In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin
Abstract
1. Introduction
2. Results
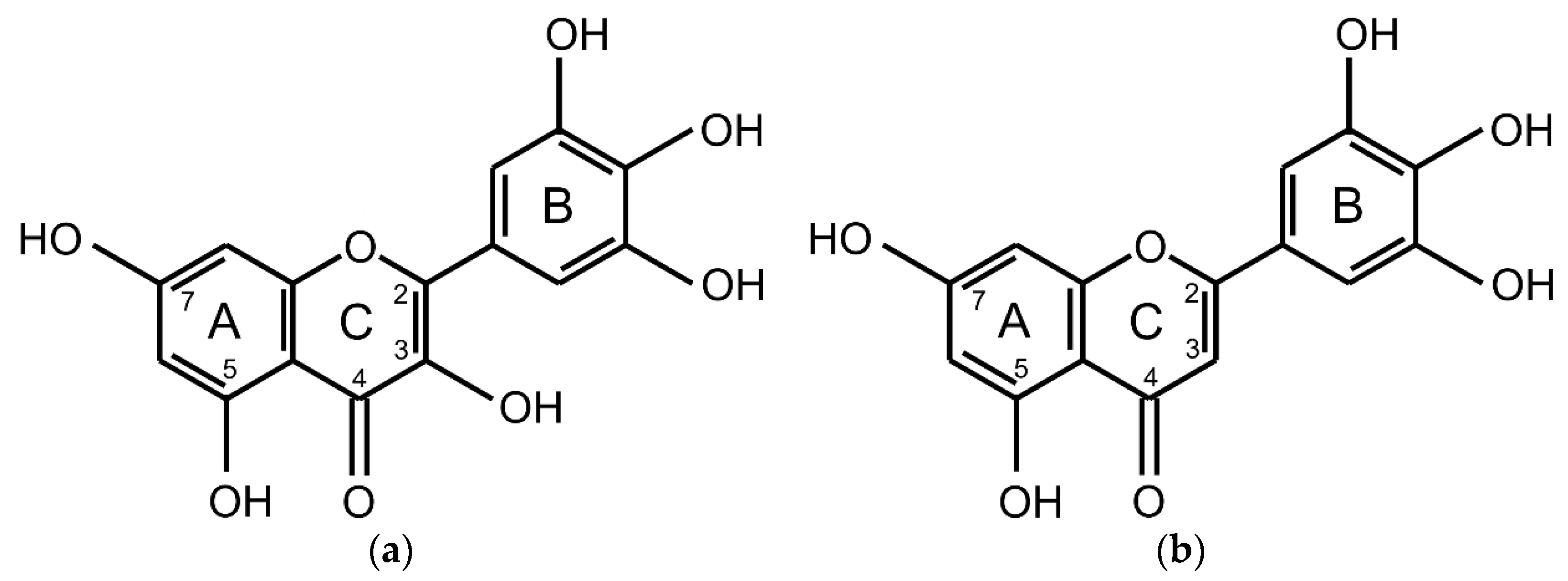
2.1. Differential Pulse Voltammetry
2.2. Deoxyribose Degradation Assay
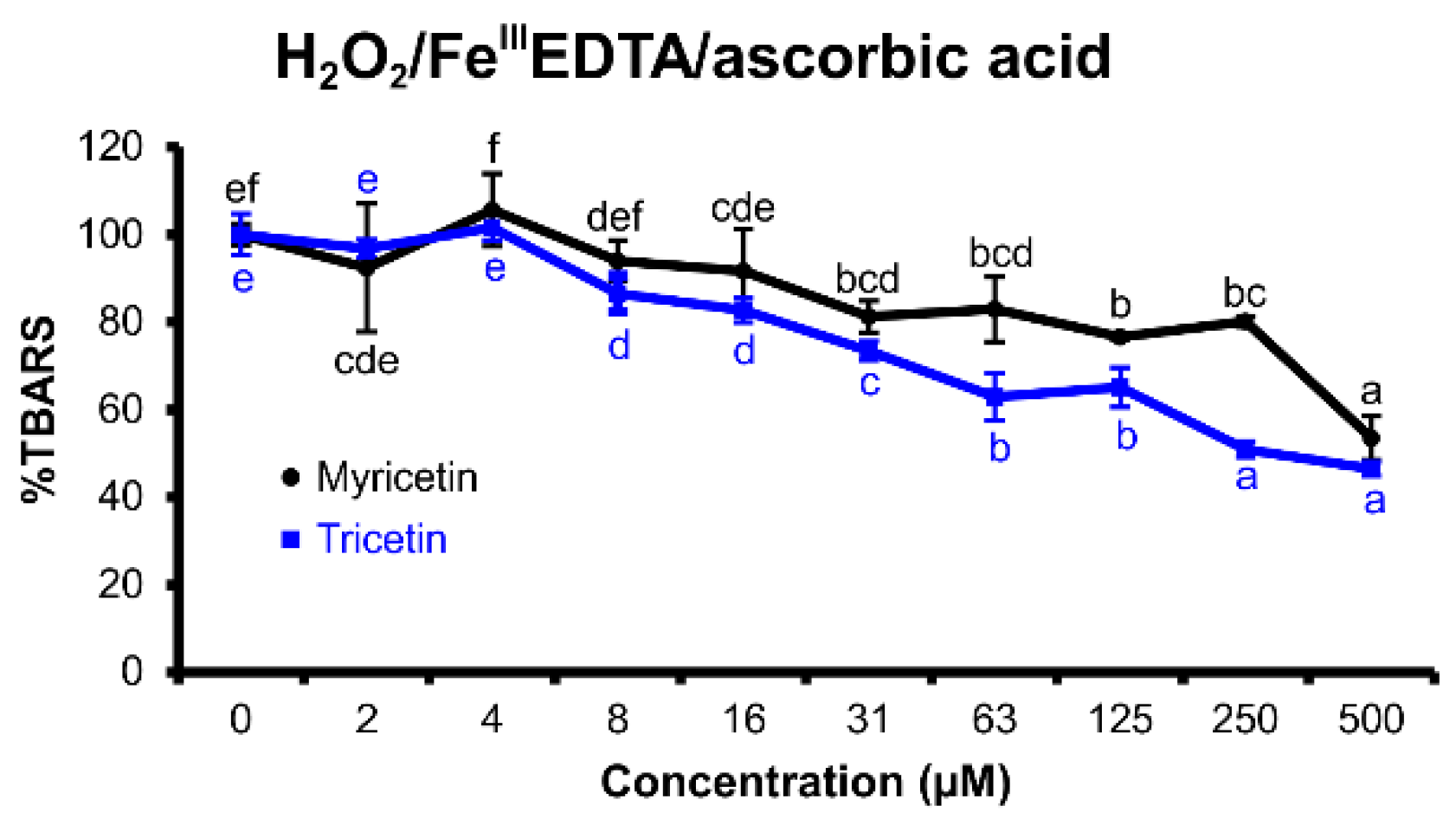
2.2.1. H2O2/FeIIIEDTA/Ascorbic Acid Variant
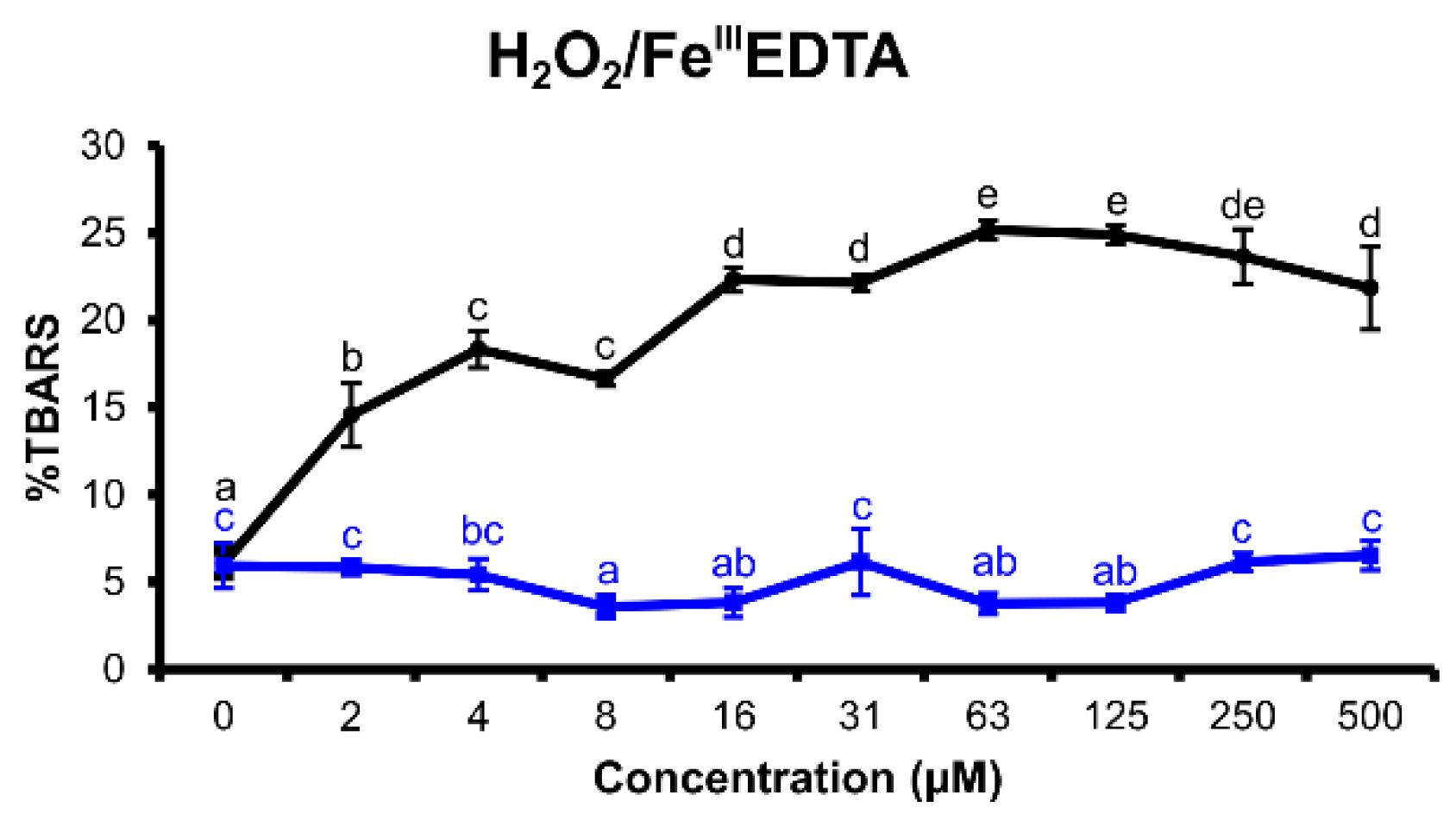
2.2.2. H2O2/FeIIIEDTA Variant
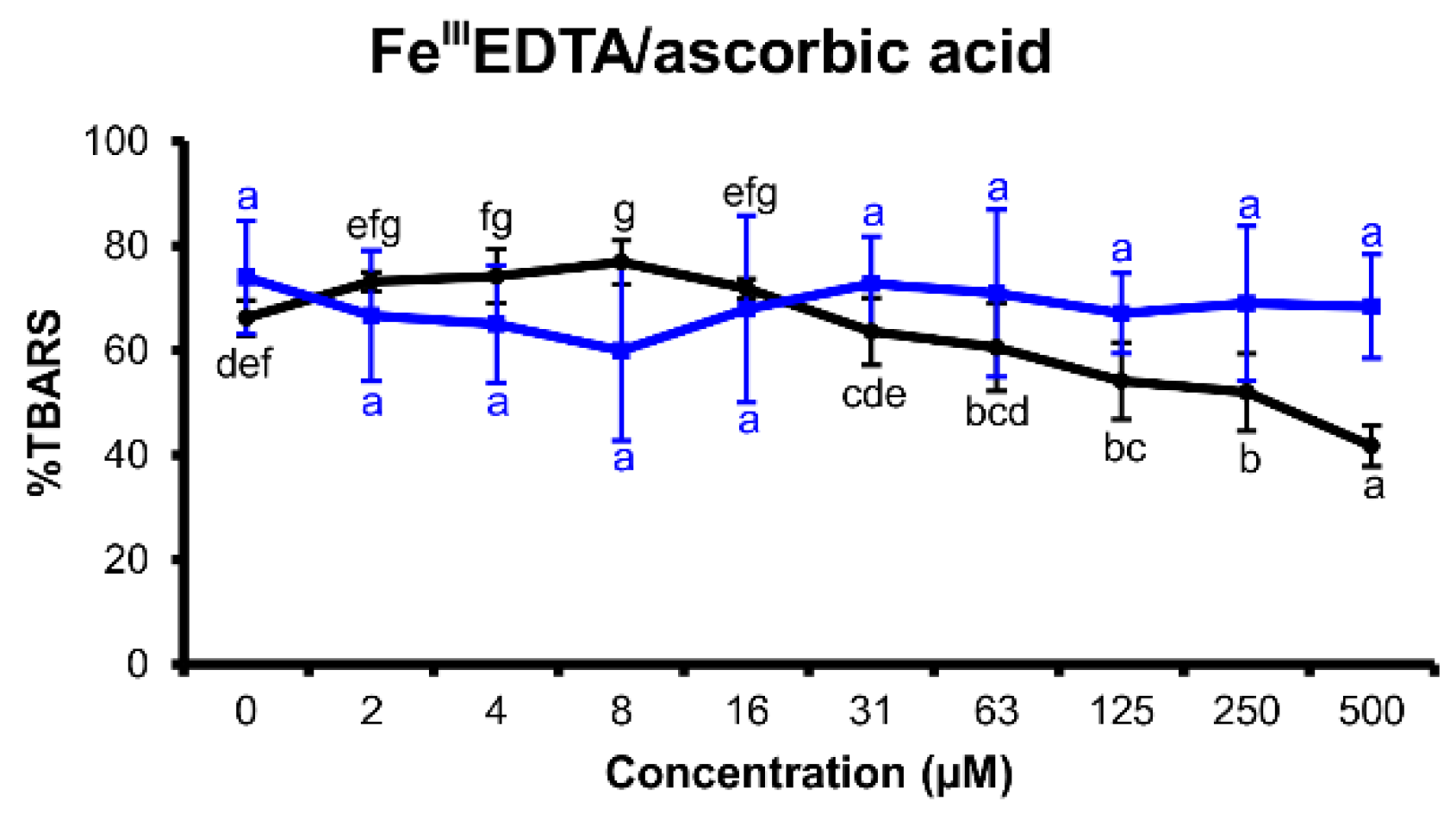
2.2.3. FeIIIEDTA/Ascorbic Acid Variant
2.2.4. FeIIIEDTA Variant
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Differential Pulse Voltammetry
4.3. Deoxyribose Degradation Assay
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Kanner, J. Polyphenols by generating H2O2, affect cell redox signaling, inhibit PTPs and activate Nrf2 axis for adaptation and cell surviving: In vitro, in vivo and human health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, F.; Bachmann, G. Low-molecular-weight metabolite systems chemistry. Front. Environ. Sci. 2015, 3, 12. [Google Scholar] [CrossRef]
- Kubicova, L.; Chobot, V. Potential of kynurenine metabolites in drug development against neurodegenerative diseases. Neural Regen. Res. 2021, 16, 308–309. [Google Scholar] [PubMed]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Fernandez, J.; Salazar, G. The impact of dietary supplementation of whole foods and polyphenols on atherosclerosis. Nutrients 2020, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Alberto, M.R.