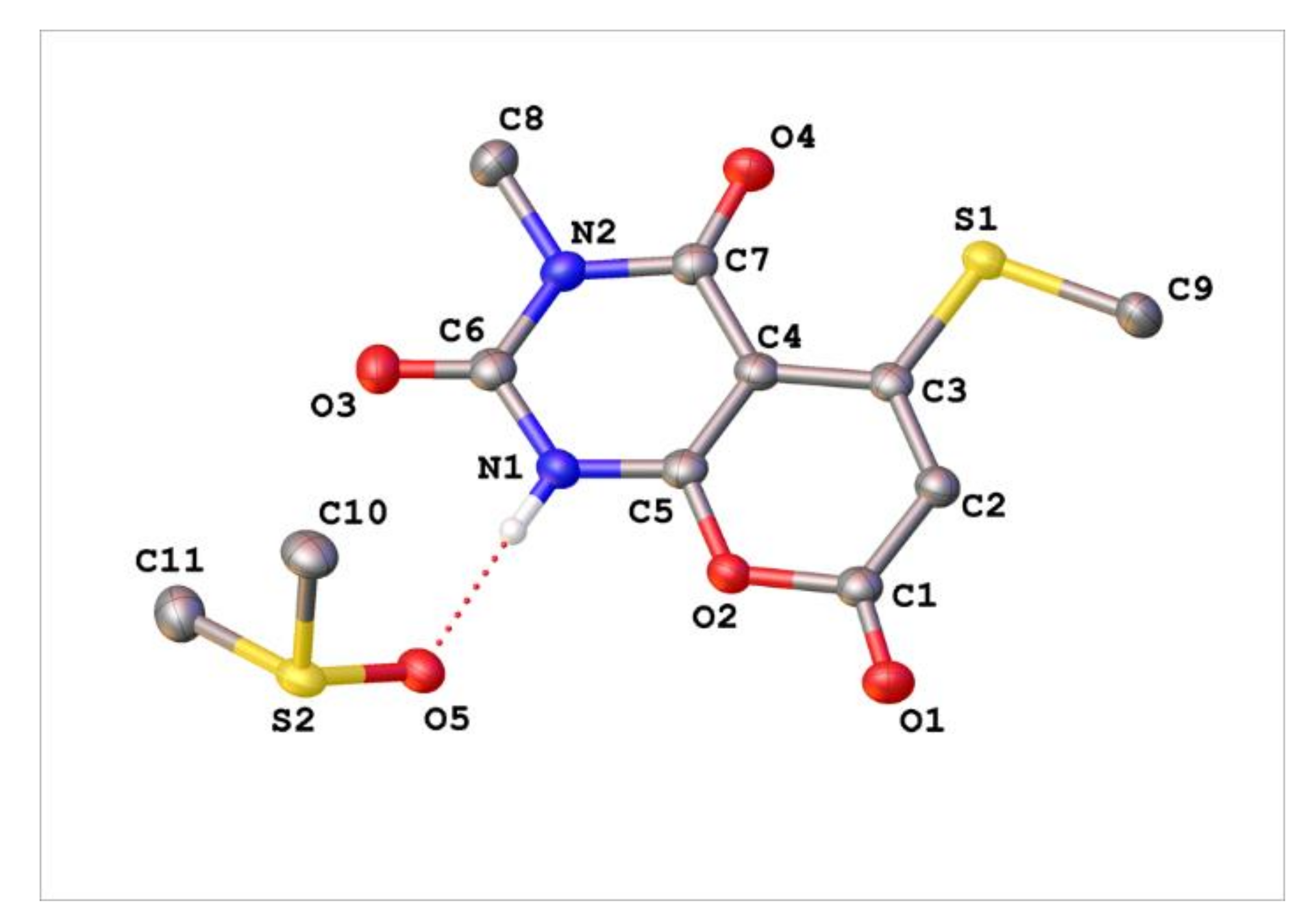
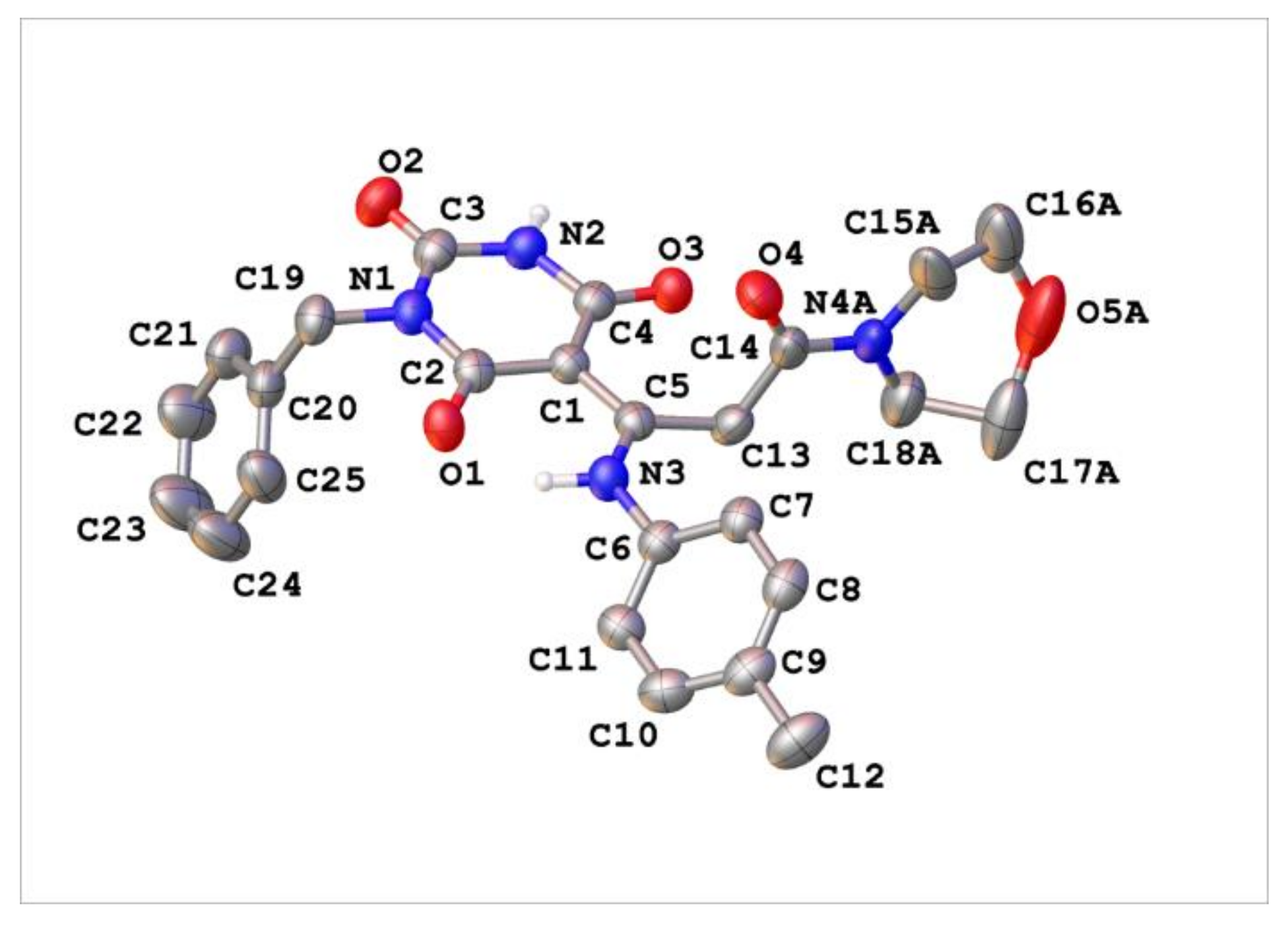
Appendix C. Synthesis
Appendix C.1. General Procedure A
Pyrano[2,3-d]pyrimidinones2a–e Barbituric acid 3a–e (4 mmol) and 5-[bis(methylthio)methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione 4a–e (4 mmol) were heated at reflux in pyridine for 1 h. The condenser was connected to a Dreschel bottle containing bleach solution to destroy methanethiol. The resulting precipitate was filtered off and washed with EtOH to give 2a–e.
