Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy
Abstract
1. Introduction
2. Reagent-Switchable Cyclizations
2.1. Endo/Exo Selective Cyclizations
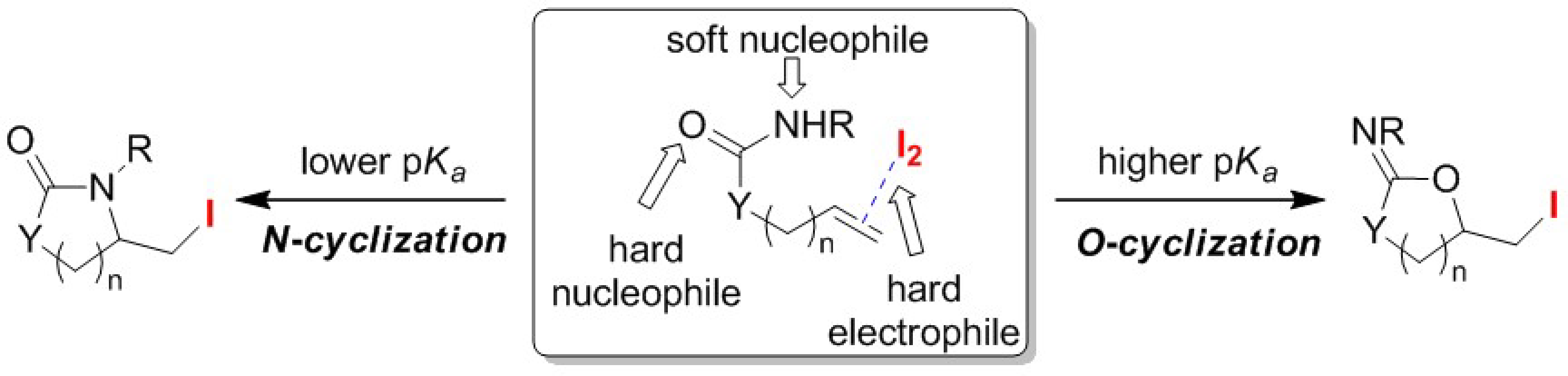
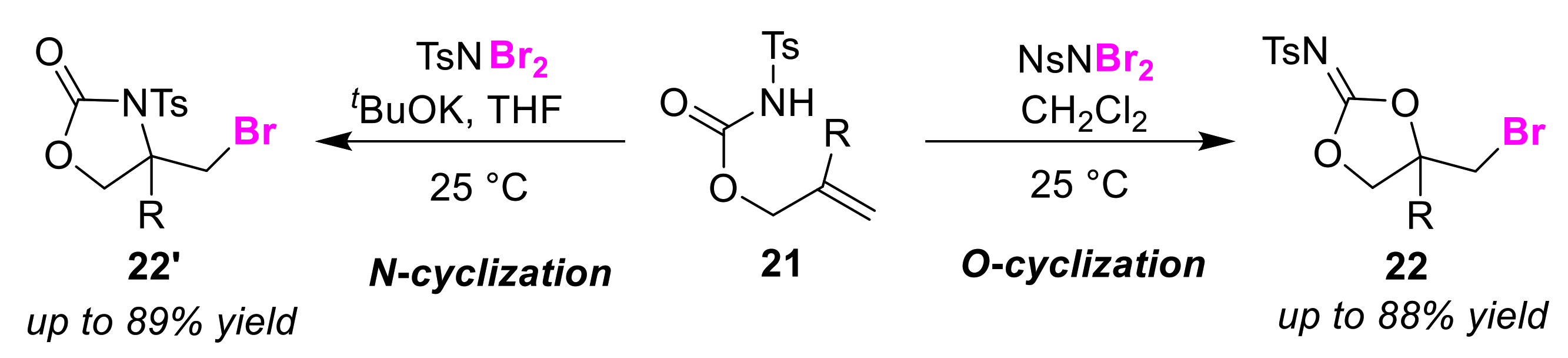
2.2. O/N Atom-Selective Cyclizations
