Comparative Evaluation of Intestinal Absorption and Functional Value of Iron Dietary Supplements and Drug with Different Delivery Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fe Titer of Tested Formulations
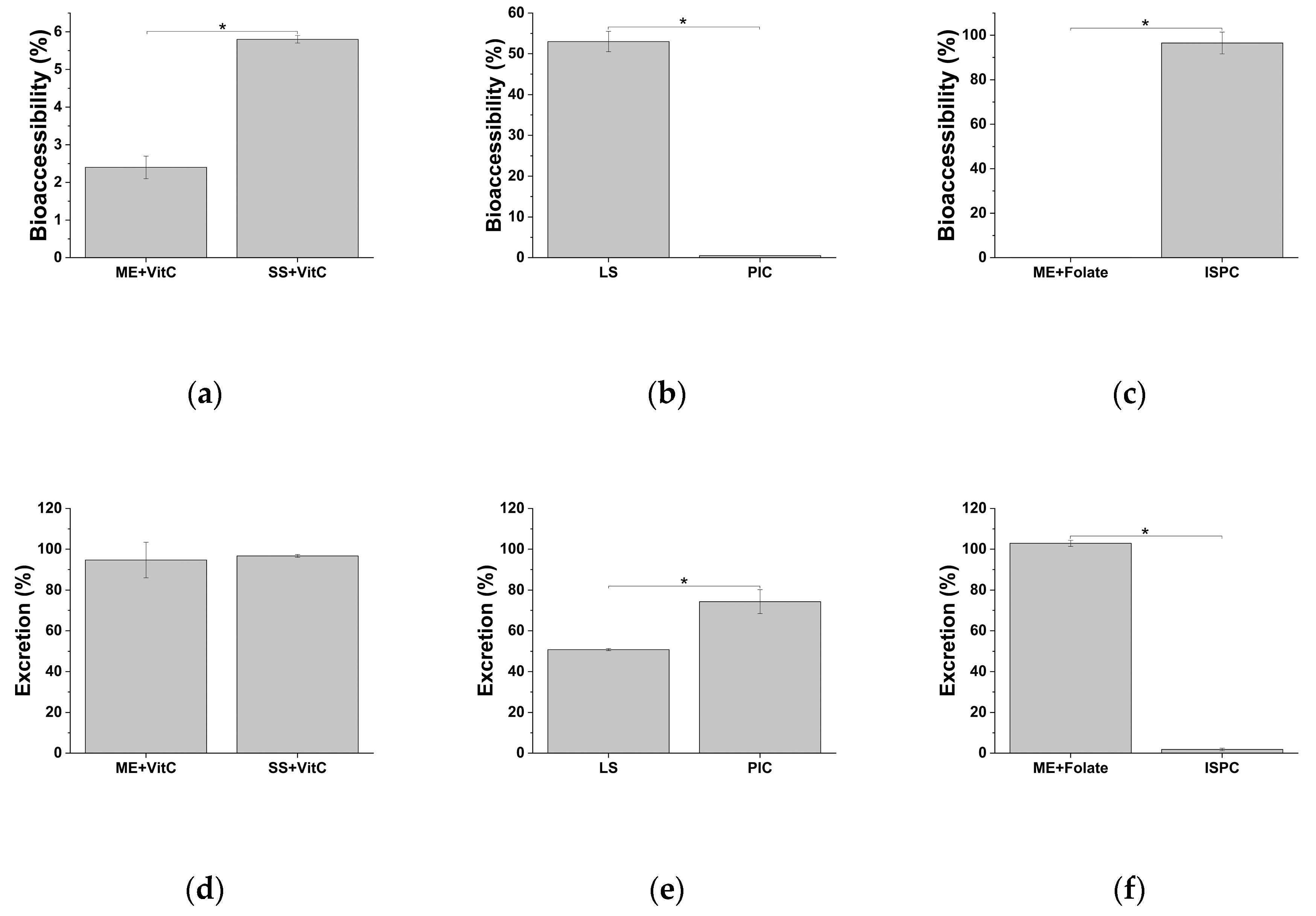
2.2. Bioaccessibility of the Fe-Based Formulations
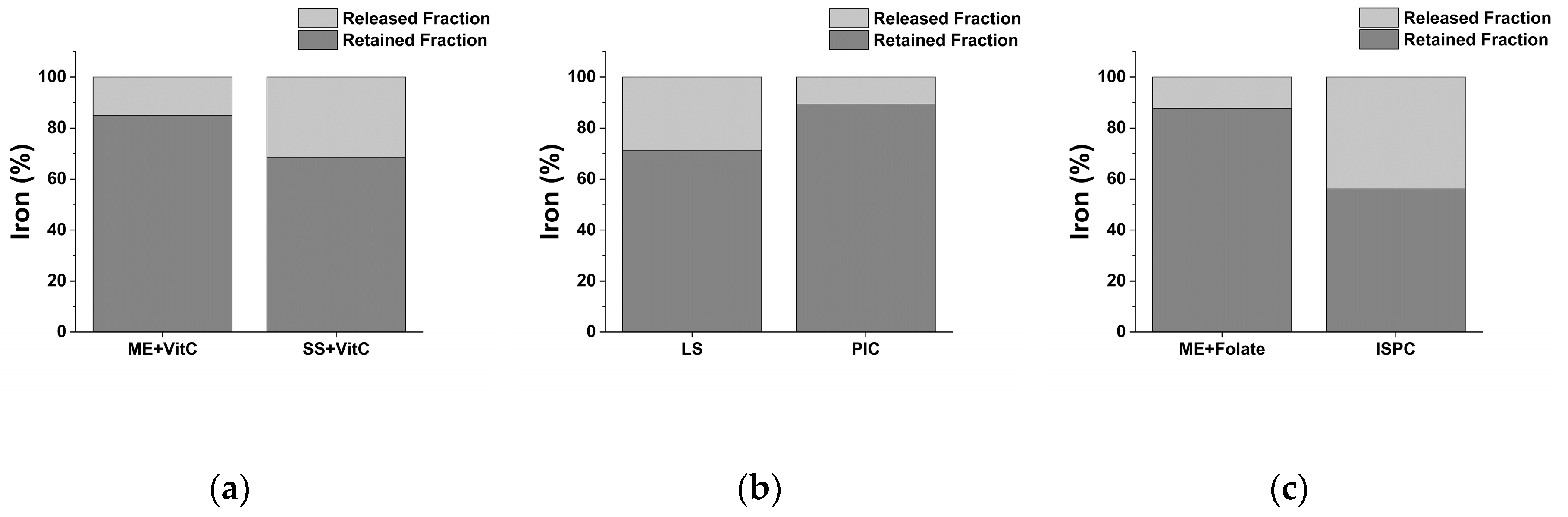
2.3. Functional Value of Iron in Tested Dietary Supplements after In Vitro Digestion
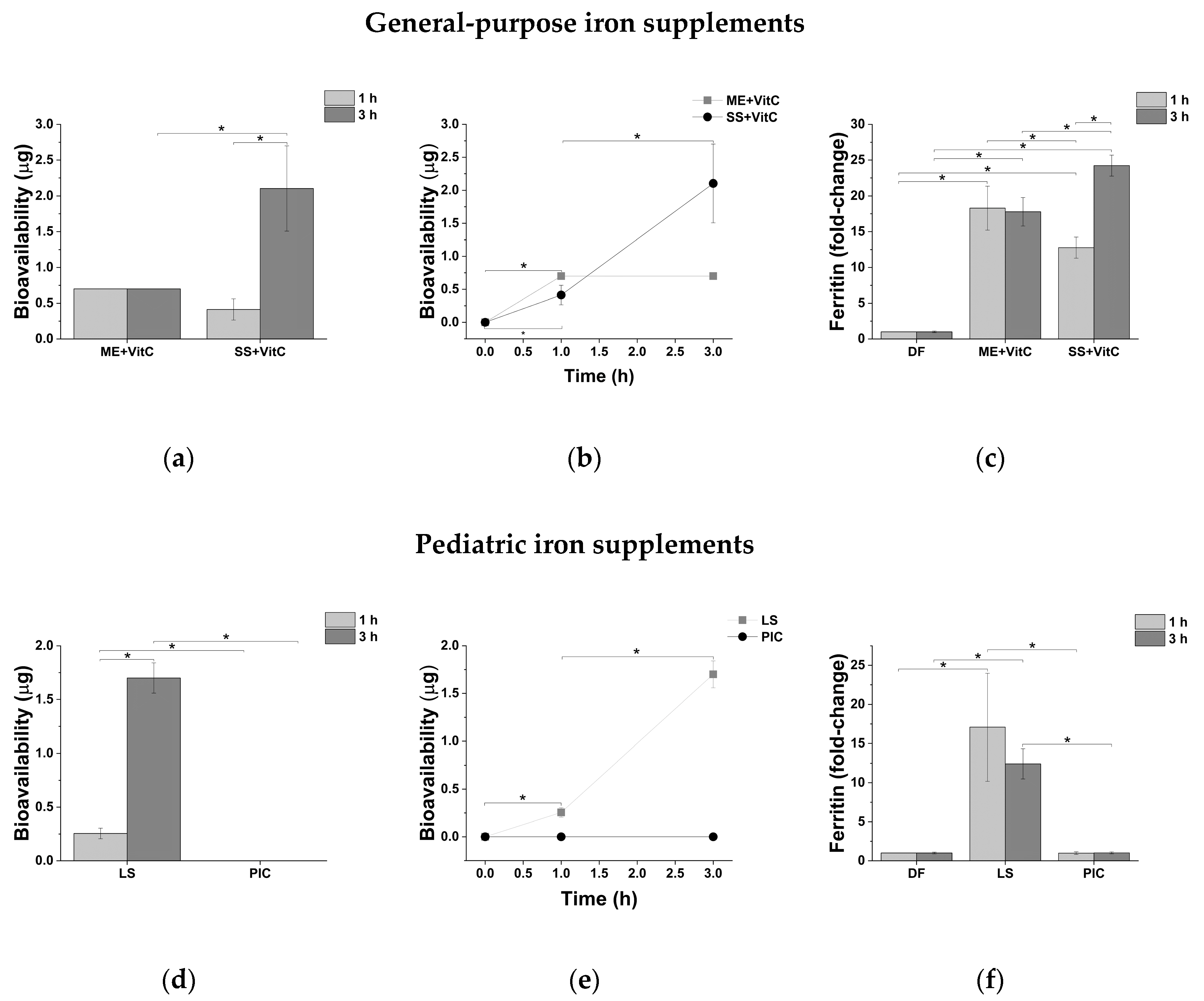
2.4. Iron Intestinal Absorption
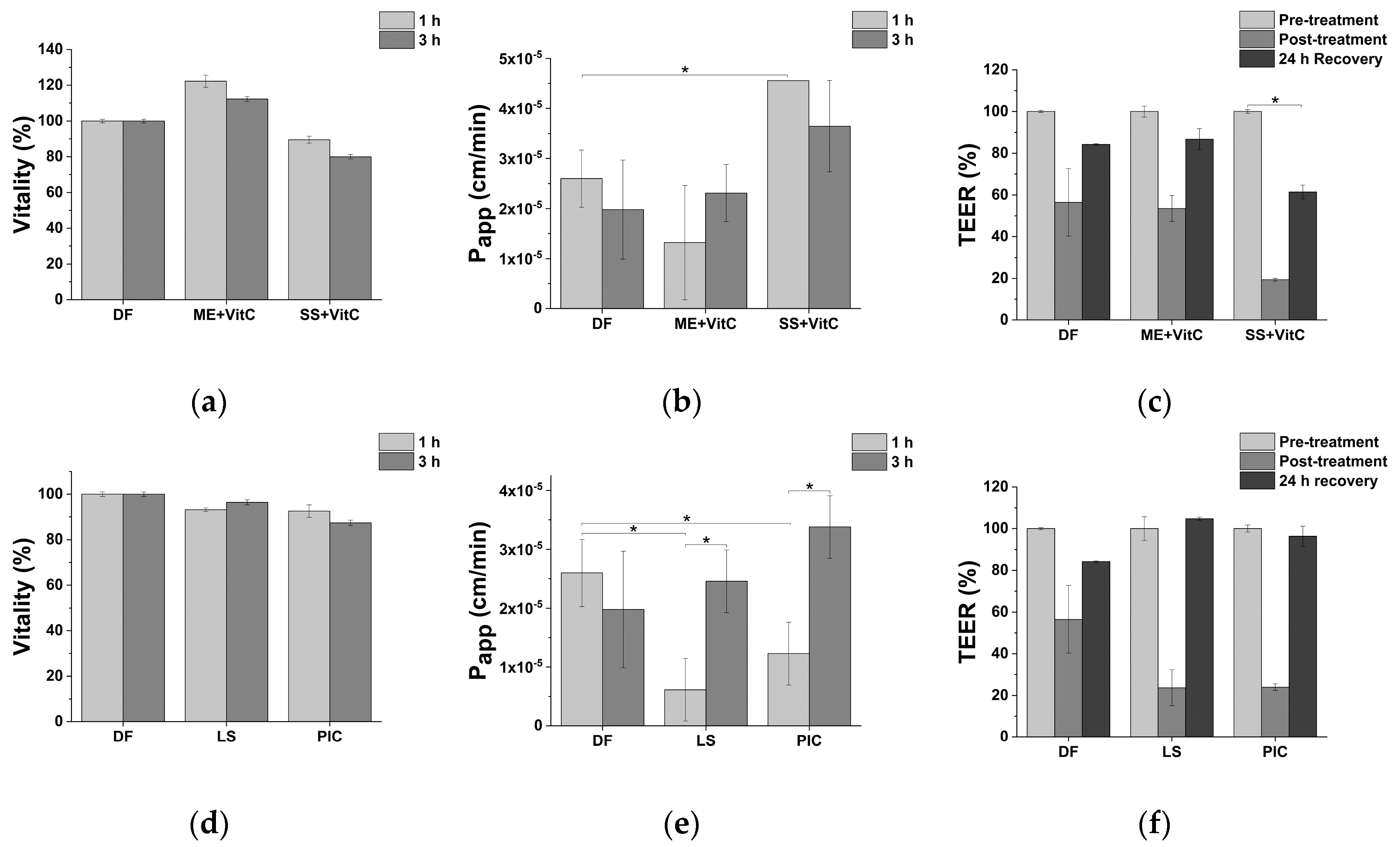
2.5. Impact of Fe-Based Formulations on Intestinal Epithelium
3. Materials and Methods
3.1. Materials
3.2. Formulations Composition
3.3. Methods
3.3.1. Cell Cultures
Caco-2 Cell Culture
Raji Cell Culture
3.3.2. Iron Content Determination in the Different Iron-Based Formulations
3.3.3. Determination of Fe-Based Formulation Bioaccessibility
3.3.4. Determination of Delivery System Resistance to the Digestive Process
3.3.5. Determination of Fe-Based Formulations Functional Iron Fraction
3.3.6. Intestinal Epithelium In Vitro Model
3.3.7. Evaluation of Fe Intestinal Absorption
3.3.8. Determination of Intracellular Ferritin
3.3.9. Vitality and Barrier Integrity of the Intestinal Epithelium Model
3.3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponka, P. Cellular iron metabolism. Kidney Int. 1999, 55, S2–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, H.; Evstatiev, R.; Kornek, G.; Aapro, M.; Bauernhofer, T.; Buxhofer-Ausch, V.; Fridrik, M.; Geissler, D.; Geissler, K.; Gisslinger, H.; et al. Iron metabolism and iron supplementation in cancer patients. Wien. Klin. Wochenschr. 