Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material
Abstract
:1. Introduction
- -
- CS-salts do not influence fungal cell growth,
- -
- the fungal cell counts on CS-salt-modified denture base material do not differ from unmodified standard material (control),
- -
- the roughness (Ra) of CS-salt-modified denture base material does not differ from unmodified standard material.
2. Results
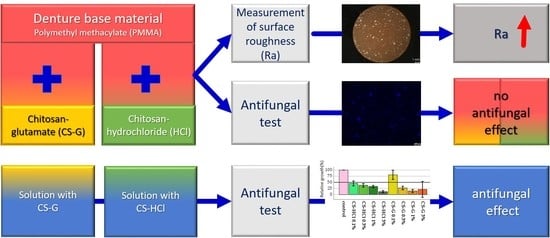
2.1. Antifungal Test: Effect of Chitosan-Salt Solutions
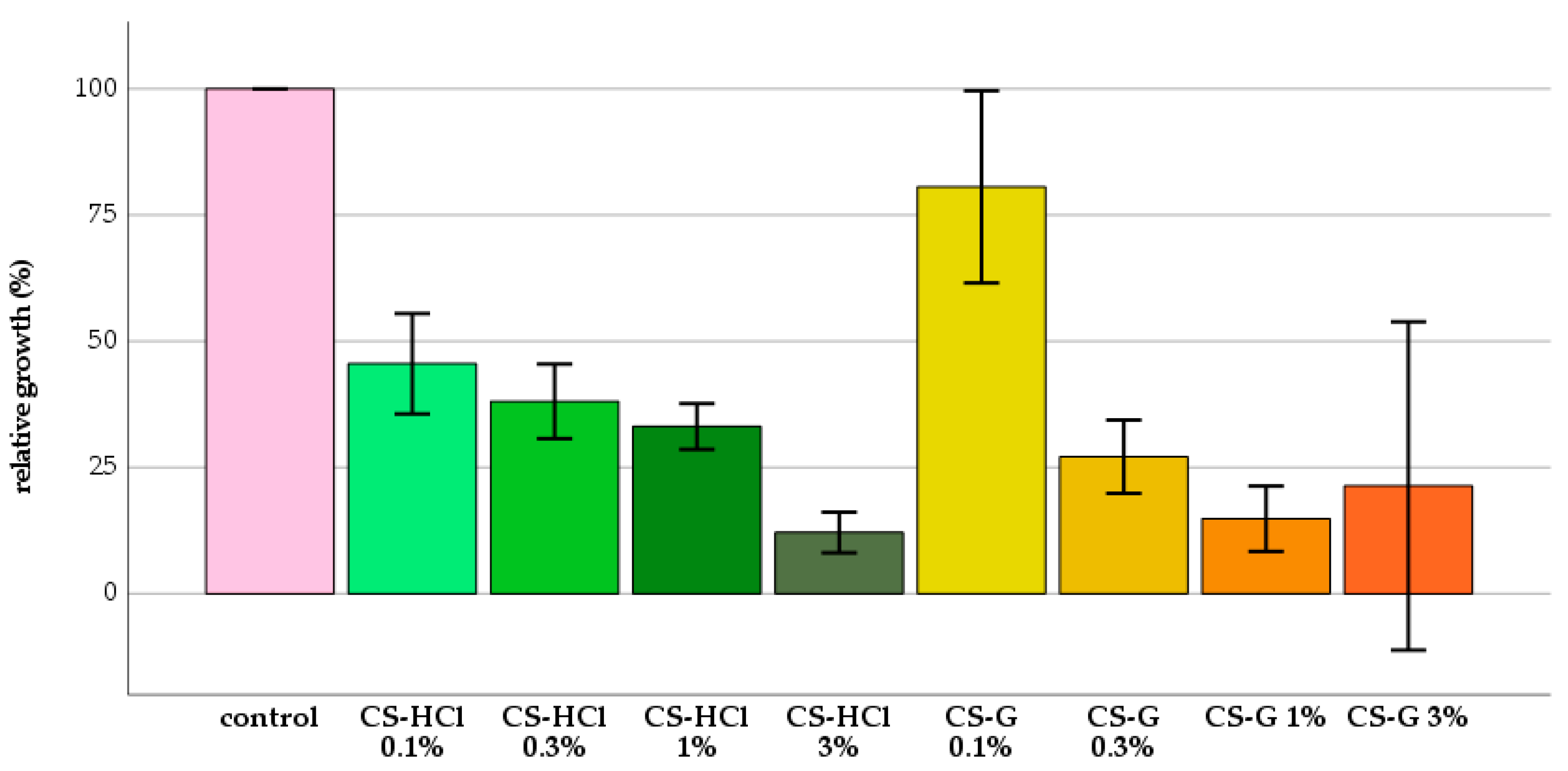
2.2. Roughness
2.3. Antifungal Test: Effect of CS-Salt Modified PMMA
3. Materials and Methods
3.1. Chitosan(CS)-Salts
3.2. Preparation of CS-Salt Modified PMMA Specimens
3.3. Roughness Measurement
3.4. Antifungal Test
3.4.1. Candida Cell Suspension
3.4.2. Chitosan-Salt Solution
3.4.3. Optical Density Measurement
3.4.4. Biofilm Assay and Fungal Cell Count
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture stomatitis: Causes, cures and prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Marrelli, M.; Mirgaldi, R.; De Vito, D.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar] [PubMed]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonca, G.; McGraw, K.; Stoner, L. Evidence regarding the treatment of denture stomatitis. J. Prosthodont. 2016, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Truhlar, M.R.; Shay, K.; Sohnle, P. Use of a new assay technique for quantification of antifungal activity of nystatin incorporated in denture liners. J. Prosthet. Dent. 1994, 71, 517–524. [Google Scholar] [CrossRef]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Kawala, M.; Smardz, J.; Adamczyk, L.; Grychowska, N.; Wieckiewicz, M. Selected applications for current polymers in prosthetic dentistry - state of the art. Curr. Med. Chem. 2018, 25, 6002–6012. [Google Scholar] [CrossRef]
- Sivakumar, I.; Arunachalam, K.S.; Sajjan, S.; Ramaraju, A.V.; Rao, B.; Kamaraj, B. Incorporation of antimicrobial macromolecules in acrylic denture base resins: A research composition and update. J. Prosthodont. 2014, 23, 284–290. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. Pmma: An essential material in medicine and dentistry. J. Long Term Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, T.; Ueda, T.; Sakurai, K. Inhibition of denture plaque by tio2 coating on denture base resins in the mouth. J. Prosthet. Dent. 2017, 118, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Fouda, S.M.; Napankangas, R.; Raustia, A. Flexural and surface properties of pmma denture base material modified with thymoquinone as an antifungal agent. J. Prosthodont. 2018. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777. [Google Scholar]
- Hamid, S.K.; Al-Dubayan, A.H.; Al-Awami, H.; Khan, S.Q.; Gad, M.M. In vitro assessment of the antifungal effects of neem powder added to polymethyl methacrylate denture base material. J. Clin. Exp. Dent 2019, 11, e170–e178. [Google Scholar] [CrossRef]
- Iqbal, Z.; Zafar, M.S. Role of antifungal medicaments added to tissue conditioners: A systematic review. J. Prosthodont. Res. 2016, 60, 231–239. [Google Scholar] [CrossRef]
- Ferreira, G.L.; Perez, A.L.; Rocha, I.M.; Pinheiro, M.A.; de Castro, R.D.; Carlo, H.L.; Lima Ede, O.; Castellano, L.R. Does scientific evidence for the use of natural products in the treatment of oral candidiasis exist? A systematic review. Evid. Based Complement. Alternat. Med. 2015, 2015, 147804. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical application of chitosan in dental specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef]
- Skoskiewicz-Malinowska, K.; Kaczmarek, U.; Malicka, B.; Walczak, K.; Zietek, M. Application of chitosan and propolis in endodontic treatment: A review. Mini Rev. Med. Chem. 2017, 17, 410–434. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Kochańska, B.; Kędzia, A.; Gębska, A. Sensitivity to chitosan ascorbate microaerophilic bacteria isolated from infections of oral cavity. Prog. Chem. Appl. Chitin. Deriv. 2016, 21, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Kucharska, M.; Ciechańska, D.; Niekraszewicz, A.; Wiśniewska-Wrona, M.; Kardas, I. Potential use of chitosan–based materiale in medicine. Prog. Chem. Appl. Chitin Deriv. 2010, 15, 169–176. [Google Scholar]
- Knapczyk, J.; Macura, A.B.; Pawlik, B. Simple tests demonstrating the antimycotic effect of chitosan. Int. J. Pharm. 1992, 80, 33–38. [Google Scholar] [CrossRef]