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; Isla, M.I. Chemical and functional characterization of skin, pulp and seed powder from the Argentine native fruit mistol (Ziziphus mistol). Effects of phenolic fractions on key enzymes involved in metabolic syndrome and oxidative stress. J. Funct. Food. 2017, 37, 531–540. [Google Scholar] [CrossRef]
- Giuliano, C.; Cerri, S.; Blandini, F. Potential therapeutic effects of polyphenols in Parkinson’s disease: In vivo and in vitro pre-clinical studies. Neural Regen. Res. 2021, 16, 234–241. [Google Scholar]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in neuroprotection by phytochemicals: Bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary polyphenols: A multifactorial strategy to target Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Ledda, A.; Cacchio, M.; Ruffini, I.; Ricci, A.; Ippolito, E.; Di Renzo, A.; Dugall, M.; Corsi, M.; et al. 5-Year control and treatment of edema and increased capillary filtration in venous hypertension and diabetic microangiopathy using O-(β-hydroxyethyl)-rutosides: A prospective comparative clinical registry. Angiology 2008, 59, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Ruan, Y.; Li, Z.H.; Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2850–2862. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Mendez-Marin, I.; Baget-Bernaldiz, M.; Fernéndez-Ballart, J.; Santos-Blanco, E. Review of the relationship between renal and retinal microangiopathy in diabetes mellitus patients. Curr. Diabetes