Stability of Alum-Containing Paper under Alkaline Conditions
Abstract
:1. Introduction
- (a)
- How does the mechanism of hydrolytic reactions of Al(III) compounds affect pH and, thus, hydrolytic degradation?
- (b)
- How do otherwise redox-stable Al(III) compounds participate in the redox reactions occurring in paper and, therefore, contribute to redox degradation?
- (c)
- How can sulfuric acid and organic acids be formed by the redox reactions occurring in the paper and the reactions stimulated by nitrogen and sulfur oxides present in the environment?
- (d)
- What is the effect of present rosin on the course of redox and hydrolytic reactions?
- (e)
- Does the oxidation capacity of actual or potential oxidizing agents depend on pH and, if so, how?
- What is the homogeneity of alum and rosin distribution in the paper and to what extent does the inhomogeneity affect the paper degradation process?
- What is the role of alkaline hydrolysis and related oxidative processes in paper degradation?
- What is the impact of deacidification on the stability/fragility of alum-containing paper?
- Which levels of pH and alkaline reserve can be reached in the treated alum-containing paper?
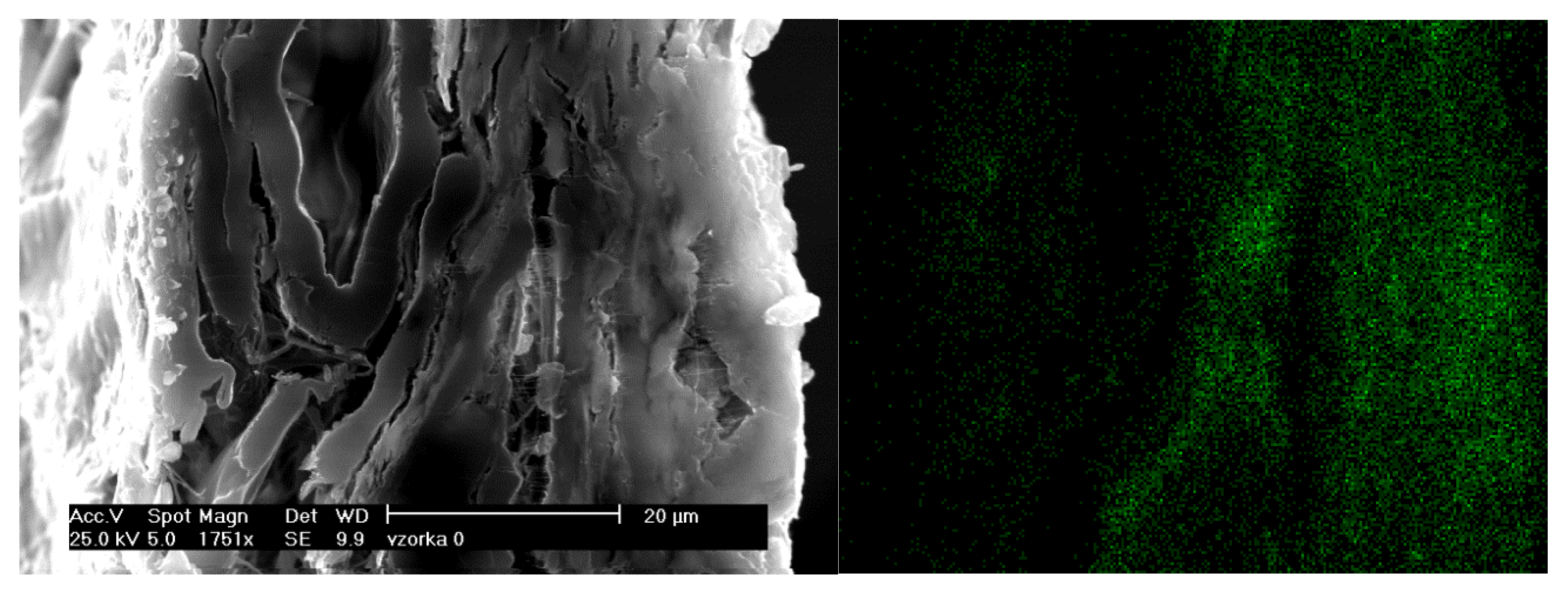
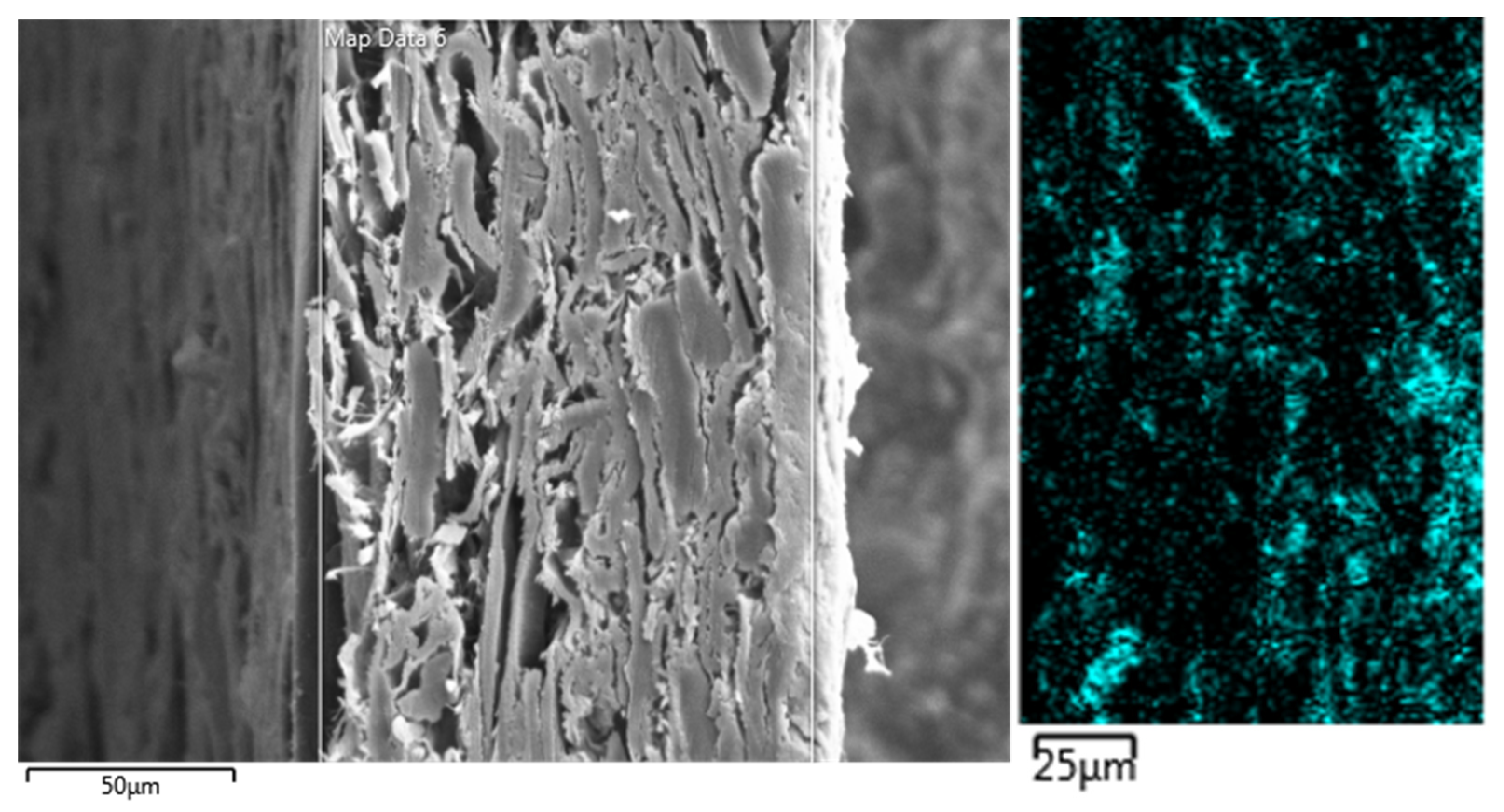
2. Homogeneity of Alum Dispersion in Paper
3. Cellulose and Paper Degradation by Alkaline Hydrolysis
4. Oxidation of Cellulose and Paper in an Alkaline Environment
5. Impact of Deacidification on the Stability of Alum-Containing Paper