2015, 127, 907–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, F.; Rocha, S.; Fernandes, R. Iron Metabolism: From Health to Disease. J. Clin. Lab. Anal. 2014, 28, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. Plenary Paper Red Cells, Iron, and Erythropoiesis a systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, F.; Yip, R.; Dallman, P.R.; Olivares, M.; Hertrampf, E.; Walter, T. Iron status with different infant feeding regimens: Relevance to screening and prevention of iron deficiency. J. Pediatr. 1991, 118, 687–692. [Google Scholar] [CrossRef]
- Cantor, A.G.; Bougatsos, C.; Dana, T.; Blazina, I.; McDonagh, M. Routine Iron Supplementation and Screening for Iron Deficiency Anemia in Pregnancy: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2015, 162, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Shashiraj, A.; Faridi, M.M.; Singh, O.; Rusia, U. Mother’s iron status, breastmilk iron and lactoferrin—are they related? Eur. J. Clin. Nutr. 2006, 60, 903–908. [Google Scholar] [CrossRef]
- De Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Brissot, P.; Loréal, O. Iron metabolism and related genetic diseases: A cleared land, keeping mysteries. J. Hepatol. 2016, 64, 505–515. [Google Scholar] [CrossRef]
- Liu, K.; Kaffes, A.J. Iron deficiency anaemia: A review of diagnosis, investigation and management. Eur. J. Gastroenterol Hepatol. 2011, 24, 109–116. [Google Scholar] [CrossRef]
- Harvey, L.J.; Armah, C.N.; Dainty, J.R.; Foxall, R.J.; Lewis, D.J.; Langford, N.J.; Fairweather-Tait, S.J. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br. J. Nutr. 2005, 94, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Bourgeois, T.; Berlan, E.D.; Fischer, A.N.; O’Brien, S.; Klima, J. Iron deficiency and fatigue in adolescent females with heavy menstrual bleeding. Haemophilia 2012, 19, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auerbach, M.; Adamson, J.W. How we diagnose and treat iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Reddy, M.B. Efficacy of weekly compared with daily iron supplementation. Am. J. Clin. Nutr. 1995, 62, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.A.; Fisher, S.A.; Doree, C.; Di Angelantonio, E.; Roberts, D.J. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst. Rev. 2014, 2014, CD009532. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; Goran, E.K.N.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Armah, S.M.; Carriquiry, A.L.; Reddy, M.B. Total Iron Bioavailability from the US Diet Is Lower Than the Current Estimate. J. Nutr. 2015, 145, 2617–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, G.; Mondello, P.; Tambaro, R.; De Maria, M.; D’Amico, F.; Sticca, G.; Di Falco, C. 341 Intravenous iron support vs oral liposomal iron support in patients with refractory anemia treated with Epo alpha. Monocentric prospective study. Leuk. Res. 2011, 35, S137. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Wang, H.; Gao, G.; Yu, P.; Chang, Y. Encapsulation of Iron in Liposomes Significantly Improved the Efficiency of Iron Supplementation in Strenuously Exercised Rats. Biol. Trace Elem. Res. 2014, 162, 181–188. [Google Scholar] [CrossRef]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2014, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mafodda, A.; Giuffrida, D.; Prestifilippo, A.; Azzarello, D.; Giannicola, R.; Mare, M.; Maisano, R. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: A pilot study. Support. Care Cancer 2017, 25, 2779–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duck, K.A.; Connor, J. Iron uptake and transport across physiological barriers. BioMetals 2016, 29, 573–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, O. Molecular mechanism of intestinal iron absorption. Metallomics 2011, 3, 103–109. [Google Scholar] [CrossRef]
- Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability. Int. J. Mol. Sci. 2018, 19, 2722. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, I.V.M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Atanassova, B.D.; Tzatchev, K.N. Ascorbic acid—important for iron metabolism. Folia Medica 2009, 50, 11–16. [Google Scholar]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: Inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and intervention measures of iron side effects on the intestine. Crit. Rev. Food Sci. Nutr. 2019, 60, 2113–2125. [Google Scholar] [CrossRef]
- Kiss, L.; Hellinger, É.; Pilbat, A.; Kittel, Á.; Török, Z.; Füredi, A.; Szakács, G.; Veszelka, S.; Sipos, P.; Ózsvári, B.; et al. Sucrose Esters Increase Drug Penetration, But Do Not Inhibit P-Glycoprotein in Caco-2 Intestinal Epithelial Cells. J. Pharm. Sci. 2014, 103, 3107–3119. [Google Scholar] [CrossRef]
- Szűts, A.; Makai, Z.; Rajkó, R.; Szabó-Révész, P. Study of the effects of drugs on the structures of sucrose esters and the effects of solid-state interactions on drug release. J. Pharm. Biomed. Anal. 2008, 48, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Fokkink, R.; Peters, R.; Tromp, P.; Rivera, Z.E.H.; Rietjens, I.M.; Hendriksen, P.J.; Bouwmeester, H. Behaviour of silver nanoparticles and silver ions in anin vitrohuman gastrointestinal digestion model. Nanotoxicology 2012, 7, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Rieux, A.D.; Ragnarsson, E.G.; Gullberg, E.; Préat, V.; Schneider, Y.-J.; Artursson, P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005, 25, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Scheers, N.; Almgren, A.B.; Sandberg, A.-S. Proposing a Caco-2/HepG2 cell model for in vitro iron absorption studies. J. Nutr. Biochem. 2014, 25, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Declared Amount/Dose (mg/dose) | Measured Amount (mg/dose) | Recovery (%) | |