2.3. Ene/Diene Selective Cyclizations
2.4. Syn/Anti Selective Cyclizations
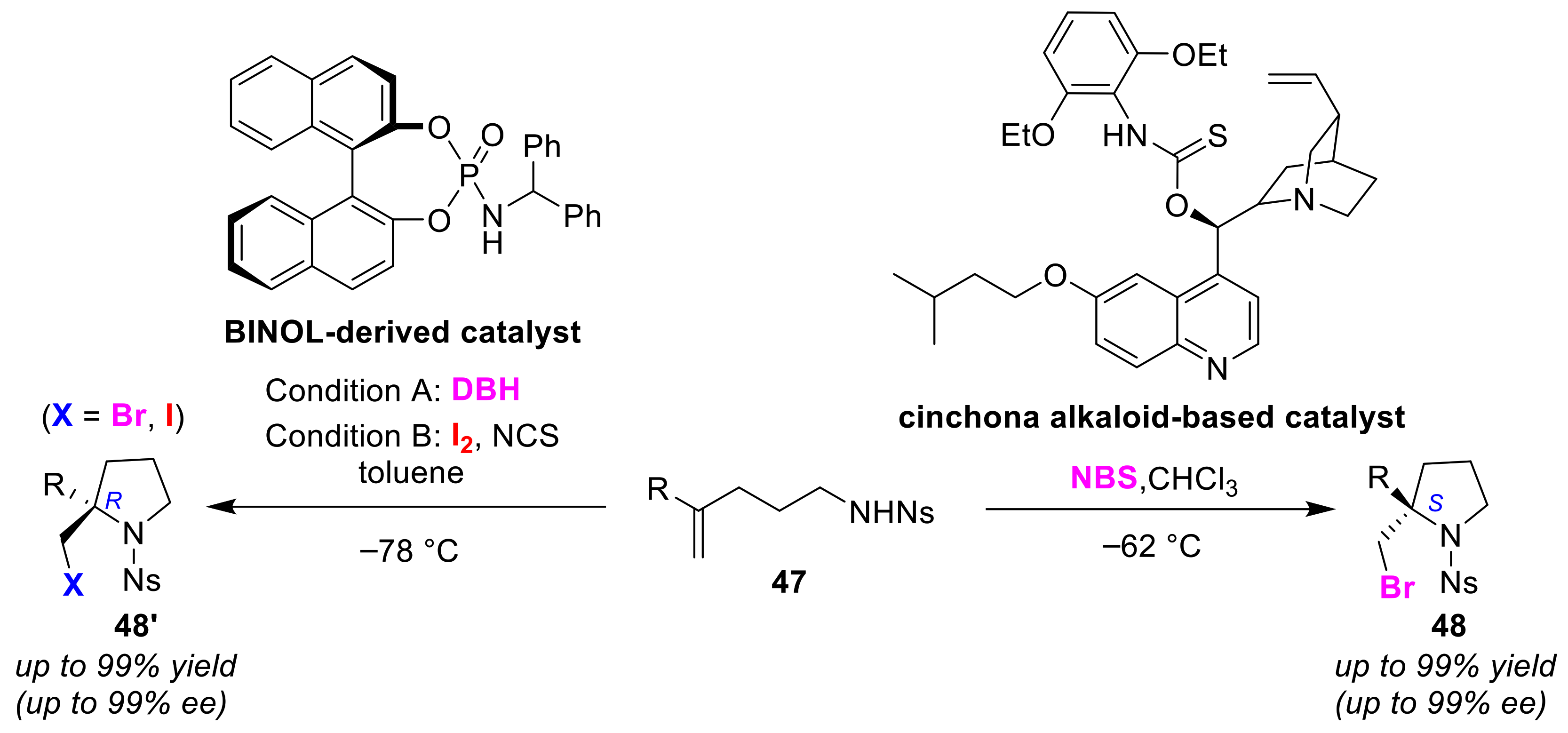
2.5. Enantioselective Cyclizations