6. Levels of pH and Alkaline Reserve Reached in Treating Alum-Containing Paper
7. Stabilization and Conservation of Paper Documents
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jablonský, M.; Šima, J.; Lelovský, M. Considerations on factors influencing the degradation of cellulose in alum-rosin sized paper. Carbohyd. Polym. 2020, 245, 116534. [Google Scholar] [CrossRef]
- Obi Reddy, K.; Maheswari, C.U.; Shukla, M.; Muzenda, E. Preparation, Chemical Composition, Characterization, and Properties of Napier Grass Paper Sheets. Sep. Sci. Technol. 2014, 49, 1527–1534. [Google Scholar] [CrossRef]
- Illing, M.F. Anleitung, auf eine sichere, einfache und wohlfeile Art Papier in der Masse zu leimen. Als Beitrag zur Papiermacherkunst; Forschungsstelle Papiergeschichte: Mainz, Germany, 1807. [Google Scholar]
- Wang, F.; Tanaka, H.; Kitaoka, T.; Hubbe, M.A. Distribution characteristics of rosin size and their effect on the internal sizing of paper. Nord. Pulp. Pap. Res. J. 2000, 15, 416–421. [Google Scholar] [CrossRef]
- Kitaoka, T.; Isogai, A.; Onabe, F.; Endo, T. Sizing mechanism of rosin emulsion size-alum systems. Part 4. Surface sizing by rosin emulsion size on alum-treated base paper. Nord. Pulp. Pap. Res. J. 2001, 16, 96–102. [Google Scholar] [CrossRef]
- Blažej, A.; Krkoška, P. Technológia Výroby Papiera; Alfa: Bratislava, Slovakia, 1989. [Google Scholar]