3-Benzyl-5-(methylthio)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (2a).
Yield: 60%; yellow solid, melting point 270–280 °C; 1H-NMR (300 MHz, DMSO-d6): 10.45 (s, 1H, NH), 7.30 (m, 5H, ArH), 5.68 (s, 1H, C=CH), 4.97 (s, 2H, ArCH2), 2.38 (s, 3H, SCH3); 13C-NMR (300 MHz, DMSO-d6): 168.8, 162.5, 155.6 and 153.3 (C=O), 149.2, 139.6, 137.2, 128.7, 127.9 and 127.4 (Ar), 96.6 (C=C), 43.6 (CH2), 15.2 (SCH3); LC-MS (ESI-ve) Found [M − H]− 314.95 (C15H11N2O4S requires 315.04).
3-Ethyl-5-(methylthio)-1H-pyrano[2,3-d]pyrimidine-2,4,7(3H)-trione (2b). Yield: 69%; pale orange solid, melting point 301–303 °C; 1H-NMR (300 MHz, DMSO-d6): 13.10 (s, 1H, NH), 5.65 (s, 1H, C=CH), 3.79 (q, 2H, J = 7.0 Hz, CH2CH3), 2.38 (s, 3H, SCH3), 1.10 (t, 3H, J = 7.0 Hz, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 162.5, 160.1, 158.6 and 155.6 (C=O), 96.5 and 91.2 (C=C), 35.7 (CH2CH3), 15.2 (SCH3), 13.7 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 252.95 (C10H9N2O4S requires 253.03).
3-Methyl-5-(methylthio)-1H-pyrano[2,3-d]pyrimidine-2,4,7(3H)-trione (2c).
Yield: 62%; yellow crystals, melting point 286–288 °C; 1H-NMR (300 MHz, DMSO-d6): 8.65 (s, 1H, NH), 5.62 (s, 1H, C=CH) 3.13 (s, 3H, NCH3), 2.38 (s, 3H, SCH3); 13C-NMR (300 MHz, DMSO-d6): 162.5, 160.4, 158.6 and 155.6 (C=O), 138.7, 125.1, 96.4 and 91.0 (C=C), 27.4 (NCH3), 15.2 (SCH3); LC-MS (ESI-ve) Found [M – H]− 238.95 (C9H7N2O4S requires 239.01).
3-(4-Fluorobenzyl)-5-(methylthio)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (2d).
Yield: 61%; yellow-orange solid, melting point 204–206 °C; 1H-NMR (400 MHz, DMSO-d6): 8.66 (s, 1H, NH), 7.34-7.11 (m, 4H, ArH), 5.66 (s, 1H, C=CH) 4.94 (s, 2H, ArCH2), 2.37 (s, 3H, SCH3); 13C-NMR (400 MHz, DMSO-d6): 163.0, 162.5, 160.3 and 155.6 (C=O), 149.2, 133.4, 130.3 and 115.4 (Ar), 96.6 and 91.3 (C=C), 43.0 (CH2), 15.2 (SCH3); LC-MS (ESI-ve) Found [M − H]− 332.90 (C15H10FN2O4S requires 333.03).
1,3-Dimethyl-5-(methylthio)-1H-pyrano[2,3-d]pyrimidine-2,4,7(3H)-trione (2e).
Yield: 67%; yellow crystals, melting point 275–278 °C; 1H-NMR (400 MHz, DMSO-d6): 5.56 (s, 1H,C=CH), 3.26, 3.46 (2s, 6H, NCH3), 2.31 (s, 3H, SCH3); 13C-NMR (400 MHz, DMSO-d6): 164.0 (C=O), 96.5 (C=C), and 29.8 28.5 (NCH3), 16.0 (SCH3). LC-MS (ESI + ve) Found [M + H]+ 254.90 (C10H11N2O4S requires 255.04).
Appendix C.2. General Procedure B
3-Amino-3-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propanamides5a–d. A solution of 1,3-dimethyl-5-(methylthio)-1H-pyrano[2,3-d]pyrimidine-2,4,7(3H)-trione 2e (2 mmol) and benzylamine (4 mmol) was heated at reflux in EtOH (10 mL) for 1 h. The EtOH was removed, and the residue was washed with diethyl ether to give 5(a–d).
N-benzyl-3-(benzylamino)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propenamide (5a).
Yield: 79%; white crystals, melting point 186–188 °C; 1H-NMR (400 MHz, CDCl3): 13.02 (s, 1H, NH), 7.93 (s, 1H, NH), 7.33–7.22 (m, 10H, ArH), 5.04 (s, 2H, NCH2Ar), 4.36 (s, 2H, CONCH2Ar), 4.17 (s, 2H, COCH2), 3.24 and 3.20 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 169.0, 166.6, 166.3 and 164.0 (C=O), 151.0, 137.9, 135.4, 129.1, 128.6, 128.2, 127.4 and 127.3 (Ar), 90.5 (C=C), 48.2, 43.7 and 37.8 (CH2), 28.2 and 27.8 (CH3); LC-MS (ESI-ve) Found [M − H]− 419.15 (C23H23N4O4 requires 419.17).
N-(4-chloro-3-(trifluoromethyl)benzyl)-3-((4-chloro-3-(trifluoromethyl)benzyl)amino)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propenamide (5b).
Yield: 78%; white crystals, melting point 209–211 °C; 1H-NMR (400 MHz, CDCl3): 13.08 (s, 1H, NH), 8.16 (s, 1H, NH), 7.58–7.31 (m, 6H, ArH), 5.07 (s, 2H, NCH2Ar), 4.37 (s, 2H, CONCH2Ar), 4.14 (s, 2H, COCH2), 3.26 and 3.24 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 168.7, 166.8, 166.4 and 164.0 (C=O), 150.8, 137.2, 134.6, 132.3, 131.8, 131.7, 126.8, 126.7, 126.2 and 126.1 (Ar), 96.2 (C=C), 47.2, 42.7 and 37.7 (CH2), 28.3 and 27.9 (CH3); LC-MS (ESI-ve) Found [M − H]− 623.00 (C25H1935Cl2F6N4O4 requires 623.07).