|---|---|---|---|
| ME with vitamin C (ME + VitC) | 30 | 29.5 ± 2.5 | 98.3 |
| SS with vitamin C (SS + VitC) | 30 | 27.7 ± 0.8 | 92.3 |
| LS | 11.3 | 12.7 ± 0.1 | 112.9 |
| PIC | 30 | 27.7 ± 0.1 | 92.2 |
| ME with folate (ME + Folate) | 30 | 27.8 ± 0.1 | 92.5 |
| ISPC | 40 | 34.9 ± 0.1 | 87.0 |
| Complete | Supernatant | Pellet | |||
|---|---|---|---|---|---|
| Formulation | mg | mg | % | mg | % |
| General-Purpose Iron Supplements | |||||
| ME + VitC | 28.35 ± 2.14 | 0.74 ± 0.12 | 2.43 ± 0.32 | 26.84 ± 2.52 | 94.71 ± 8.72 |
| SS + VitC | 27.75 ± 0.85 | 1.61 ± 0.02 | 5.78 ± 0.07 | 26.83 ± 0.20 | 96.70 ± 0.72 |
| Pediatric Iron Supplements | |||||
| LS | 11.8 ± 0.0 | 6.2 ± 0.3 | 53.0 ± 2.5 | 6.0 ± 0.1 | 50.8 ± 0.5 |
| PIC | 29.2 ± 2.2 | 0.1 ± 0.0 | 0.5 ± 0.0 | 21.7 ± 1.7 | 74.3 ± 5.9 |
| Obstetrics and Gynecology Iron Supplements | |||||
| ME + Folate | 27.76 ± 0.08 | 0.02 ± 0.00 | 0.07 ± 0.00 | 28.62 ± 0.41 | 103.11 ± 1.46 |
| ISPC | 34.85 ± 0.03 | 32.54 ± 1.73 | 96.55 ± 4.93 | 0.53 ± 0.19 | 1.82 ± 0.55 |
| Formulation | Sample | Primary Peak (nm) | d10 (nm) | d50 (nm) | d90 (nm) |
|---|---|---|---|---|---|
| General-Purpose Iron Supplements | |||||
| ME + VitC | UPW | 396.1 ± 64.3 | 17.1 | 267.7 | 511.2 |
| Surnatant | 396.1 ± 60.4 | 131.8 | 369.5 | 571.5 | |
| SS + VitC | UPW | 91.3 ± 6.3 | 41.0 | 92.2 | 210.5 |
| Surnatant | 122.4 ± 21.6 | 64.2 | 325.6 | 4447.2 | |
| Pediatric Iron Supplements | |||||
| LS | UPW | 825.0 ± 115.9 | 627.9 | 892.7 | 4381.5 |
| Surnatant | 458.7 ± 86.2 | 140.6 | 425.5 | 1013.0 | |
| PIC | UPW | 50.8 ± 7.9 | 26.6 | 75.5 | 422.4 |
| Surnatant | 122.4 ± 74.8 | 40.3 | 117.6 | 369.5 | |
| Obstetrics and Gynecology Iron Supplements | |||||
| ME + Folate | UPW | 255.0 ± 5.4 | 133.1 | 206.4 | 347.3 |