- Hagiopol, C.; Johnston, J.W. Chemistry of Modern Papermaking; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Xu, L.; Myers, J.; Hart, P.W. How to use alum with cationic dispersed rosin size. Tappi J. 2016, 15, 331–335. [Google Scholar] [CrossRef]
- Özgörüş, N.K.; Ünlü, C.H.; Grupče, O.; Bakan, F.; Sezen, M. Analysis of deterioration phenomena in a Koran by nineteenth-century ottoman Calligrapher Mehmed Şevki. Restaurator 2017, 38, 331–354. [Google Scholar] [CrossRef]
- Jablonský, M. Distribution of Elements in Paper. Unpublished Results; 2020. [Google Scholar]
- Katuščák, S.; Jablonský, M.; Holúbková, S. Comparative evaluation of deacidification processes. Z Bibl. Bibl. 2012, 106, 149–176. [Google Scholar]
- Bicchieri, M. Ferric and Cupric Ions: Interactions with Cellulose as a Function of pH. In Proceedings of the International Conference on Conservation and Restoration of Archive and Library Materials, Erice, Sicily, Italy, 22–29 April 1996; Carlo, F., Paola, F.M., Eds.; Biblioteca Centrale della Regione Siciliana: Palermo, Italy, 1998; pp. 695–709. [Google Scholar]
- Glaus, M.A.; van Loon, R.L. Cellulose Degradation at Alkaline Conditions: Long-Term Experiments at Elevated Temperatures; Paul Scherrer Institute, PSI Bericht Nr.: Villigen, Switzerland, 2004. [Google Scholar]
- Humphreys, P.N.; Laws, A.; Holton, D. A Review of Cellulose Degradation and the Fate of Degradation Products Under Repository Conditions; SERCO/TAS/002274/001 Issue 2; Serco Contractors Report for the Nuclear Decommissioning Authority, Serco Technical and Assurance Services: Oxfordshire, UK, 2010.
