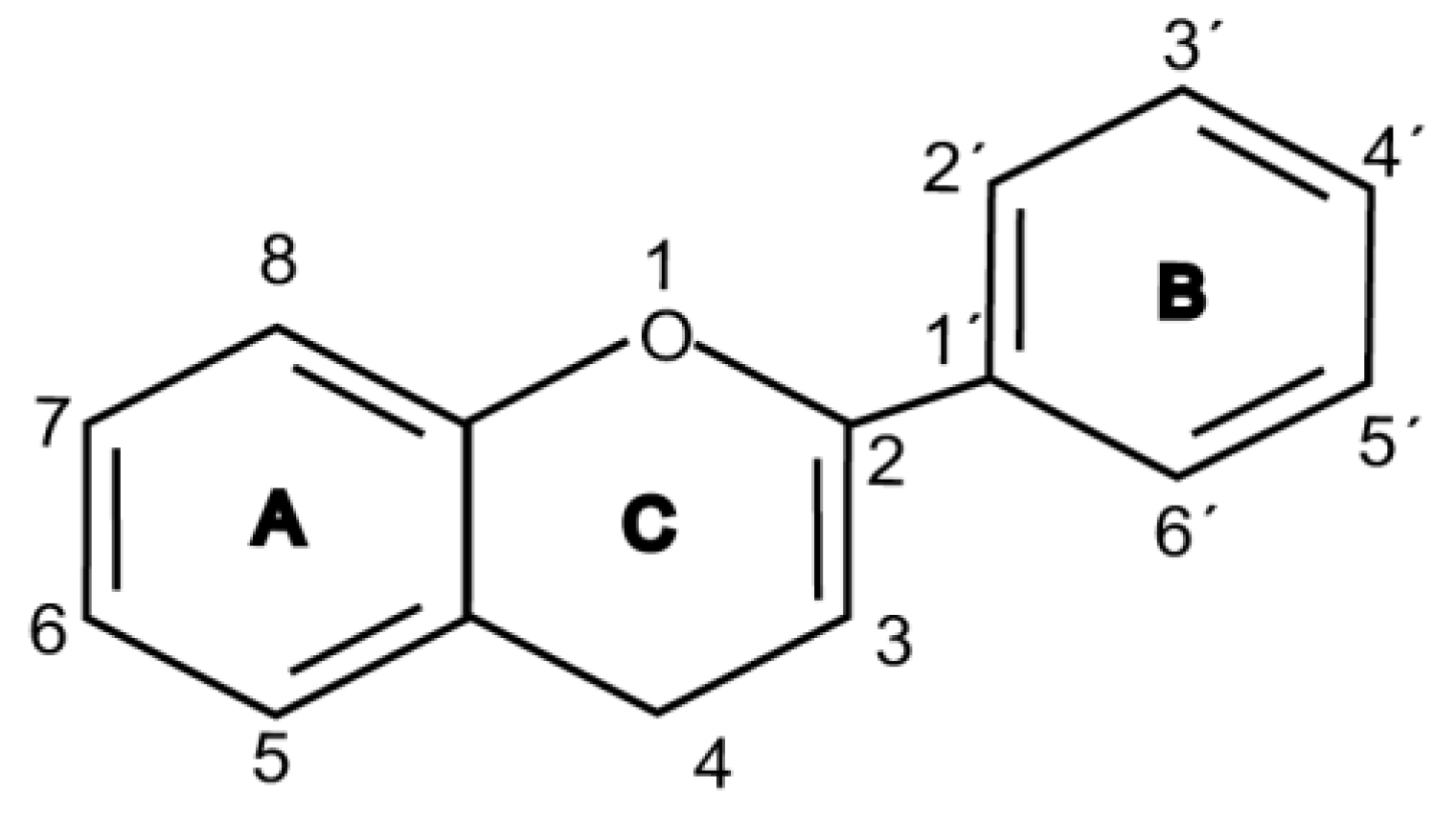
| Flavonoid Class | Structure Backbone | Example | Concentration | Radiation Type/Dose | Model | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Flavonol |  | Quercetin | 50 mg/kg/d | CT (20 Gy) | Rat | Antioxidant | [21] |
| 5–100 μM | γ ray (2 Gy) | Neuron | Downregulates TNF-α | [85] | |||
| Flavone | 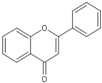 | Baicalein | 1–10 μM | γ ray (16 Gy) | neural progenitor cell | Antioxidant; neuroprotective | [22] |
| 10 mg/kg/d | γ ray (5 Gy) | Mouse | |||||
| Flavanol |  | EGCG | 2.5 and 5 mg/kg/d | γ ray (4 Gy) | Rat | Downregulates TNF-α, IL-6; protects hippocampus | [89] |
| Anthocyanin |  | Cyanidin | 200, 100 and 50 mg/kg/d | γ ray (6 Gy) | Mouse | Against immuno-suppression induced by the radiation | [93] |
| Flavanone |  | Silymarin | 140 mg/kg/d | γ ray (0.2 and 0.6 Gy/d) | Rat | Repairs DNA damage | [94] |
| Isoflavone |  | Genistein | 200 mg/kg/d | γ ray (8.75 Gy) | Mouse | Protects the hematopoietic progenitor cell | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xie, C.; Xi, S.; Qian, F.; Peng, X.; Huang, J.; Tang, F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules 2020, 25, 5719. https://doi.org/10.3390/molecules25235719
Wang Q, Xie C, Xi S, Qian F, Peng X, Huang J, Tang F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules. 2020; 25(23):5719. https://doi.org/10.3390/molecules25235719
Chicago/Turabian StyleWang, Qinqi, Chenghao Xie, Shijun Xi, Feng Qian, Xiaochun Peng, Jiangrong Huang, and Fengru Tang. 2020. "Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage" Molecules 25, no. 23: 5719. https://doi.org/10.3390/molecules25235719
APA StyleWang, Q., Xie, C., Xi, S., Qian, F., Peng, X., Huang, J., & Tang, F. (2020). Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules, 25(23), 5719. https://doi.org/10.3390/molecules25235719