| Surnatant | 531.2 ± 88.1 | 186.5 | 511.2 | 1053.9 | |
| ISPC | UPW | 190.1 ± 41.2 | 46.4 | 151.0 | 402.0 |
| Surnatant | 68.1 ± 52.07 | 44.2 | 141.3 | 486.5 | |
| Recovery (%) | Functional Iron (%) | Released Iron (%) | |
|---|---|---|---|
| General-Purpose Iron Supplements | |||
| ME + VitC | 83.27 ± 0.01 | 85.02 ± 0.01 | 14.98 ± 0.00 |
| SS + VitC | 97.93 ± 0.02 | 68.45 ± 0.02 | 31.55 ± 0.00 |
| Pediatric Iron Supplements | |||
| LS | 99.16 ± 0.01 | 71.15 ± 0.01 | 28.85 ± 0.01 |
| PIC | 88.70 ± 0.01 | 89.41 ± 0.03 | 10.59 ± 0.01 |
| Obstetrics and Gynecology Iron Supplements | |||
| ME + Folate | 100 ± 0.10 | 87.71 ± 0.06 | 12.20 ± 0.01 |
| ISPC | 85.49 ± 0.02 | 56.13 ± 0.01 | 43.87 ± 0.01 |
| Bioavailability | ||||
|---|---|---|---|---|
| Formulation | Time (h) | Fe (µg) | Ferritin (Fold Change) | Papp (×10−4 cm/min) |
| General-Purpose Iron Supplements | ||||
| ME + VitC | 1 | 0.68 ± 0.04 | 18.3 ± 3.1 | 4.39 ± 0.18 |
| 3 | 0.71 ± 0.06 | 17.8 ± 2.0 | 1.48 ± 0.06 | |
| SS + VitC | 1 | 0.42 ± 0.13 | 12.8 ± 1.5 | 3.46 ± 1.65 |
| 3 | 2.09 ± 0.64 | 24.2 ± 1.5 | 6.28 ± 2.23 | |
| Pediatric Iron Supplements | ||||
| LS | 1 | 0.26 ± 0.08 | 17.1 ± 6.85 | 0.20 ± 0.04 |
| 3 | 1.70 ± 0.12 | 12.4 ± 1.86 | 0.44 ± 0.04 | |
| PIC | 1 | 0.00 ± 0.00 | 1.0 ± 0.2 | 0.00 ± 0.00 |
| 3 | 0.00 ± 0.00 | 1.0 ± 0.1 | 0.00 ± 0.00 | |
| Obstetrics and Gynecology Iron Supplements | ||||
| ME + Folate | 1 | 0.06 ± 0.02 | 0.3 ± 0.2 | 0.77 ± 0.25 |
| 3 | 0.08 ± 0.01 | 1.4 ± 0.0 | 0.35 ± 0.05 | |
| ISPC | 1 | 0.03 ± 0.02 | 4.3 ± 1.8 | 0.03 ± 0.02 |
| 3 | 0.06 ± 0.04 | 6.4 ± 0.9 | 0.02 ± 0.01 | |
| Dietary Supplement | Total Fe Per Day (mg) | Iron | Delivery Technology | Other Active Principles |
|---|---|---|---|---|
| General-Purpose Iron Supplements | ||||
| ME + VitC | 30.0 | Fe3+ pyrophosphate | Microencapsulation | Vitamin C |