Rev. 2010, 6, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile redox chemistry complicates antioxidant capacity assessment: Flavonoids as milieu-dependent anti- and pro-oxidants. Int. J. Mol. Sci. 2013, 14, 11830–11841. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Rasekh, H.R.; Salimi, A.; Mohsenifar, Z.; Pourahmad, J. Myricetin selectively induces apoptosis on cancerous hepatocytes by directly targeting their mitochondria. Basic Clin. Pharmacol. Toxicol. 2016, 119, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.Y.; Yang, F.; Dong, W.B. The Protective Effects of Myricetin against Cardiovascular Disease. J. Nutr. Sci. Vitaminol. 2019, 65, 470–476. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.F.; Hu, P.F.; Xiong, Y.; Bao, J.P.; Qian, J.; Wu, L.D. Tricetin protects rat chondrocytes against IL-1 b-induced inflammation and apoptosis. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Chow, J.M.; Lee, W.J.; Chen, H.Y.; Tan, P.; Wen, Y.C.; Lin, Y.W.; Hsiao, P.C.; Yang, S.F. Tricetin induces apoptosis of human leukemic HL-60 cells through a reactive oxygen species-mediated c-Jun N-terminal kinase activation pathway. Int. J. Mol. Sci. 2017, 18, 1667. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Lin, F.C.; Chen, P.N.; Chen, M.K.; Hsin, C.H.; Yang, S.F.; Lin, C.W. Tricetin suppresses migration and presenilin-1 expression of nasopharyngeal carcinoma through Akt/GSK-3b pathway. Am. J. Chin. Med. 2020, 48, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. A new flavone glucoside together with known ellagitannins and flavones with anti-diabetic and anti-obesity activities from the flowers of pomegranate (Punica granatum). Nat. Prod. Res. 2019, 33, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yuan, L.; Wang, W.B.; Zhang, M.J.; Wang, Q.; Li, S.M.; Zhang, L.; Hu, K. Tricetin protects against 6-OHDA-induced neurotoxicity in Parkinson’s disease model by activating Nrf2/HO-1 signaling pathway and preventing mitochondria-dependent apoptosis pathway. Toxicol. Appl. Pharm. 2019, 378, 114617. [Google Scholar] [CrossRef]
- Blasco, A.J.; Crevillen, A.G.; Gonzalez, M.C.; Escarpa, A. Direct electrochemical sensing and detection of natural antioxidants and antioxidant capacity in vitro systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Chobot, V. Simultaneous detection of pro- and antioxidative effects in the variants of the deoxyribose degradation assay. J. Agric. Food Chem. 2010, 58, 2088–2094. [Google Scholar] [CrossRef]
- Aruoma, O.I. Deoxyribose Assay for Detecting Hydroxyl Radicals. In Oxygen Radicals in Biological Systems Part C; Siels, H., Abelson, J., Melvin, S., Eds.; Academic Press Inc.: San Diego, CA, USA, 1994; Volume 233, pp. 57–66. [Google Scholar]