- de Freitas Fernandes, F.S.; Pereira-Cenci, T.; da Silva, W.J.; Filho, A.P.R.; Straioto, F.G.; Del Bel Cury, A.A. Efficacy of denture cleansers on candida spp. Biofilm formed on polyamide and polymethyl methacrylate resins. J. Prosthet. Dent. 2011, 105, 51–58. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.T.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118, 881–885. [Google Scholar] [CrossRef]
- Sadeghi Ardestani, Z.; Falahati, M.; Sayah Alborzi, S.; Ashrafi Khozani, M.; Rostam Khani, F.; Bahador, A. The effect of nanochitosans particles on candida biofilm formation. Curr. Med. Mycol. 2016, 2, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Haider, A.; Zahid, S.; Khan, S.A.; Faryal, R.; Kaleem, M. In-vitro antifungal efficacy of tissue conditioner-chitosan composites as potential treatment therapy for denture stomatitis. Int. J. Biol. Macromol. 2019, 125, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Azcurra, A.I.; Barembaum, S.R.; Bojanich, M.A.; Calamari, S.E.; Aguilar, J.; Battellino, L.J.; Dorronsoro, S.T. Effect of the high molecular weight chitosan and sodium alginate on candida albicans hydrophobicity and adhesion to cells. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E120–E125. [Google Scholar] [PubMed]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common candida species and candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e1–181.e7. [Google Scholar] [CrossRef] [Green Version]
- Atai, Z.; Atai, M.; Amini, J.; Salehi, N. In vivo study of antifungal effects of low-molecular-weight chitosan against candida albicans. J. Oral. Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, M.W.; Ungphaiboon, S.; Phadoongsombut, N.; Pangsomboon, K.; Chelae, S.; Mahattanadul, S. Effectiveness of an alcohol-free chitosan-curcuminoid mouthwash compared with chlorhexidine mouthwash in denture stomatitis treatment: A randomized trial. J. Altern. Complement. Med. 2019. [Google Scholar] [CrossRef]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.-C. Antifungal effect of high-and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and n-acetyl-d-glucosamine against candida albicans, candida krusei and candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmüller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- Hemmerich, W. Rechner zur adjustierung des α-niveaus: Statistikguru. Available online: https://statistikguru.de/rechner/adjustierung-des-alphaniveaus.html (accessed on 28 August 2020).