- Pavasars, I.; Hagberg, J.; Borén, H.; Allard, B. Alkaline Degradation of Cellulose: Mechanisms and Kinetics. J. Polym. Environ. 2003, 11, 39–47. [Google Scholar] [CrossRef]
- Area, M.C.; Cheradame, H. Paper aging and degradation: Recent findings and research methods. BioResource 2011, 6, 5307–5337. [Google Scholar]
- Ahn, K.; Hennniges, U.; Banik, G.; Potthast, A. Is cellulose degradation due to β-elimination processes a threat in mass deacidification of library books? Cellulose 2012, 19, 1149–1159. [Google Scholar] [CrossRef]
- Ahn, K.; Rosenau, T.; Potthast, A. The influence of alkaline reserve on the aging behavior of book papers. Cellulose 2013, 20, 1989–2001. [Google Scholar] [CrossRef]
- Stephens, C.H.; Whitmore, P.M.; Morris, H.R.; Smith, T. Assessing the Risks of Alkaline Damage During Deacidification Treatments of Oxidized Paper. J. Am. Inst. Conserv. 2009, 48, 235–249. [Google Scholar] [CrossRef]
- Golova, O.P.; Nosova, N.I. Degradation of cellulose by alkaline oxidation. Russ. Chem. Rev. 1973, 42, 327–338. [Google Scholar] [CrossRef]
- Knill, J.C.; Kennedy, J.F. Degradation of Cellulose Under Alkaline Conditions. Carbohyd. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- Jablonský, M.; Šima, J. Oxidative degradation of paper—A minireview. J. Cult. Herit. 2020, submitted. [Google Scholar]
- Dufour, J.; Havermans, J.B.G.A. Study of the Photo-Oxidation of Mass-Deacidified Papers. Restaurator 2001, 22, 20–40. [Google Scholar] [CrossRef]
- Conte, A.M.; Pulci, O.; Knapik, A.; Bagniuk, J.; Del Sole, R.; Lojewska, J.; Missori, M. Role of cellulose oxidation in the yellowing of ancient paper. Phys. Rev. Lett. 2012, 108, 158301. [Google Scholar] [CrossRef]
- Ahn, K.; Zaccaron, S.; Zwirchmayr, N.S.; Hettegger, H.; Hofinger, A.; Bacher, M.; Henniges, U.; Hosoya, T.; Potthast, A.; Rosenau, T. Yellowing and brightness reversion of celluloses: CO or COOH, who is the culprit? Cellulose 2019, 26, 429–444. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, P.M.; Bogaard, J. Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator 1994, 15, 26–45. [Google Scholar] [CrossRef]
- Margutti, S.; Conio, G.; Calvini, P.; Pedemonte, E. Hydrolytic and oxidative degradation of paper. Restaurator 2001, 22, 67–83. [Google Scholar] [CrossRef]
- Emsley, A.M.; Stevens, G.C. Kinetics and mechanisms of the low-temperature degradation of cellulose. Cellulose 1994, 1, 26–56. [Google Scholar] [CrossRef]
- Haskins, J.F.; Hogsed, M.J. The Alkaline Oxidation of Cellulose. I. Mechanism of the Degradative Oxidation of Cellulose by Hydrogen Peroxide in Presence of Alkali. J. Org. Chem. 1950, 15, 1264–1274. [Google Scholar] [CrossRef]
- Nuopponen, M.; Pääkkönen, T.; Pönni, R.; Vuorinen, T. Method for Catalytic Oxidation of Cellulose and Method for Making a Cellulose Product. Patent No. WO2015028719A1, 5 March 2015. [Google Scholar]
- Imamura, A.H.; Segato, T.P.; de Oliveira, L.J.M.; Hassan, A.; Crespilho, F.N.; Carrilho, E. Monitoring cellulose oxidation for protein immobilization in paper-based low-cost biosensors. Microchim. Acta 2020, 187, 272. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Lin, X.; Xiao, H.; Miao, Q.; Huang, L.; Chen, L.; Wu, H. TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers 2017, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Strong, E.B.; Kirschbaum, C.W.; Martinez, A.W.; Martinez, N.W. Paper miniaturization via periodate oxidation of cellulose. Celulose 2018, 25, 3211–3217. [Google Scholar] [CrossRef]