- Komorsky-Lovric, S.; Jovanovic, I.N. Abrasive stripping square wave voltammetry of some natural antioxidants. Int. J. Electrochem. Sci. 2016, 11, 836–842. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 471–528. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Defining the role of flavonoid structure on cottonseed oil stabilization: Study of A- and C-ring substitution. J. Am. Oil Chem. Soc. 2007, 84, 129–136. [Google Scholar] [CrossRef]
- Oliveira-Brett, A.M.; Ghica, M.E. Electrochemical oxidation of quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Kubicova, L. Pro- and antioxidant activity of three selected flavan type flavonoids: Catechin, eriodictyol and taxifolin. Int. J. Mol. Sci. 2016, 17, 1986. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Lisnund, S.; Blay, V.; Chansaenpak, K.; Pinyou, P. Voltammetric determination of gallic acid with a glassy carbon electrode modified with reduced graphene oxide. Int. J. Electrochem. Sci. 2020, 15, 7214–7227. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Ferreira, M.; Varela, H.; Torresi, R.M.; Tremiliosi, G. Electrode passivation caused by polymerization of different phenolic compounds. Electrochim. Acta 2006, 52, 434–442. [Google Scholar] [CrossRef]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R.S. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricerin. Effects on lipid-peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Jovanovic, I.N.; Milicevic, A. A model for the estimation of oxidation potentials of polyphenols. J. Mol. Liq. 2017, 241, 255–259. [Google Scholar] [CrossRef]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.P.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef]