3-(1,3-Dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(3-(trifluoromethyl)benzyl)-3-((3-(trifluoromethyl)benzyl)amino)propenamide (5c).
Yield: 55%; white crystals, melting point 148–149 °C; 1H-NMR (400 MHz, CDCl3): 13.10 (s, 1H, NH), 8.11 (s, 1H, NH), 7.54–7.38 (m, 8H, ArH), 5.11 (s, 2H, NCH2Ar), 4.43 (s, 2H, CONCH2Ar), 4.18 (s, 2H, COCH2), 3.26 and 3.23 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 168.9, 166.7, 166.4 and 164.1 (C=O), 150.8, 139.0, 136.5, 130.6, 129.8, 129.1, 125.2, 124.3, 124.2 and 123.8 (Ar), 90.8 (C=C), 47.7, 43.1 and 37.7 (CH2), 28.2 and 27.9 (CH3); LC-MS (ESI-ve) Found [M − H]− 555.05 (C25H21F6N4O4 requires 555.15), HRMS (ESI + ve) Found [M + H]+ 557.1234 (C25H23F6N4O4 requires 557.1623).
N-(4-chlorobenzyl)-3-((4-chlorobenzyl)amino)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propenamide (5d).
Yield: 80%; white crystals, melting point 216–217 °C; 1H-NMR (400 MHz, CDCl3): 12.99 (s, 1H, NH), 8.00 (s, 1H, NH), 7.28–7.10 (m, 8H, ArH), 5.00 (s, 2H, NCH2Ar), 4.31 (s, 2H, CONCH2Ar), 4.13 (s, 2H, COCH2), 3.25 and 3.22 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 168.8, 166.6, 166.3 and 164.0 (C=O), 150.9, 136.4, 134.3, 133.8, 133.2, 129.4, 128.9, 128.8 and 128.7 (Ar), 90.7 (C=C), 47.6, 43.1 and 37.8 (CH2), 28.3 and 27.9 (CH3); LC-MS (ESI-ve) Found [M − H]− 487.10 (C23H21Cl2N4O4 requires 487.09).
(E/Z)-3-(1-(4-fluorobenzyl)-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-methoxyphenyl)-3-((4-methoxyphenyl)amino)propanamide5e.
A solution of 3-(4-fluorobenzyl)-5-(methylthio)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione 2d (1 mmol) and 4-methoxyaniline (1 mmol) was heated at 150 °C in DMSO for 2 h. After cooling to RT, the resulting precipitate was filtered off and washed with diethyl ether to give 5e.
Yield: 82%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 13.99 and 13.72 (s, 1H, NH, E,Z), 11.33 and 10.96 (s, 1H, NH, E,Z), 9.93 and 9.91 (s, 1H, NH, E,Z), 7.39–6.85 (m, 12H, ArH), 4.98 and 4.90 (s, 2H, ArCH2, E,Z), 4.04 and 4.01 (s, 2H, COCH2, E,Z), 3.77 and 3.72 (s, 6H, 2 × OCH3); 13C-NMR (300 MHz, DMSO-d6): 170.1, 169.5, 167.2, 165.7, 163.1 and 162.8 (C=O), 159.4, 155.5, 150.3, 132.6, 130.1, 130.0, 129.8, 129.7, 128.8, 127.6, 121.0, 120.9, 115.7, 115.5, 115.4, 115.2 and 114.3 (Ar), 91.0 (C=C), 55.9 and 55.6 (OCH3), 42.3 (CH2); LC-MS (ESI-ve) Found [M − H]− 531.15 (C28H24FN4O6 requires 531.17).
Appendix C.3.General Procedure C
3-Amino-3-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propanamides5f–l. A solution of 1,3-dimethyl-5-(methylsulfinyl)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione 6 (2 mmol) and aniline (4 mmol) was heated at reflux in EtOH (10 mL) for 1 h. The ethanol was removed and the residue was washed with diethyl ether to give 5f–l.
3-((4-Chloro-3-(trifluoromethyl)phenyl)amino)-N-(3-chloro-4-(trifluoromethyl)phenyl)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propenamide (5f).
Yield: 61%; white crystals, melting point 212–215 °C; 1H-NMR (300 MHz, DMSO-d6): 14.02 (s, 1H, NH), 10.61 (s, 1H, NH), 8.12-7.63 (m, 6H, ArH), 4.12 (s, 2H, COCH2), 3.24 and 3.14 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 168.7, 167.2, 166.2 and 162.4 (C=O), 150.9, 138.8, 136.0, 133.4, 132.6, 132.3, 126.4, 124.1 and 117.8 (Ar), 95.9 and 91.8 (C=C), 28.2 and 28.0 (CH3); LC-MS (ESI-ve) Found [M − H]− 594.95 (C23H1535Cl2F6N4O4 requires 595.04).
N-3-((trifluoromethyl)phenyl)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((3-(trifluoromethyl)phenyl)amino)propenamide (5g).
Yield: 72%; white crystals, melting point 215–218 °C; 1H-NMR (400 MHz, CDCl3): 14.53 (s, 1H, NH), 8.96 (s, 1H, NH), 7.74–7.26 (m, 8H, ArH), 4.06 (s, 2H, COCH2), 3.31 and 3.27 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 167.9, 166.6, 164.9 and 163.8 (C=O), 150.7, 138.2, 136.4, 130.4, 129.5, 125.5, 123.6, 122.7, 121.0 and 116.5 (Ar), 91.8 (C=C), 39.8 (CH2), 28.4 and 28.0 (CH3); LC-MS (ESI-ve) Found [M − H]− 527.00 (C23H17F6N4O4 requires 527.12).
N-(4-chlorophenyl)-3-((4-chlorophenyl)amino)-3-(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propenamide (5h).
Yield: 60%; white solid, melting point 273–276 °C; 1H-NMR (400 MHz, CDCl3): 14.36 (s, 1H, NH), 8.79 (s, 1H, NH), 7.39-7.15 (m, 8H, ArH), 4.06 (s, 2H, COCH2), 3.30 and 3.26 (s, 6H, 2 × NCH3); 13C-NMR (400 MHz, CDCl3): 168.1, 166.6, 164.8 and 163.7 (C=O), 150.8, 136.3, 134.8, 134.2, 129.8, 128.9, 128.0 and 121.0 (Ar), 91.5 (C=C), 39.7 (CH2), 28.3 and 28.0 (CH3); LC-MS (-ve) Found [M − H]− 459.05 (C21H1735Cl2N4O4 requires 459.06).
3-(1,3-Dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-phenoxyphenyl)-3-((4-phenoxyphenyl)amino)propenamide (5i).