- Rychlý, J.; Matisová-Rychlá, L.; Lazár, M.; Janigová, I.; Strlič, M.; Kočar, D.; Hanus, J.; Mináriková, J.; Katuščák, S. Thermal oxidation of cellulose investigated by chemiluminescence. The effect of magnesium and calcium carbonates and of different pHs. Comptes Rendus Chim. 2006, 9, 1425–1432. [Google Scholar] [CrossRef]
- Bukovský, V. The influence of light on ageing of newsprint paper. Restaurator 2000, 21, 55–76. [Google Scholar] [CrossRef]
- Lull, W.P.; Banks, P.N. Conservation Environment Guidelines for Libraries and Archives; Canadian Council of Archives: Ottawa, ON, Canada, 1995; p. 102. [Google Scholar]
- Goswami, M. Effects of Environmental factors on preservation of library documents. Int. J. Libr. Inf. Stud. 2018, 8, 42–47. [Google Scholar]
- Havermans, J.B.; Dufour, J. Photo oxidation of paper documents. Restaurator 1997, 18, 103–114. [Google Scholar]
- Phillips, G.O.; Hinojosa, O.; Arthur, J.C.; Mares, T. Photochemical initiation of free radicals in cotton cellulose. Text. Res. J. 1966, 36, 822–827. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, Z.; Qu, J.; Wang, C.; Wang, A. Evaluation of Ultraviolet Light and Hydrogen Peroxide Enhanced Ozone Oxidation Treatment for the Production of Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2020, 8, 2688–2697. [Google Scholar] [CrossRef]
- Henniges, U.; Banik, G.; Reibke, R.; Potthast, A. Studies into the Early Degradation Stages of Cellulose by Different Iron Gall Ink Components. Macromol. Symp. 2008, 262, 150–161. [Google Scholar] [CrossRef]
- Henniges, U.; Reibke, R.; Banik, G.; Huhsmann, E.; Hähner, U.; Prohaska, T.; Potthast, A. Iron gall ink-induced corrosion of cellulose: Aging, degradation and stabilization. Part 2: Application on historic sample material. Cellulose 2008, 15, 861–870. [Google Scholar] [CrossRef]
- Potthast, A.; Henniges, U.; Banik, G. Iron gall ink-induced corrosion of cellulose: Aging, degradation and stabilization. Part 1: Model paper studies. Cellulose 2008, 15, 849–859. [Google Scholar] [CrossRef]
- Kolar, J.; Strlič, M.; Budnar, M.; Malešič, J.; Šelih, V.S.; Simčič, J. Stabilisation of Corrosive Iron Gall Inks. Acta Chim. Slov. 2003, 50, 763–770. [Google Scholar]
- Šelih, V.S.; Strlič, M.; Kolar, J.; Pilhar, B. The role of transition metals in oxidative degradation of cellulose. Polym. Degrad. Stabil. 2007, 92, 1476–1481. [Google Scholar] [CrossRef]
- Malešič, J.; Kolar, J.; Strilič, M.; Polanc, S. The use of halides for stabilisation of iron gall ink containing paper: The pronounced effect of cation. e-Preserv. Sci. 2005, 2, 13–18. [Google Scholar]
- Zappalá, A.; De Stefani, C. Evaluation of the Effectiveness of Stabilization Methods. Treatments by Deacidification, Trehalose, Phytates on Iron Gall Inks. Restaurator 2005, 26, 36–43. [Google Scholar] [CrossRef]
- Spence, D.L.; Baker, A.T.; Byrne, J.P. Characterization of document paper using elemental compositions determined by inductively coupled plasma mass spectroscopy. J. Anal. Atom. Spectrom. 2000, 15, 813–819. [Google Scholar] [CrossRef]
- Hanson, V.F. Determinantion of Trace Elements in Paper by Energy Dispersive X-Ray Fluorescence. In Preservation of Paper and Textiles of Historic and Artistic Value II; Williams, J., Ed.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1981. [Google Scholar]
- Tanase, I.G.; Udristioiu, F.M.; Bunaciu, A.A.; Aboul-Enein, H.Y. Analysis of trace elements in paper using Inductively coupled plasma—Mass spectrometry (ICP—MS). Gazi Univ. J. Sci. 2012, 25, 843–851. [Google Scholar]
- Elmas, G.M. The effect of colorants on the content of heavy metals in recycled corrugated board papers. Bioresorces 2018, 12, 2690–2698. [Google Scholar]