- Lee, H.-L.; Wang, R.-S.; Hsu, Y.-C.; Chuang, C.-C.; Chan, H.-R.; Chiu, H.-C.; Wang, Y.-B.; Chen, K.-Y.; Fu, E. Antifungal effect of tissue conditioners containing poly(acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on candida albicans growth in vitro. J. Dent. Sci. 2018, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Tavaria, F.; Pintado, M. Insights into chitosan antibiofilm activity against methicillin-resistant staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Gondim, B.L.C.; Castellano, L.R.C.; de Castro, R.D.; Machado, G.; Carlo, H.L.; Valenca, A.M.G.; de Carvalho, F.G. Effect of chitosan nanoparticles on the inhibition of candida spp. Biofilm on denture base surface. Arch. Oral. Biol. 2018, 94, 99–107. [Google Scholar] [CrossRef]
- Cobrado, L.; Azevedo, M.M.; Silva-Dias, A.; Ramos, J.P.; Pina-Vaz, C.; Rodrigues, A.G. Cerium, chitosan and hamamelitannin as novel biofilm inhibitors? J. Antimicrob. Chemother. 2012, 67, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.G.S.; Guedes, G.M.M.; da Silva, M.L.Q.; Castelo-Branco, D.; Sidrim, J.J.C.; Cordeiro, R.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against candida spp. In planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, A.; Zheng, Y.; Ye, B. In vitro damage of candida albicans biofilms by chitosan. Exp. Ther. Med. 2014, 8, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Raman, N.; Marchillo, K.; Lee, M.R.; Rodriguez Lopez, A.L.; Andes, D.R.; Palecek, S.P.; Lynn, D.M. Intraluminal release of an antifungal beta-peptide enhances the antifungal and anti-biofilm activities of multilayer-coated catheters in a rat model of venous catheter infection. ACS Biomater. Sci. Eng. 2016, 2, 112–121. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Lamfon, H.; Porter, S.R.; McCullough, M.; Pratten, J. Formation of candida albicans biofilms on non-shedding oral surfaces. Eur. J. Oral Sci. 2003, 111, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Marechal, M.; Busscher, H.; Weerkamp, A.; Darius, P.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation: An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Tanner, J.; Vallittu, P.; Haapasalo, M. Adherence of candida albicans to the surface of polymethylmethacrylate-e glass fiber composite used in dentures. Int. J. Prosthodont. 1999, 12, 83–86. [Google Scholar] [PubMed]