Yield: 75%; white crystals, melting point 207–210 °C; 1H-NMR (400 MHz, CDCl3): 14.28 (s, 1H, NH), 8.63 (s, 1H, NH), 7.37–6.98 (m, 18H, ArH), 4.12 (s, 2H, COCH2), 3.30 and 3.27 (s, 6H, 2 × NCH3); 13C-NMR (400 MHz, CDCl3): 168.5, 166.6, 164.9 and 163.7 (C=O), 157.9, 156.0, 153.5, 150.9, 133.4, 129.7, 128.1, 124.3, 123.0, 121.5, 119.7 and 118.8 (Ar), 96.2 and 91.0 (C=C), 39.6 (CH2), 28.3 and 27.9 (NCH3); LC-MS (ESI-ve) Found [M − H]− 575.10 (C33H27N4O6 requires 575.19).
3-(1,3-Dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-methoxyphenyl)-3-((4-methoxyphenyl)amino)propenamide (5j).
Yield: 77%; white solid, no clear melting point (decomposed); 1H-NMR (400 MHz, CDCl3): 14.19 (s, 1H, NH), 8.50 (s, 1H, NH), 7.28–6.71 (m, 8H, ArH), 4.07 (s, 2H, COCH2), 3.76 and 3.69 (s, 6H, 2 × OCH3), 3.29 and 3.25 (s, 6H, NCH3); 13C-NMR (400 MHz, CDCl3): 168.7, 166.5, 164.7 and 163.6 (C=O), 159.6, 156.4, 151.0, 131.1, 128.4, 127.8, 121.5, 114.7 and 114.0 (Ar), 96.2 and 91.0 (C=C), 55.5 (OCH3), 39.6 (CH2), 28.2 and 27.9 (NCH3); LC-MS (ESI-ve) Found [M − H]− 451.15 (C23H23N4O6 requires 451.16).
3-(1,3-Dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(p-tolyl)-3-(p-tolylamino)propenamide (5k).
Yield: 64%; white crystals, melting point decomposed; 1H-NMR (400 MHz, CDCl3): 14.26 (s, 1H, NH), 8.49 (s, 1H, NH), 7.27–6.98 (m, 8H, ArH), 4.08 (s, 2H, COCH2), 3.29 and 3.25 (s, 6H, 2 × NCH3), 2.31 and 2.21 (s, 6H, ArCH3); 13C-NMR (400 MHz, CDCl3): 168.5, 166.6, 164.8 and 163.6 (C=O), 151.0, 138.7, 135.3 133.9, 133.2, 130.2, 129.4, 126.3 and 119.8 (Ar), 96.2 and 91.1 (C=C), 39.7 (CH2), 28.2 and 27.9 (NCH3), 21.1 and 20.9 (ArCH3); LC-MS (ESI-ve) Found [M − H]− 419.15 (C23H23N4O4 requires 419.17).
3-(1,3-Dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-fluorophenyl)-3-((4-fluorophenyl)amino)propenamide (5l).
Yield: 80%; white solid, melting point 249-252 °C; 1H-NMR (300 MHz, CDCl3): 14.303 (s, 1H, NH), 8.69 (s, 1H, NH), 7.33–6.90 (m, 8H, ArH), 4.06 (s, 2H, COCH2), 3.30 and 3.27 (s, 6H, 2 × NCH3); 13C-NMR (300 MHz, CDCl3): 168.4, 166.6, 164.0 and 163.6 (C=O), 150.8, 128.6, 121.6, 116.8, 116.4, 115.7 and 115.5 (Ar), 39.5 (CH2), 28.3 and 27.9 (CH3); LC-MS (ESI-ve) Found [M − H]− 427.05 (C21H17F2N4O4 requires 427.12).
3-Dimethyl-5-(methylsulfinyl)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione6. To a solution of 2e (2.6 g, 10 mmol) in DCM (20 mL), m-chloroperbenzoic acid (1.73 g, 10 mmol) was added at RT. The mixture was stirred for 2 h. The DCM was removed, and the residual solid was briefly stirred in diethyl ether (20 mL). The mixture was filtered, and the solid collected was dried under a vacuum. The crude product was recrystallized from DCM-Et2O.
Yield: 58%; pale yellow crystals, melting point 188–190 °C; 1H-NMR (400 MHz, DMSO-d6): 6.66 (s, 1H, C=CH), 3.31, 3.53 (2s, 6H, 2 × NCH3), 2.78 (s, 3H, S(O)CH3); 13C-NMR (400 MHz, DMSO-d6): 167.6 and 154.9 (C=O), 104.0 (C=C), 44.8 (S(O)CH3), 30.2 and 31.6 (NCH3). LC-MS (ESI + ve) Found [M + H]+ 270.90 (C10H11N2O5S requires 271.03).
Appendix C.4.General Procedure D
(E/Z)-3-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(methylthio)propanoates7a–b. To a solution of pyrano[2,3-d]pyrimidine 2a–b (2 mmol) in MeOH (5 mL) was added sodium methoxide (2 mmol, 25% solution in MeOH), and the resulting solution was heated at reflux overnight. The MeOH was removed, and H2O (25 mL) was added. The solution was neutralized with aq. 1 M HCl. The resulting precipitate was filtered off and washed with water to give 7a–b.
(E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(methylthio)propanoate (7a).
Yield: 71%; pale yellow solid, melting point 172–175 °C; 1H-NMR (400 MHz, CDCl3): 8.75 and 8.67 (s, 1H, NH, E,Z), 7.38–7.19 (m, 5H, ArH), 5.00 and 5.01 (s, 2H, ArCH2, E,Z), 4.430 and 4.40 (s, 2H, COCH2, E,Z), 3.68 and 3.65 (s, 3H, OCH3), 2.38 (s, 3H, SCH3); 13C-NMR (400 MHz, CDCl3): 182.8, 167.8, 162.9 and 160.2 (C=O), 149.4, 136.5, 129.2, 129.0, 128.6, 128.4, 128.1 and 127.6 (Ar), 112.7 (C=CSCH3), 60.0 (C=CSCH3), 52.8 (OCH3), 44.1 (ArCH2), 39.6 (COCH2), 17.1 (SCH3); LC-MS (ESI-ve) Found [M − H]− 347.00 (C16H15N2O5S requires 347.07), HRMS (ESI + ve) Found [M + Na]+ 371.07 (C16H16N2O5SNa requires 371.07).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(methylthio)propanoate (7b).
Yield: 77%; orange solid, melting point 189–191 °C; 1H-NMR (300 MHz, CDCl3): 8.55 and 8.37 (s, 1H, NH, E,Z), 4.47 and 4.30 (s, 2H, COCH2), 3.90 and 3.78 (q, J = 7.1 Hz, 2H, CH3CH2, E,Z), 3.71 (s, 3H, OCH3), 2.41 (s, 3H, SCH3), 1.28 and 1.17 (t, J = 7.0 Hz, 3H, CH3CH2); 13C-NMR (300 MHz, CDCl3): 182.3, 167.9, 160.1 and 160.1 (C=O), 52.8 (COOCH3), 39.5 (COCH2), 36.3 (CH2CH3), 17.1 (SCH3), 13.4 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 284.90 (C11H13N2O5S requires 285.05).