| SS + VitC | 30.0 | Fe3+ pyrophosphate | Sucrosomial Iron | Vitamin C |
| Pediatric Iron Supplements | ||||
| LS | 11.3 | Fe2+ fumarate | Liposomial Solution | - |
| PIC | 30.0 | Fe3+ | Polydextrose-iron complex | - |
| Obstetrics and Gynecology Iron Supplements | ||||
| ME + Folate | 30.0 | Fe3+ pyrophosphate | Microencapsulation | Folate |
| ISPC | 40.0 | Fe3+ | Iron-succinylated proteins complex | - |
Sample Availability: Analyzed iron-based dietary supplements and drug are commercially available. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastore, P.; Roverso, M.; Tedesco, E.; Micheletto, M.; Mantovan, E.; Zanella, M.; Benetti, F. Comparative Evaluation of Intestinal Absorption and Functional Value of Iron Dietary Supplements and Drug with Different Delivery Systems. Molecules 2020, 25, 5989. https://doi.org/10.3390/molecules25245989
Pastore P, Roverso M, Tedesco E, Micheletto M, Mantovan E, Zanella M, Benetti F. Comparative Evaluation of Intestinal Absorption and Functional Value of Iron Dietary Supplements and Drug with Different Delivery Systems. Molecules. 2020; 25(24):5989. https://doi.org/10.3390/molecules25245989
Chicago/Turabian StylePastore, Paolo, Marco Roverso, Erik Tedesco, Marta Micheletto, Etienne Mantovan, Michela Zanella, and Federico Benetti. 2020. "Comparative Evaluation of Intestinal Absorption and Functional Value of Iron Dietary Supplements and Drug with Different Delivery Systems" Molecules 25, no. 24: 5989. https://doi.org/10.3390/molecules25245989
APA StylePastore, P., Roverso, M., Tedesco, E., Micheletto, M., Mantovan, E., Zanella, M., & Benetti, F. (2020). Comparative Evaluation of Intestinal Absorption and Functional Value of Iron Dietary Supplements and Drug with Different Delivery Systems. Molecules, 25(24), 5989. https://doi.org/10.3390/molecules25245989