- Susewind, S.; Lang, R.; Hahnel, S. Biofilm formation and candida albicans morphology on the surface of denture base materials. Mycoses 2015, 58, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Pujia, A.; Palmieri, F.; Gatto, R.; Falisi, G.; Gargari, M.; Caruso, S.; Apicella, D.; Rastelli, C.; Nardi, G.M.; et al. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with cad-cam technology. Int J. Immunopathol. Pharmacol. 2016, 29, 778–783. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
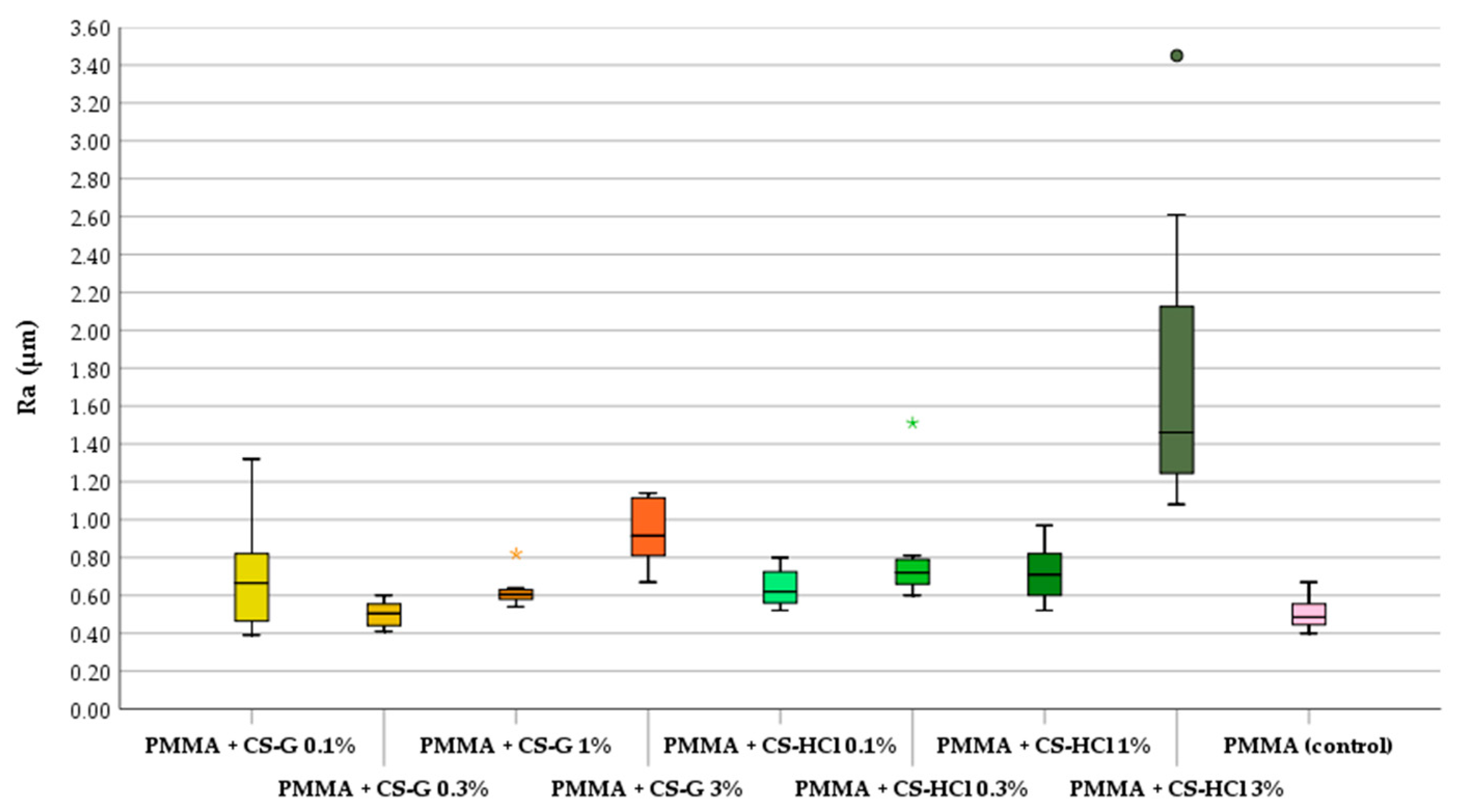
| Group | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|
| PMMA + CS-G 0.1% | 1.79 × 104 | 1.03 × 104 | 1.90 × 104 | 1.00 × 103 | 3.50 × 104 |
| PMMA + CS-G 0.3% | 4.48 × 104 | 1.00 × 105 | 1.05 × 104 | 1.00 × 103 | 2.93 × 105 |
| PMMA + CS-G 1% | 1.26 × 104 | 1.89 × 104 | 3.50 × 103 *A | 2.00 × 103 | 5.70 × 104 |
| PMMA + CS-G 3% | 3.76 × 105 | 1.85 × 105 | 3.41 × 105 | 1.52 × 105 | 7.20 × 105 |
| PMMA + CS-HCl 0.1% | 2.83 × 104 | 3.34 × 104 | 2.00 × 104 | 8.00 × 103 | 1.10 × 105 |
| PMMA + CS-HCl 0.3% | 2.88 × 104 | 1.28 × 104 | 2.90 × 104 | 8.00 × 103 | 4.60 × 104 |
| PMMA + CS-HCl 1% | 1.30 × 104 | 1.04 × 104 | 1.25 × 104 * | 2.00 × 103 | 3.00 × 104 |
| PMMA + CS-HCl 3% | 7.43 × 104 | 4.39 × 104 | 6.85 × 104 | 2.90 × 104 | 1.69 × 105 |
| PMMA (control) | 5.08 × 104 | 4.48 × 104 | 3.85 × 104 *A | 1.00 × 104 | 1.31 × 105 |
Sample Availability: Samples of the compounds used are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczak, K.; Schierz, G.; Basche, S.; Petto, C.; Boening, K.; Wieckiewicz, M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules 2020, 25, 5899. https://doi.org/10.3390/molecules25245899
Walczak K, Schierz G, Basche S, Petto C, Boening K, Wieckiewicz M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules. 2020; 25(24):5899. https://doi.org/10.3390/molecules25245899
Chicago/Turabian StyleWalczak, Katarzyna, Georg Schierz, Sabine Basche, Carola Petto, Klaus Boening, and Mieszko Wieckiewicz. 2020. "Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material" Molecules 25, no. 24: 5899. https://doi.org/10.3390/molecules25245899