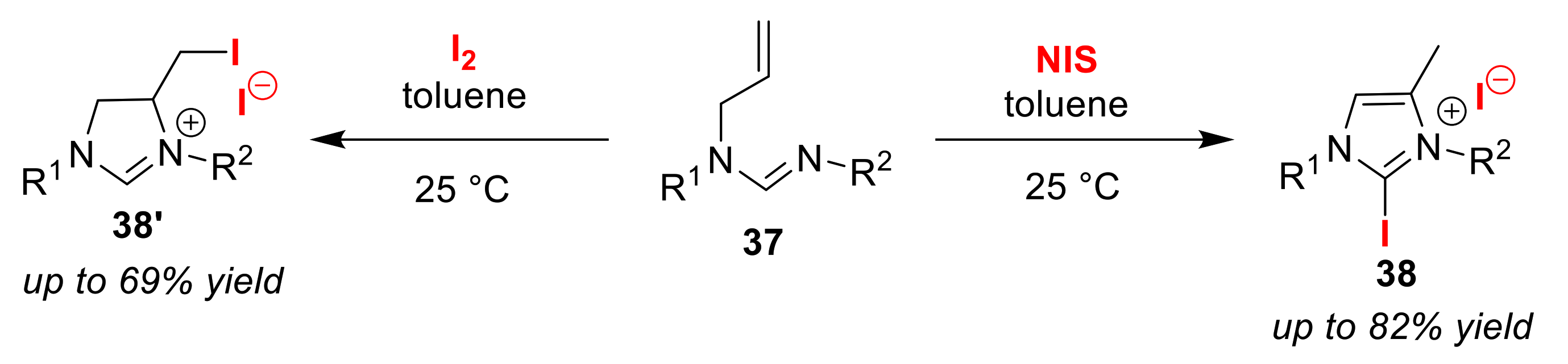
2.6. Other Miscellaneous Reactions
3. Substrate-Switchable Cyclizations
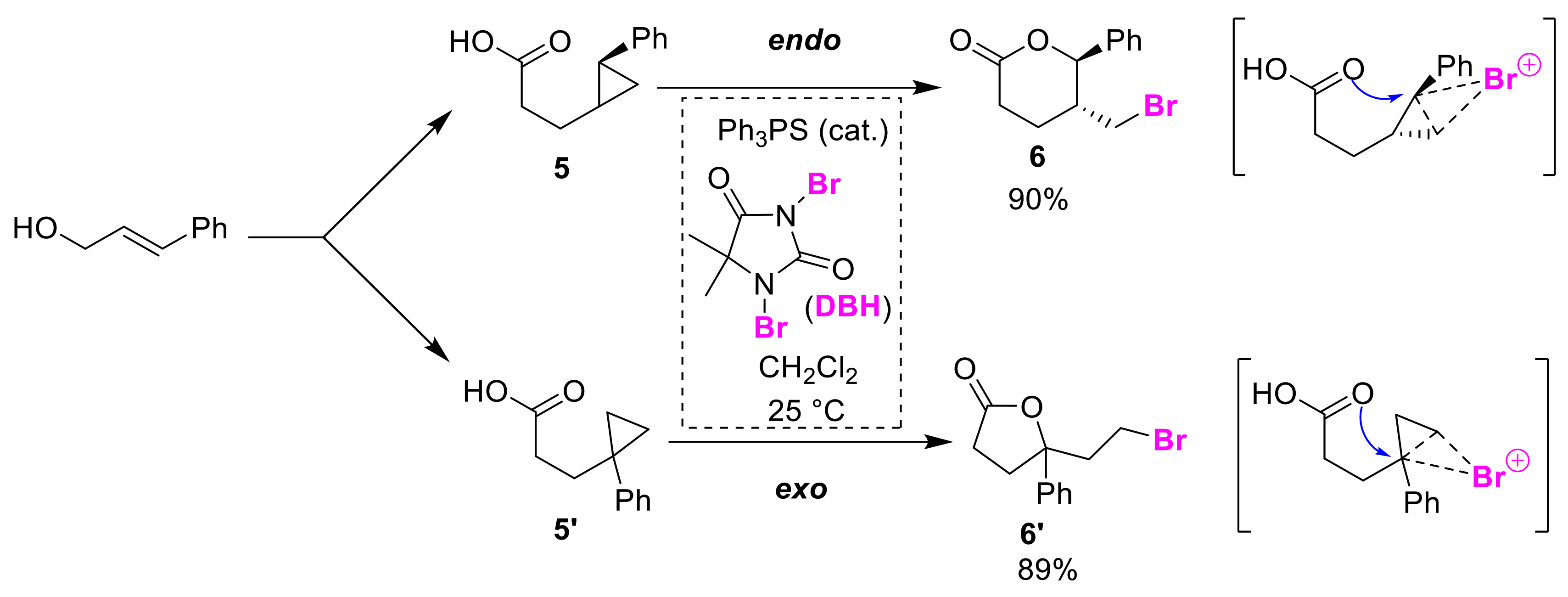