Appendix C.5. General Procedure E
Methyl (E/Z)-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(amino)propanoates8a–f. A solution of (E,Z)-3-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(methylthio)propanoate 7a–b (1 mmol) and amine (2 mmol) in EtOH (15 mL) was heated at reflux overnight. After cooling to RT, the precipitate was filtered to give 8a–f.
Methyl (E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(phenylamino)propanoate (8a).
Yield: 61%; off-white solid, melting point 162–165 °C; 1H-NMR (300 MHz, CDCl3): 13.93 and 13.82 (s, 1H, NH, E,Z), 8.52 and 8.40 (s, 1H, NH, E,Z), 7.25–7.12 (m, 10H, ArH), 5.03 and 5.00 (s, 2H, ArCH2, E,Z), 3.90 and 3.80 (s, 2H, COCH2, E,Z), 3.66 and 3.63 (s, 3H, OMe); 13C-NMR (300 MHz, CDCl3): 168.2, 167.6, 166.3 and 162.7 (C=O), 150.0, 136.8, 135.5, 129.9, 128.8, 128,.3, 127.6, 125.9 (Ar), 91.2 (C=C), 52.6(OCH3), 43.7 (ArCH2), 38.0 (COCH2). LC-MS (ESI-ve) Found [M − H]− 392.05 (C21H18N3O5 requires 392.12).
Methyl (E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino) propanoate (8b).
Yield: 82%; off-white solid, melting point 189–191 °C; 1H-NMR (300 MHz, CDCl3): 13.75 and 13.62 (s, 1H, NH, E,Z), 8.13 and 8.03 (s, 1H, NH, E,Z), 7.39–6.84 (m, 9H, ArH), 5.03 and 5.00 (s, 2H, ArCH2, E,Z), 3.89 and 3.86 (s, 2H, COCH2, E,Z), 3.76 and 3.67 (s, 3H, ArOCH3) 3.66 and 3.63 (s, 3H, OCH3); 13C-NMR (300 MHz, CDCl3): 168.2, 167.6, 166.3 and 162.7 (C=O), 150.0, 128.5, 128.3, 127.6, 127.2, 127.1, 115.0 and 114.7 (Ar), 91.2 (C=C), 55.6 (ArOCH3), 52.6 (COOCH3), 43.7 (ArCH2), 37.9 (COCH2); LC-MS (ESI-ve) Found [M − H]− 422.10 (C22H20N3O6 requires 422.13).
Methyl (E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((p-tolylphenyl)amino)-propanoate (8c).
Yield: 57%; orange crystals, melting point 171–173 °C; 1H-NMR (300 MHz, CDCl3): 13.93 and 13.82 (s, 1H, NH, E,Z), 8.53 and 8.43 (s, 1H, NH, E,Z), 7.49–7.12 (m, 9H, ArH), 5.13 and 5.10 (s, 2H, ArCH2, E,Z), 3.97 and 3.96 (s, 2H, COCH2, E,Z), 3.76 and 3.72 (s, 3H, ArOCH3) 2.4 (s, 3H, ArCH3); 13C-NMR (300 MHz, CDCl3): 168.5, 167.6, 166.4, 162.8 (C=O), 151.1, 130.4, 128.5, 127.6 and 125.7 (Ar), 52.5 (COOCH3), 43.7 (ArCH2), 37.9 (COCH2), 21.1 (ArCH3); LC-MS (ESI-ve) Found [M − H]− 406.10 (C22H20N3O5 requires 406.14).
Methyl (E,Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino) propanoate (8d).
Yield: 53%; off-white crystals, melting point 199–202 °C; 1H-NMR (300 MHz, CDCl3): 13.86 and 13.67 (s, 1H, NH, E,Z), 8.47 and 8.30 (s, 1H, NH, E,Z), 7.06–6.86 (m, 4H, ArH), 3.93 and 3.92 (q, J = 7.1 Hz, 2H, CH3CH2, E,Z), 3.89 and 3.88 (s, 2H, COCH2), 3.77 and 3.69 (s, 3H, ArOCH3), 1.20 and 1.11 (t, J = 7.1 Hz, 3H, CH3CH2); 13C-NMR (300 MHz, CDCl3): 168.6, 166.3, 162.7 and 159.6 (C=O), 149.7, 128.1, 127.3, 127.2 and 115.0 (Ar), 91.1 (C=C), 55.6 (ArOCH3), 52.6 (COOCH3), 37.9 (COCH2), 35.8 (CH2CH3), 13.4 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 360.10 (C17H18N3O6 requires 360.12).
Methyl (E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((p-tolylphenyl)amino)-propanoate (8e).
Yield: 82%; pale yellow solid, melting point 191–192 °C; 1H-NMR (300 MHz, CDCl3): 14.03 and 13.84 (s, 1H, NH, E,Z), 8.68 and 8.50 (s, 1H, NH, E,Z), 7.28–7.11 (m, 4H, ArH), 4.01 and 3.98 (q, J = 7.1 Hz, 2H, CH3CH2, E,Z), 3.94 and 3.93 (s, 2H, COCH2), 2.40 (s, 3H, ArCH3), 1.29 and 1.18 (t, J = 7.1 Hz, 3H, CH3-CH2); 13C-NMR (300 MHz, CDCl3): 168.5, 167.6, 166.4 and 162.8 (C=O), 149.7, 138.9, 133.0, 130.4, 125.8 and 125.7 (Ar), 91.2 (C=C), 52.5 (COOCH3), 37.9 (COCH2), 35.8 (CH2CH3), 21.1 (ArCH3), 13.4 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 344.05 (C17H18N3O5 requires 344.12).
Methyl (E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-(trifluoromethyl)phenyl) amino)propanoate (8f).
Yield: 56%; white crystals, melting point 193–194 °C; 1H-NMR (300 MHz, CDCl3): 14.03 and 13.84 (s, 1H, NH, E,Z), 8.68 and 8.50 (s, 1H, NH, E,Z), 7.28–7.11 (m, 4H, ArH), 4.01 and 3.98 (q, J = 7.1 Hz, 2H, CH3CH2, E,Z), 3.94 and 3.93 (s, 2H, COCH2), 1.29 and 1.18 (t, J = 7.1 Hz, 3H, CH3CH2); LC-MS (ESI-ve) Found [M − H]− 398.00 (C17H15F3N3O5 requires 398.10).
Appendix C.6. General Procedure F
5-(Amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-triones9a–f. To a stirred solution of methyl(E, Z)-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(amino)propanoate 8a–f (1 mmol) in MeOH (10 mL) was added aq. 1 M NaOH (1 mL). The mixture was heated to 70 °C for 1 h. The MeOH was removed, and H2O (20 mL) was added. The mixture was cooled in an ice bath and acidified with aq. 1 M HCl. The precipitate was filtered off and dried to give 9a–f.
3-Benzyl-5-(phenylamino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (9a).
