Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period
Abstract
:1. Introduction
2. Results and Discussion
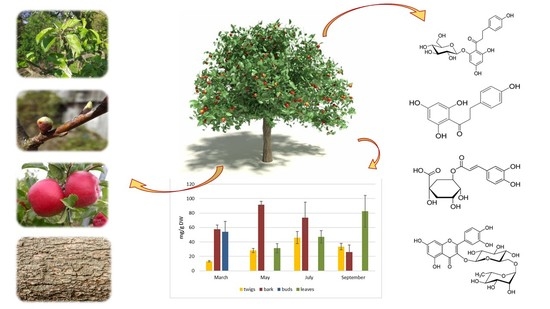
2.1. Variations of Phloridzin, Phloretin, Chlorogenic Acid and Rutin in the Individual Parts of Apple Tree Cultivars during the Vegetation Period
2.2. Content of Phloridzin and Chlorogenic Acid in Whole Apple Fruits
2.3. Statistical Comparison of the Analysed Cultivars
3. Materials and Methods
3.1. Plant Material
3.2. Preparation and Extraction of the Samples
3.3. Chromatography Analysis and the Evaluation of the Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Han, L.; Fang, C.; Zhu, R.; Peng, Q.; Li, D.; Wang, M. Inhibitory effect of phloretin on α-glucosidase: Kinetics, interaction mechanism and molecular docking. Int. J. Biol. Macromol. 2017, 95, 520–527. [Google Scholar] [CrossRef]
- Masumoto, S.; Akimoto, Y.; Oike, H.; Kobori, M. Dietary Phloridzin Reduces Blood Glucose Levels and Reverses Sglt1 Expression in the Small Intestine in Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2009, 57, 4651–4656. [Google Scholar] [CrossRef]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 39, 5299–5306. [Google Scholar] [CrossRef]
- Mudaliar, S.; Polidori, D.; Zambrowicz, B.; Henry, R.R. Sodium–Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport. Diabetes Care 2015, 38, 2344–2353. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-Y.; Liang, J.; Chen, S.-X.; Wang, B.-X.; Yuan, H.; Li, C.-T.; Wu, Y.-Y.; Wu, Y.-F.; Shi, X.-G.; Gao, J.; et al. Phloridzin alleviate colitis in mice by protecting the intestinal brush border and improving the expression of sodium glycogen transporter 1. J. Funct. Foods 2018, 45, 348–354. [Google Scholar] [CrossRef]
- Choi, C.-I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef] [Green Version]
- Blaschek, W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Medica 2017, 83, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phloridzin: A review. Diabetes Metabol. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus × domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Walia, M.; Kumar, S.; Agnihotri, V.K. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J. Sci. Food Agric. 2016, 96, 1440–1450. [Google Scholar] [CrossRef]
- Xü, K.; Lü, H.; Qü, B.; Shan, H.; Song, J. High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep. Purif. Technol. 2010, 72, 406–409. [Google Scholar] [CrossRef]
- Ma, Y.; Ban, Q.; Shi, J.; Dong, T.; Jiang, C.-Z.; Wang, Q. 1-Methylcyclopropene (1-MCP), storage time, and shelf life and temperature affect phenolic compounds and antioxidant activity of ‘Jonagold’ apple. Postharvest Biol. Technol. 2019, 150, 71–79. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.S.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Aliomrani, M.; Sepand, M.R.; Mirzaei, H.R.; Kazemi, A.R.; Nekoonam, S.; Sabzevari, O. Effects of phloretin on oxidative and inflammatory reaction in rat model of cecal ligation and puncture induced sepsis. DARU J. Pharm. Sci. 2016, 24, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Kumar, S.; Rana, A.; Sharma, V.; Katoch, P.; Padwad, Y.; Bhushan, S. Phenolic constituents from apple tree leaves and their in vitro biological activity. Ind. Crop. Prod. 2016, 90, 118–125. [Google Scholar] [CrossRef]
- Kim, J.; Durai, P.; Jeon, D.; Jung, I.D.; Lee, S.J.; Park, Y.-M.; Kim, Y. Phloretin as a Potent Natural TLR2/1 Inhibitor Suppresses TLR2-Induced Inflammation. Nutrients 2018, 10, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondonno, N.; Bondonno, C.P.; Ward, N.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Bakr, A.G.; Osman, A.T. Protective effects of phloridzin against methotrexate-induced liver toxicity in rats. Biomed. Pharmacother. 2017, 95, 529–535. [Google Scholar] [CrossRef]
- De Oliveira, M.R. Phloretin-induced cytoprotective effects on mammalian cells: A mechanistic view and future directions. BioFactors 2016, 42, 13–40. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure–Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Zhang, Y.; Zhang, J.; Wang, J.; Kang, W. Coagulatory active constituents of Malus pumila Mill. flowers. Chem. Cent. J. 2018, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Liu, J.; Zhang, C.; Liu, J.; Jiao, Z. Enzymatic preparation and antioxidant activity of the phloridzin oxidation product. J. Food Biochem. 2017, 42, e12475. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Miosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Li, P.; Gong, X.; Ma, F. Genome-wide identification of glycosyltransferases converting phloretin to phloridzin in Malus species. Plant Sci. 2017, 265, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Martens, S.; Gang, D.R. Biosynthetic Pathway and Metabolic Engineering of Plant Dihydrochalcones. J. Agric. Food Chem. 2018, 66, 2273–2280. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on its Anti-Obesity Property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef]