- Elmas, G.M.; Çınar, G. Toxic metals in paper and paperboard food packagings. Bioresorces 2018, 13, 7560–7580. [Google Scholar] [CrossRef]
- Kuokkanen, T.; Nurmesniemi, H.; Pöykiö, R.; Kujala, K.; Kaakinen, J.; Kuokkanen, M. Chemical and leaching properties of paper mill sludge. Chemi Spec. Bioavailab. 2008, 20, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Kinnarinen, T.; Golmaei, M.; Jernström, E.; Häkkinen, A. Removal of hazardous trace elements from green liquor dregs by mechanical separation methods. Nord. Pulp. Pap. Res. J. 2018, 33, 420–429. [Google Scholar] [CrossRef]
- Barbusinski, K. Fenton reaction—Controversy concerning the chemistry. Ecol. Chem. Eng. S 2009, 16, 347–358. [Google Scholar]
- Yamamoto, N.; Koga, N.; Nagaoka, M. Ferryl-oxo Species produced from Fenton’s reagent via a two-step pathway: Minimun free energy path analysis. J. Phys. Chem. B 2012, 116, 14178–14182. [Google Scholar] [CrossRef]
- Duca, G. Homogeneous Catalysis with Metal Complexes: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 105–120. [Google Scholar]
- Liland, K.B.; Ese, M.H.; Lundgaard, L.E.; Kes, M. Oxidation of Cellulose. In Proceedings of the Conference Record of the 2008 IEEE International Symposium on Electrical Insulation, Vancouver, BC, Canada, 9–12 June 2008; pp. 304–307. [Google Scholar]
- Hanus, J.; Bakos, D.; Vrska, M.; Jablonsky, M.; Katuscak, S.; Holubkova, S.; Bajzíková, M.; Bukovsky, V.; Rychly, J. The Kniha Project in Slovakia. In Proceedings of the 2nd International Symposium and Workshops, Durability of Paper and Writing, Ljubljana, Slovenia, 5–7 July 2008; Volume 2, pp. 17–19. [Google Scholar]
- Buchanan, S.; Bennett, W.; Domach, M. An Evaluation of the Bookkeeper Mass Deacidification Process, Technical Evaluation Team Report for the Preservation Directorate; Library of Congress: Pittsburgh, PA, USA, 1994; pp. 1–33.
- Čížová, K.; Jablonský, M.; Briškárová, A.; Vizárová, K.; Kačík, F.; Šima, J. Kinetic Study of Artefact Paper Degradation. Assessment of Deacidification Effects by Folding Endurance. Cell Chem. Technol. 2018, 52, 99–104. [Google Scholar]
- Holubkova, S. Porovnávacie hodnotenia známych procesov konzervovanie kníh. Diploma Thesis, FCHPT STU, Radlinského 9, Bratislava, Slovakia, 2007. [Google Scholar]
- ISO 9706: 2000. Information and Documentation—Paper for Documents—Requirements for Permanence; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- Strlič, M.; Kolar, J. Aging and Stabilization of Paper; National and University Library: Ljubljana, Slovenia, 2005. [Google Scholar]
- Baty, J.W.; Maitland, C.L.; Minter, W.; Hubbe, M.A.; Jordan-Mowery, S.K. Deacidification for the conservation and preservation of paper-based works: A review. Bioresources 2010, 5, 1955–2023. [Google Scholar] [CrossRef]
- Holúbková, S.; Jablonský, M. The efficacy of newsprint paper deacidification by carbonated magnesium propylate dissolved in heptafluoropropane. Acta Chim. Slov. 2008, 1, 124–133. [Google Scholar]
- Potthast, A.; Ahn, K. Critical evaluation of approaches toward mass deacidification of paper by dispersed particles. Cellulose 2017, 24, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Hubbe, M.A.; Smith, R.D.; Zou, X.; Katuscak, S.; Potthast, A.; Ahn, K. Deacidification of acidic books and paper by means of non-aqueous dispersions of alkaline particles: A review focusing on completeness of the reaction. Bioresources 2017, 12, 4410–4477. [Google Scholar] [CrossRef] [Green Version]