Yield: 58%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 10.41 (s, 1H, NH, E,Z), 7.49–7.28 (m, 10H, ArH), 5.03 (s, 2H, ArCH2, E,Z), 4.91 (s, 1H, C=CH); 13C-NMR (300 MHz, DMSO-d6): 163.2, 160.9, 158.2 and 155.6 (C=O), 149.1, 137.3, 137.0, 130.1, 128.8, 127.8, 126.8 and 125.0 (Ar), 75.6 (C=C), 43.2 (ArCH2); LC-MS (ESI-ve) Found [M − H]− 360.10 (C20H14N3O4 requires 360.10).
3-Benzyl-5-((4-methoxyphenyl)amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (9b).
Yield: 79%; white crystals, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 10.18 (s, 1H, NH), 7.33–6.99 (m, 9H, ArH), 5.02 (s, 2H, ArCH2), 4.70 (s, 1H, C=CH); 13C-NMR (300 MHz, DMSO-d6): 163.2, 161.1, 158.3 and 156.5 (C=O), 149.4, 137.1, 129.9, 128.8, 127.8, 127.6, 127.0 and 115.1 (Ar), 84.4 and 75.0 (C=C), 55.8 (ArOCH3), 43.6 (ArCH2); LC-MS (ESI-ve) Found [M-H]− 390.05 (C21H16N3O5 requires 390.11), HRMS (ESI + ve) Found [M + H]+ 392.09 (C21H18N3O5 requires 392.12).
3-Benzyl-5-((p-tolylphenyl)amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (9c).
Yield: 75%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 10.31 (s, 1H, NH), 7.34–7.22 (m, 9H, ArH), 5.02 (s, 2H, ArCH2), 4.84 (s, 1H, C=CH), 2.33 (s, 3H, ArCH3); 13C-NMR (300 MHz, DMSO-d6): 163.1, 160.8, 158.1 and 155.8 (C=O), 149.0, 137.0, 136.3, 134.6, 130.5, 128.8, 127.8 and 125.0 (Ar), 75.3 (C=C), 43.6 (ArCH2), 21.0 (ArCH3); LC-MS (ESI-ve) Found [M-H]− 374.10 (C21H16N3O4 requires 374.11), HRMS (ESI + ve) Found [M + H]+ 376.10 (C21H18N3O4 requires 376.13).
3-Ethyl-5-((4-methoxyphenyl)amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione (9d).
Yield: 53%; pale grey solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 10.79 (s, 1H, NH), 7.26–6.93 (m, 4H, ArH), 4.57 (s, 1H, C=CH), 3.84 (q, J = 7.0 Hz, 2H, CH2CH3), 3.77 (s, 3H, ArOCH3), 1.05 (t, J = 7.0 Hz, 3H, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 167.8, 165.2, 162.1 and 157.6 (C=O), 131.3, 126.3 and 115.0 (Ar), 82.6 and 75.3 (C=C), 55.7 (ArOCH3), 34.8 (CH2CH3), 13.7 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 328.05 (C16H14N3O5 requires 328.09).
3-Ethyl-5-((p-tolylphenyl)amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7-(1H,3H)-trione (9e).
Yield: 46%; yellow-orange crystals, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 13.10 (s, 1H, NH), 10.40 (s, 1H, NH), 7.26 (m, 4H, ArH), 4.84 (s, 1H, C=CH), 3.86 (q, J = 7.0 Hz, 2H, CH2CH3), 2.32 (s, 3H, ArCH3), 1.15 (t, J = 7.0 Hz, 3H, J = 7.0 Hz, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 163.1, 160.6, 158.2 and 155.8 (C=O), 148.7, 136.3, 134.7, 130.5 and 124.9 (Ar), 75.2 (C=C), 35.7 (CH2CH3), 21.0 (ArCH3), 13.1 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 312.00 (C16H14N3O4 requires 312.10).
3-Ethyl-5-((4-(trifluoromethyl)phenyl)amino)-1H-pyrano[2,3-d]pyrimidine-2,4,7(3H)-trione (9f).
Yield: 63%; pale yellow crystals, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 13.10 (s, 1H, NH), 10.40 (s, 1H, NH), 7.26 (m, 4H, ArH), 4.84 (s, 1H, C=CH), 3.86 (m, 2H, CH2CH3), 1.15 (m, 3H, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 163.1, 160.6, 158.2 and 155.8 (C=O), 155.9, 125.0 (Ar), 75.2 (C=C), 35.7 (CH2CH3), 13.1 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 366.00 (C16H11F3N3O4 requires 366.07).
Appendix C.7. General Procedure G
3-Amino-3-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)propanamides10a–q. A solution of 5-(amino)-2H-pyrano[2,3-d]pyrimidine-2,4,7(1H,3H)-trione 9 and amine in 2,2,2-trifluoroethanol was irradiated in a microwave reactor at 150 °C for 1 h. After cooling, the solvent was removed, and acetone was added. The resulting precipitate was filtered off to give pure 10a–q.
(E/Z)-1-benzyl-5-(1-((4-methoxyphenyl)amino)-3-morpholino-3-oxopropyl-idene)pyrimidine-2,4,6(1H,3H,5H)-trione (10a).
Yield: 20%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, CDCl3): 13.88 and 13.76 (s, 1H, NH, E,Z), 8.41 (s, 1H, NH, E,Z), 7.37–6.84 (m, 9H, ArH), 5.02 and 4.69 (s, 2H, ArCH2, E,Z), 3.93 (s, 2H, COCH2, E,Z), 3.76 (s, 3H, OCH3), 3.67–3.35 (m, 8H, morpholine); 13C-NMR (300 MHz, CDCl3): 169.9, 166.3, 162.0 and 159.5 (C=O), 150.0, 128.4, 128.2, 127.5, 127.2 and 114.7 (Ar), 90.8 (C=C), 65.5 (OCH2, morpholine), 55.6 (OCH3), 46.5 (NCH2, morpholine), 43.6 (CH2); LC-MS (ESI-ve) Found [M − H]− 477.20 (C25H25N4O6 requires 477.18).
(E/Z)-1-benzyl-5-(3-morpholino-3-oxo-1-(p-tolylamino)propylidene)pyrimidine-2,4,6(1H,3H,5H)-trione (10b).
Yield: 50%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, CDCl3): 13.96 and 13.82 (s, 1H, NH, E,Z), 7.87 (s, 1H, NH, E,Z), 7.38–7.10 (m, 9H, ArH), 5.02 and 4.97 (s, 2H, ArCH2, E,Z), 3.93 (s, 2H, COCH2, E,Z), 3.67-3.33 (m, 8H, morpholine), 2.32 (s, 3H, ArCH3); 13C-NMR (500 MHz, CDCl3): 170.3, 167.2, 166.3 and 162.5 (C=O), 150.0, 138.7, 137.4, 136.9, 133.2, 130.0, 128.4, 127.5 and 124.7 (Ar), 90.8 (C=C), 66.8 (OCH2, morpholine), 46.5 (NCH2, morpholine), 42.3 (ArCH2), 36.9 (COCH2), 21.2 (CH3Ar); LC-MS (ESI-ve) Found [M − H]− 461.15 (C25H25N4O5 requires 461.18).