- Grunewald, R.A. Ascorbic acid in the brain. Brain Res. Rev. 1993, 18, 123–133. [Google Scholar] [CrossRef]
- Badu-Boateng, C.; Naftalin, R.J. Ascorbate and ferritin interactions: Consequences for iron release in vitro and in vivo and implications for inflammation. Free Radic. Biol. Med. 2019, 133, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]


| Peak Number | Myricetin (mV) | Tricetin (mV) |
|---|---|---|
| 1 | −1 | 72 |
| 2 | shoulder | 175 |
| 3 | 191 | 316 |
| 4 | 513 | 575 |
| 5 | 798 | 876 |
Sample Availability: Not available. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chobot, V.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Kubicova, L. In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin. Molecules 2020, 25, 5850. https://doi.org/10.3390/molecules25245850
Chobot V, Hadacek F, Bachmann G, Weckwerth W, Kubicova L. In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin. Molecules. 2020; 25(24):5850. https://doi.org/10.3390/molecules25245850
Chicago/Turabian StyleChobot, Vladimir, Franz Hadacek, Gert Bachmann, Wolfram Weckwerth, and Lenka Kubicova. 2020. "In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin" Molecules 25, no. 24: 5850. https://doi.org/10.3390/molecules25245850
APA StyleChobot, V., Hadacek, F., Bachmann, G., Weckwerth, W., & Kubicova, L. (2020). In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin. Molecules, 25(24), 5850. https://doi.org/10.3390/molecules25245850