- Cigala, R.M.; Crea, F.; De Stefano, C.; Lando, G.; Milea, D.; Sammartano, S. Electrochemical Study on the Stability of Phytate Complexes with Cu2+, Pb2+, Zn2+, and Ni2+: A Comparison of Different Techniques. J. Chem. Eng. Data 2010, 55, 4757–4767. [Google Scholar] [CrossRef]
- Zając, A.; Dymińska, L.; Lorenc, J.; Kaczmarek, S.M.; Leniec, G.; Ptak, M.; Hanuza, J. Spectroscopic properties and molecular structure of copper phytate complexes: IR, Raman, UV–Vis, EPR studies and DFT calculations. J. Biol. Inorg. Chem. 2019, 24, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Quiñone, D.; Veiga, N.; Torres, J.; Castiglioni, J.; Bazzicalupi, C.; Bianchi, A.; Kremer, C. Synthesis, solid-state characterization and solution studies of new phytate compounds with Cu(II) and 1,10-phenanthroline: Progress in the structural elucidation of phytate coordinating ability. Dalton. Trans. 2016, 45, 12156–12166. [Google Scholar] [CrossRef]
- Malešič, J.; Kolar, J. The influence of halide and pseudo-halide antioxidants in Fenton-like reaction systems. Acta Chim. Slov. 2006, 53, 450–456. [Google Scholar]
- Botti, L.; Mantovani, O.; Ruggiero, D. Calcium phytate in the treatment of corrosion caused by iron gall inks: Effects on paper. Restaurator 2005, 26, 44–62. [Google Scholar] [CrossRef]
- Havlinová, B.; Mináriková, J.; Hanus, J.; Jančovičová, V.; Szabóová, Z. The conservation of historical documents carrying iron gall ink by antioxidants. Restaurator 2007, 28, 112–128. [Google Scholar] [CrossRef]
- Kolar, J.; Mozir, A.; Strlic, M.; de Bruin, G.; Pihlar, B.; Steemers, T. Stabilisation of iron gall ink: Aqueous treatment with magnesium phytate. e-Preserv. Sci. 2007, 4, 19–24. [Google Scholar]
- Henniges, U.; Krämer, M.; Gille, L.; Brückle, I. Calcium Phytate as a Pretreatment for Iron-contaminated Papers Prior to Hydrogen Peroxide Bleaching. Stud. Conser. 2020, 1–8. [Google Scholar] [CrossRef]
- Keraitė, G.; Sivakova, B.; Kiuberis, J. Investigation of the impact of organic and inorganic halides on the ageing stability of paper with iron gall ink. Chemija 2017, 28, 137–147. [Google Scholar]
- Shahani, C. Accelerated Aging of Paper: Can It Really Foretell the Permanence of Paper? Preservation Research and Testing Series No. 9503; Library of Congress, Preservation Directorate: Washington, DC, USA, 1995.
- Pospíšil, J. Antioxidanty; Academia: Prague, Czech Republic, 1968; p. 274. [Google Scholar]
- Scott, G. Antioxidants. Chem. Ind. 1963, 7, 271–281. [Google Scholar]

| Process, Aging 96 °C, Up to 15 days | A | B | t(log w = 0) | Sτ(log w = 0) |
|---|---|---|---|---|
| Papersave Swiss, NCW | 3.27579 | −0.0182 | 180 | 7.9 |
| Papersave, BI | 3.31894 | −0.02214 | 150 | 6.6 |
| SoBu, Fürth | 3.11813 | −0.02937 | 106.2 | 4.7 |
| Papersave, ZFB | 3.26621 | −0.03121 | 104.7 | 4.6 |
| CSC Booksaver, IPC | 3.20072 | −0.05512 | 58.1 | 2.6 |
| CSC Booksaver, PAL * | 3.3919 | −0.18753 | 18.1 | 1.3 |
| Control 1 | 3.27024 | −0.14361 | 22.8 | 1.0 |
| Control 2 * | 2.88573 | −0.20292 | 14.2 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonský, M.; Šima, J. Stability of Alum-Containing Paper under Alkaline Conditions. Molecules 2020, 25, 5815. https://doi.org/10.3390/molecules25245815
Jablonský M, Šima J. Stability of Alum-Containing Paper under Alkaline Conditions. Molecules. 2020; 25(24):5815. https://doi.org/10.3390/molecules25245815
Chicago/Turabian StyleJablonský, Michal, and Jozef Šima. 2020. "Stability of Alum-Containing Paper under Alkaline Conditions" Molecules 25, no. 24: 5815. https://doi.org/10.3390/molecules25245815