(E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)-N-(4-(trifluoromethyl)phenyl)propenamide (10c).
Yield: 15%; yellow solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.00 and 13.47 (s, 1H, NH, E,Z), 11.36 and 10.98 (s, 1H, NH, E,Z), 10.51 and 10.31 (s, 1H, NH, E,Z), 7.99–6.53 (m, 13H, ArH), 5.01 and 4.91 (s, 2H, ArCH2, E,Z), 4.08 and 4.06 (s, 2H, COCH2, E,Z), 3.77 (s, 3H, OCH3); 13C-NMR (300 MHz, DMSO-d6): 164.5 (C=O), 152.6, 127.6, 126.6 and 113.4 (Ar), 92.2 (C=C), 55.9 (OCH3), 46.2 (CH2); LC-MS (ESI-ve) Found [M − H]− 551.05 (C28H22F3N4O5 requires 551.15).
(E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-methoxyphenyl)-3-(phenylamino)propenamide (10d).
Yield: 76%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.17 and 13.89 (s, 1H, NH, E,Z), 11.36 and10.98 (s, 1H, NH, E,Z), 9.93 and 9.92 (s, 1H, NH, E,Z), 7.49–6.84 (m, 14H, ArH), 5.01 and 4.93 (s, 2H, ArCH2, E,Z), 4.06 and 4.03 (s, 2H, COCH2, E,Z); 13C-NMR (300 MHz, DMSO-d6): 167.3, 162.8 and 155.6 (C=O), 150.2, 135.0, 132.5, 130.1, 128.8, 127.5, 126.3, 120.9 and 114.2 (Ar), 92.2 (C=C), 55.6 (OCH3), 43.2 (CH2); LC-MS (ESI-ve) Found [M − H]− 483.15 (C27H23N4O5 requires 483.17).
(E/Z)-3-(1-benzyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-chlorophenyl)-3-(phenylamino)propenamide (10e).
Yield: 50%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.16 and 13.88 (s, 1H, NH, E,Z), 11.38 and 10.99 (s, 1H, NH, E,Z), 10.24 and 10.26 (s, 1H, NH, E,Z), 7.51–7.22 (m, 14H, ArH), 5.01 and 4.92 (s, 2H, ArCH2, E,Z), 4.06 and 4.04 (s, 2H, COCH2, E,Z); 13C-NMR (300 MHz, DMSO-d6): 167.3, 166.4, 166.3 and 162.8 (C=O), 150.3, 138.3, 136.2, 129.0, 128.6, 127.7, 127.5, 126.3 and 120.9 (Ar), 91.18 (C=C), 43.2 (CH2); LC-MS (ESI-ve) Found [M − H]− 487.15 (C26H2035ClN4O4 requires 487.12).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)-N-(p-tolyl)propenamide (10f).
Yield: 30%; white crystals, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 13.98 and 13.85 (s, 1H, NH, E,Z), 11.23 and 10.84 (s, 1H, NH, E,Z), 9.99 and 9.96 (s, 1H, NH, E,Z), 7.44–6.87 (m, 8H, ArH), 4.04 and 4.02 (s, 2H, COCH2, E,Z), 3.83 and 3.81 (m, 2H, CH2CH3), 3.76 (s, 3H, OCH3), 2.24 (s, 3H, ArCH3), 1.15 and 1.05 (t, J = 7.0 Hz, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.7, 166.0, 162.9 and 159.3 (C=O), 150.0, 137.0, 132.4, 130.6, 129.5, 128.8, 119.4 and 115.2 (Ar), 91.2 (C=C), 55.9 (OCH3), 35.1 (CH2CH3), 20.9 (ArCH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 435.15 (C23H23N4O5 requires 435.17).
(E/Z)-N-(4-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)propenamide (10g).
Yield: 70%; white solid, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 13.98 and 13.84 (s, 1H, NH, E,Z), 11.22 and 10.83 (s, 1H, NH, E,Z), 10.29 and 10.20 (s, 1H, NH, E,Z), 7.55–7.05 (m, 8H, ArH), 4.04 and 4.02 (s, 2H, COCH2, E,Z), 3.83 and 3.82 (m, 2H, CH2CH3), 3.77 (s, 3H, OCH3), 1.13 and 1.04 m, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.4, 167.2, 162.9 and 159.4 (C=O), 150.0, 138.4, 129.1, 128.8, 127.6, 127.0, 120.9 and 115.3 (Ar), 91.1 (C=C), 55.9 (OCH3), 36.8 (CH2CH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 455.05 (C22H2035ClN4O5 requires 455.11).
(E/Z)-N-(3-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)propenamide (10h).
Yield: 52%; white crystals, melting point 258–261 °C; 1H-NMR (400 MHz, DMSO-d6): 13.98 and 13.84 (s, 1H, NH, E,Z), 11.21 and 10.82 (s, 1H, NH, E,Z), 10.33 and 10.30 (s, 1H, NH, E,Z), 7.76 – 6.88 (m, 8H, ArH), 4.04 and 4.02 (s, 2H, COCH2, E,Z), 3.86 and 3.85 (m, 2H, CH2CH3), 3.77 (s, 3H, OCH3), 1.14 and 1.04 (m, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.4, 167.2, 166.7 and 162.9 (C=O), 150.0, 140.9, 133.5, 130.9, 128.8, 123.2, 117.7 and 115.3 (Ar), 91.2 (C=C), 55.9 (OCH3), 34.9 (CH2CH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 455.05 (C22H2035ClN4O5 requires 455.11).
(E/Z)-N-(2-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)propenamide (10i).
Yield: 55%; white crystals, melting point 266–269 °C; 1H-NMR (400 MHz, DMSO-d6): 13.96 and 13.83 (s, 1H, NH, E,Z), 11.20 and 10.85 (s, 1H, NH, E,Z), 9.67 and 9.63 (s, 1H, NH, E,Z), 7.69–7.06 (m, 8H, ArH), 4.14 and 4.12 (s, 2H, COCH2, E,Z), 3.86 and 3.85 (m, 2H, CH2CH3), 3.79 (s, 3H, OCH3), 1.15 and 1.05 (m, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.5, 167.2, 163.0 and 159.4 (C=O), 150.0, 135.3, 129.9, 128.8, 126.6 and 115.2 (Ar), 91.2 (C=C), 55.9 (OCH3), 35.1 (CH2CH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 455.05 (C22H2035ClN4O5 requires 455.11).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-methoxyphenyl)amino)-N-(4-(trifluoromethyl)phenyl)propenamide (10j).
Yield: 73%; white crystals, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 13.98 and 13.84 (s, 1H, NH, E,Z), 11.22 (s, 1H, NH, E,Z), 10.84 and 10.51 (s, 1H, NH, E,Z), 7.72–6.62 (m, 8H, ArH), 4.07 (s, 2H, COCH2, E,Z), 3.86 (m, 2H, CH2CH3, E,Z), 3.77 (s, 3H, OCH3, E,Z), 1.14 and 1.03 (m, 3H, CH2CH3); LC-MS (ESI-ve) Found [M − H]− 489.15 (C23H20F3N4O5 requires 489.14).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-phenyl-3-(p-tolylamino)propanamide (10k).