- Kalinowska, M. Kwasy chlorogenowe w produktach naturalnych oraz ich właściwości antyutleniające i przeciwdrobnoustrojowe. Przemysl Chem. 2017, 1, 77–82. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A.; van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A.; van der Plas, L.H.; van der Krol, A.R. Flavonoid and chlorogenic acid changes in skin of ‘Elstar’ and ‘Jonagold’ apples during development and ripening. Sci. Hortic. 2001, 90, 69–83. [Google Scholar] [CrossRef]
- Vondráková, Z.; Trávníčková, A.; Malbeck, J.; Haisel, D.; Černý, R.; Cvikrová, M. The effect of storage conditions on the carotenoid and phenolic acid contents of selected apple cultivars. Eur. Food Res. Technol. 2020, 246, 1783–1794. [Google Scholar] [CrossRef]
- Parvaneh, T.; Abedi, B.; Davarynejad, G.H.; Moghadam, E.G. Enzyme activity, phenolic and flavonoid compounds in leaves of Iranian red flesh apple cultivars grown on different rootstocks. Sci. Hortic. 2019, 246, 862–870. [Google Scholar] [CrossRef]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of Rutin from Buckwheat (Fagopyrum esculentum Moench) Seeds and Determination by Capillary Electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef] [PubMed]
- Shafi, W.; Mansoor, S.; Jan, S.; Singh, D.B.; Kazi, M.; Raish, M.; AlWadei, M.; Mir, J.I.; Ahmad, P. Variability in Catechin and Rutin Contents and Their Antioxidant Potential in Diverse Apple Genotypes. Molecules 2019, 24, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Malagutti, A.R.; Zuin, V.G.; Cavalheiro, É.T.G.; Mazo, L.H. Determination of Rutin in Green Tea Infusions Using Square-Wave Voltammetry with a Rigid Carbon-Polyurethane Composite Electrode. Electroanalysis 2006, 18, 1028–1034. [Google Scholar] [CrossRef]
- Morling, J.R.; Yeoh, S.E.; Kolbach, D.N. Rutosides for treatment of post-thrombotic syndrome. Cochrane Database Syst. Rev. 2015, 9, CD005625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, B.L.; Arro, J.; Zhong, G.-Y.; Brown, S.K. Linkage and association analysis of dihydrochalcones phloridzin, sieboldin, and trilobatin in Malus. Tree Genet. Genomes 2018, 14, 91. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Li, L.; Gao, Y.; Wang, F.; Zhang, Y.; Fan, Y.; Wu, C. Protective effect of apple polyphenols on chronic ethanol exposure-induced neural injury in rats. Chem. Interact. 2020, 326, 109113. [Google Scholar] [CrossRef] [PubMed]
- Wandjou, J.G.N.; Lancioni, L.; Barbalace, M.C.; Hrelia, S.; Papa, F.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Beghelli, D.; et al. Comprehensive characterization of phytochemicals and biological activities of the Italian ancient apple ‘Mela Rosa dei Monti Sibillini’. Food Res. Int. 2020, 137, 109422. [Google Scholar] [CrossRef]
- Tschida, A.; Stadlbauer, V.; Schwarzinger, B.; Maier, M.; Pitsch, J.; Stübl, F.; Müller, U.; Lanzerstorfer, P.; Himmelsbach, M.; Wruss, J.; et al. Nutrients, bioactive compounds, and minerals in the juices of 16 varieties of apple (Malus domestica) harvested in Austria: A four-year study investigating putative correlations with weather conditions during ripening. Food Chem. 2021, 338, 128065. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viskelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic Composition and Antioxidant Activity of Malus domestica Leaves. Sci. World J. 2014, 2014, 306217. [Google Scholar] [CrossRef] [Green Version]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild apple (Malus spp.) by-products as a source of phenolic compounds and vitamin C for food applications. Food Biosci. 2020, 38, 100744. [Google Scholar] [CrossRef]
- Sowa, A.; Zgórka, G.; Szykuła, A.; Franiczek, R.; Żbikowska, B.; Gamian, A.; Sroka, Z. Analysis of Polyphenolic Compounds in Extracts from Leaves of Some Malus domestica Cultivars: Antiradical and Antimicrobial Analysis of These Extracts. BioMed Res. Int. 2016, 2016, 6705431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, F.; He, C.; Yu, Y.; Wang, M. Optimization of Ultrasonic-Assisted Aqueous Two-Phase Extraction of Phloridzin from Malus Micromalus Makino with Ethanol/Ammonia Sulfate System. J. Food Sci. 2017, 82, 2944–2953. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zhang, D.; Wang, Y.-Q.; Li, P.; Ma, F.-W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Bílková, A.; Baďurová, K.; Svobodová, P.; Vávra, R.; Jakubec, P.; Chocholouš, P.; Švec, F.; Sklenářová, H. Content of major phenolic compounds in apples: Benefits of ultra-low oxygen conditions in long-term storage. J. Food Compos. Anal. 2020, 92, 103587. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules 2021, 26, 3816. https://doi.org/10.3390/molecules26133816
Táborský J, Sus J, Lachman J, Šebková B, Adamcová A, Šatínský D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules. 2021; 26(13):3816. https://doi.org/10.3390/molecules26133816
Chicago/Turabian StyleTáborský, Jan, Josef Sus, Jaromír Lachman, Barbora Šebková, Anežka Adamcová, and Dalibor Šatínský. 2021. "Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period" Molecules 26, no. 13: 3816. https://doi.org/10.3390/molecules26133816
APA StyleTáborský, J., Sus, J., Lachman, J., Šebková, B., Adamcová, A., & Šatínský, D. (2021). Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules, 26(13), 3816. https://doi.org/10.3390/molecules26133816