Yield: 73%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.06 and 13.92 (s, 1H, NH, E,Z), 11.22 and 10.84 (s, 1H, NH, E,Z), 10.11 and 10.09 (s, 1H, NH, E,Z), 7.51–7.05 (m, 8H, ArH), 4.05 and 4.04 (s, 2H, COCH2, E,Z), 3.86 and 3.75 (m, 2H, CH2CH3), 2.32 (s, 3H, ArCH3), 1.14 and 1.036 (m, 3H, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 168.9, 167.3, 166.2 and 162.8 (C=O), 150.0, 139.5, 138.4, 133.7, 130.6, 129.1, 126.3, 123.5 and 119.3 (Ar), 91.4 (C=C), 35.1 (CH2CH3), 21.1 (ArCH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 405.10 (C22H21N4O4 requires 405.16).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-methoxyphenyl)-3-(p-tolylamino)propanamide (10l).
Yield: 67%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.06 and 13.92 (s, 1H, NH, E,Z), 11.21 and 10.82 (s, 1H, NH, E,Z), 9.95 and 9.91 (s, 1H, NH, E,Z), 7.40–6.85 (m, 8H, ArH), 4.03 and 4.02 (s, 2H, COCH2, E,Z), 3.85 and 3.83 (m, 2H, CH2CH3), 3.71 (ArOCH3), 2.32 (s, 3H, ArCH3), 1.14 and 1.04 (m, 3H, CH2CH3); 13C-NMR (300 MHz, DMSO-d6): 167.3, 165.7, 162.9 and 155.6 (C=O), 150.0, 138.3, 133.7, 130.6, 126.0, 120.9 and 114.3 (Ar), 91.2 (C=C), 55.6 (ArOCH3), 35.0 (CH2CH3), 21.1 (ArCH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 435.10 (C23H23N4O5 requires 435.17).
(E/Z)-N-(4-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(p-tolylamino)propanamide (10m).
Yield: 49%; white crystals, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.06 and 13.92 (s, 1H, NH, E,Z), 11.22 and 10.83 (s, 1H, NH, E,Z), 10.29 and 10.25 (s, 1H, NH, E,Z), 7.55–7.23 (m, 8H, ArH), 4.04 and 4.02 (s, 2H, COCH2, E,Z), 3.84 and 3.73 (q, 2H, CH2CH3), 2.32 (s, 3H, ArCH3), 1.14 and 1.03 (t, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.2, 167.3, 162.9 and 155.0 (C=O), 150.0, 138.4, 133.7, 130.6, 129.1, 126.0, 120.9 and 119.4 (Ar), 91.2 (C=C), 35.1 = (CH2CH3), 211 (ArCH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 439.05 (C22H2035ClN4O4 requires 439.12).
(E/Z)-N-(3-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-(p-tolylamino)propenamide (10n).
Yield: 63%; white crystals, melting point 284–285 °C; 1H-NMR (400 MHz, DMSO-d6): 14.063 and 13.92 (s, 1H, NH, E,Z), 11.23 and 10.84 (s, 1H, NH, E,Z), 10.34 and 10.30 (s, 1H, NH, E,Z), 7.75–7.10 (m, 8H, ArH), 4.04 and 4.02 (s, 2H, COCH2, E,Z), 3.86 and 3.75 (m, 2H, CH2CH3), 2.32 (s, 3H, ACH3), 1.16 and 1.02 (m, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 169.0, 168.5, 166.6 and 162.9 (C=O), 150.0, 140.9, 138.4, 133.5, 130.9, 126.1, 123.2, 118.8 and 117.7 (Ar), 91.2 (C=C), 35.1 (CH2CH3), 21.1 (ArCH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 439.05 (C22H2035ClN4O4 requires 439.12).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(4-methoxyphenyl)-3-((4-(trifluoromethyl)phenyl)amino)propanamide (10o).
Yield: 44%; grey solid, no clear melting point (decomposed); 1H-NMR (400 MHz, DMSO-d6): 14.27 and 14.10 (s, 1H, NH, E,Z), 11.51 (s, 1H, NH, E,Z), 10.01 and 9.97 (s, 1H, NH, E,Z), 7.90–6.51 (m, 8H, ArH), 4.08 (s, 2H, COCH2, E,Z), 3.86 and 3.85 (m, 2H, CH2CH3, E,Z), 3.79-3.77 (s, 3H, OCH3, E,Z), 1.15 and 1.05 (m, 3H, CH2CH3); 13C-NMR (400 MHz, DMSO-d6): 168.7, 167.3, 166.0 and 162.8 (C=O), 150.0, 140.2, 136.9, 132.5, 130.6, 129.5, 127.2, 126.1, 119.5 and 114.5 (Ar), 91.2 (C=C), 55.9 (OCH3), 35.1 (CH2CH3), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 489.05 (C23H20F3N4O5 requires 489.14).
(E/Z)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-N-(p-tolyl)-3-((4-(trifluoromethyl)phenyl)amino)propenamide (10p).
Yield: 40%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.27 and 14.10 (s, 1H, NH, E,Z), 11.32 and 10.92 (s, 1H, NH, E,Z), 10.07 and 10.03 (s, 1H, NH, E,Z), 7.90-6.46 (m, 8H, ArH), 4.09 (s, 2H, COCH2, E,Z), 3.87 and 3.76 (m, 2H, CH2CH3), 2.25 (s, 3H, ArCH3), 1.15 and 1.05 (m, 3H, CH2CH3, E,Z); 13C-NMR (400 MHz, DMSO-d6): 168.7, 167.3, 166.0 and 162.8 (C=O), 150.0, 140.2, 136.9, 132.5, 130.6, 129.5, 127.2, 126.1, 119.5 and 114.5 (Ar), 35.1 (CH2CH3), 20.9 (CH3Ar), 13.8 (CH2CH3); LC-MS (ESI-ve) Found [M − H]− 473.1 (C23H20F3N4O4 requires 473.14).
(E/Z)-N-(4-chlorophenyl)-3-(1-ethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-((4-(trifluoromethyl)phenyl)amino)propenamide (10q).
Yield: 32%; white solid, no clear melting point (decomposed); 1H-NMR (300 MHz, DMSO-d6): 14.14 and 14.09 (s, 1H, NH, E,Z), 10.93 and 10.80 (s, 1H, NH, E,Z), 10.30 and 10.28 (s, 1H, NH, E,Z), 7.88-6.54 (m, 8H, ArH), 4.08 (s, 2H, COCH2, E,Z), 3.85 and 3.76 (m, 2H, CH2CH3), 1.14 and 1.05 (m, 3H, CH2CH3); LC-MS (ESI-ve) Found [M − H]− 493.05 (C22H1735ClF3N4O4 requires 493.09).