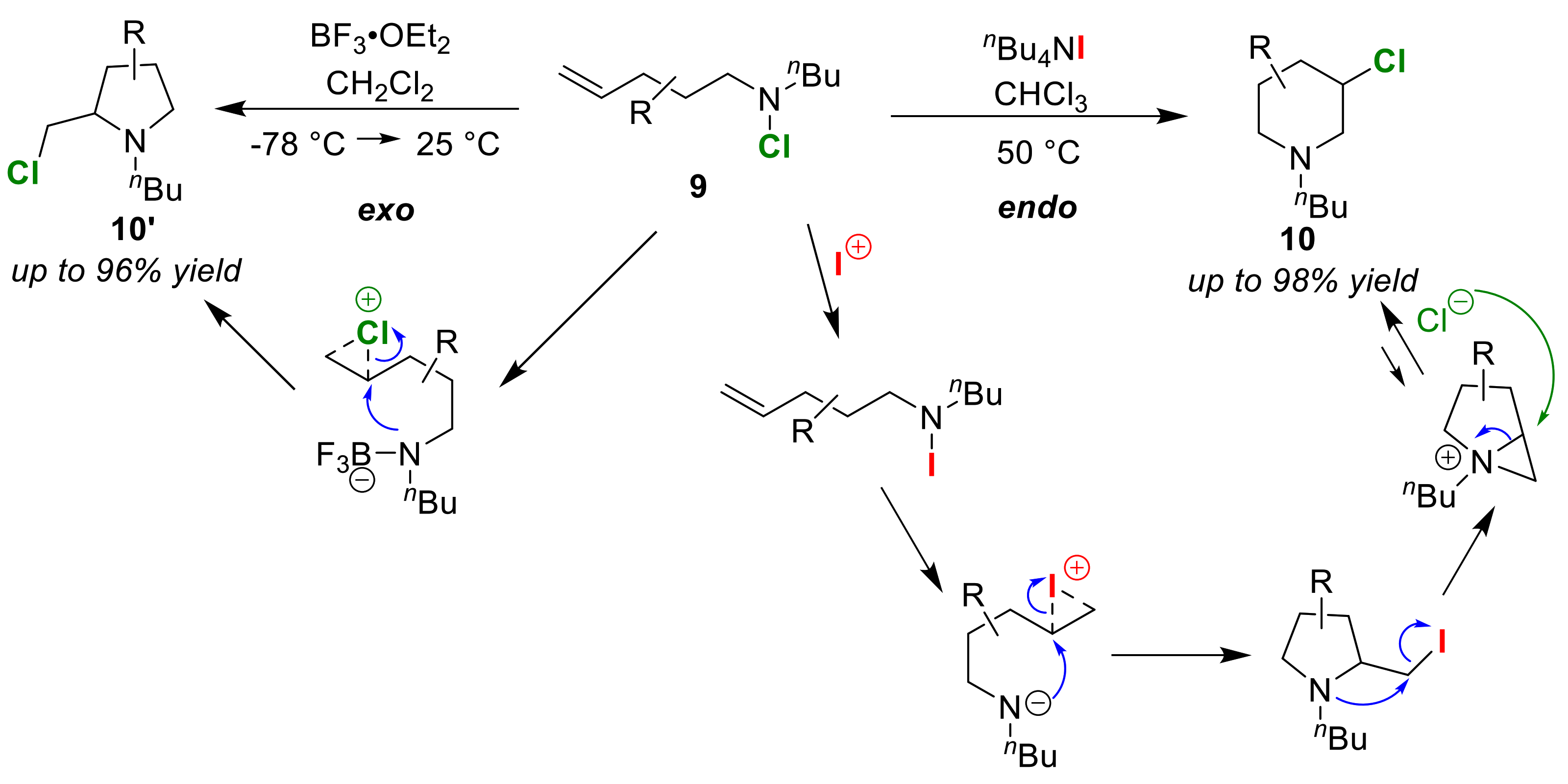
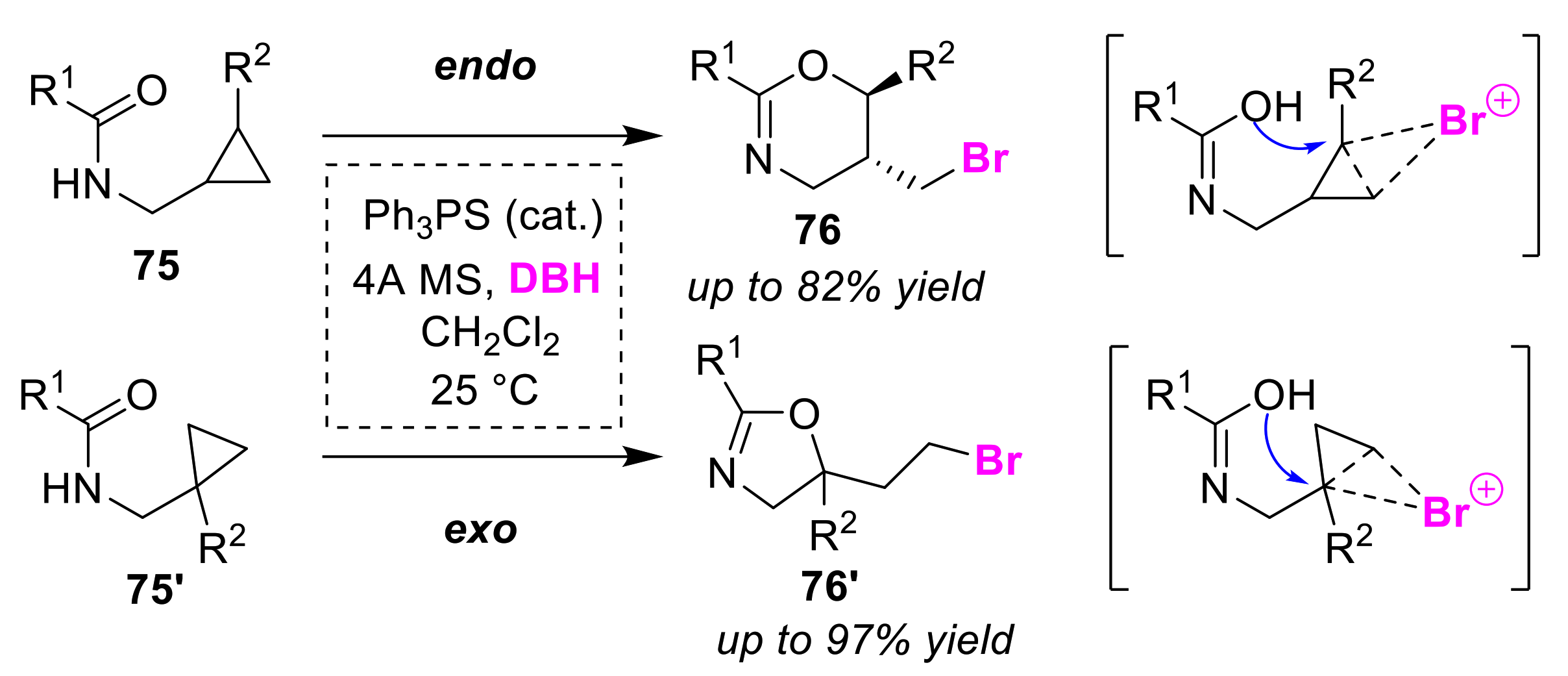
3.1. Endo/exo Selective Cyclizations
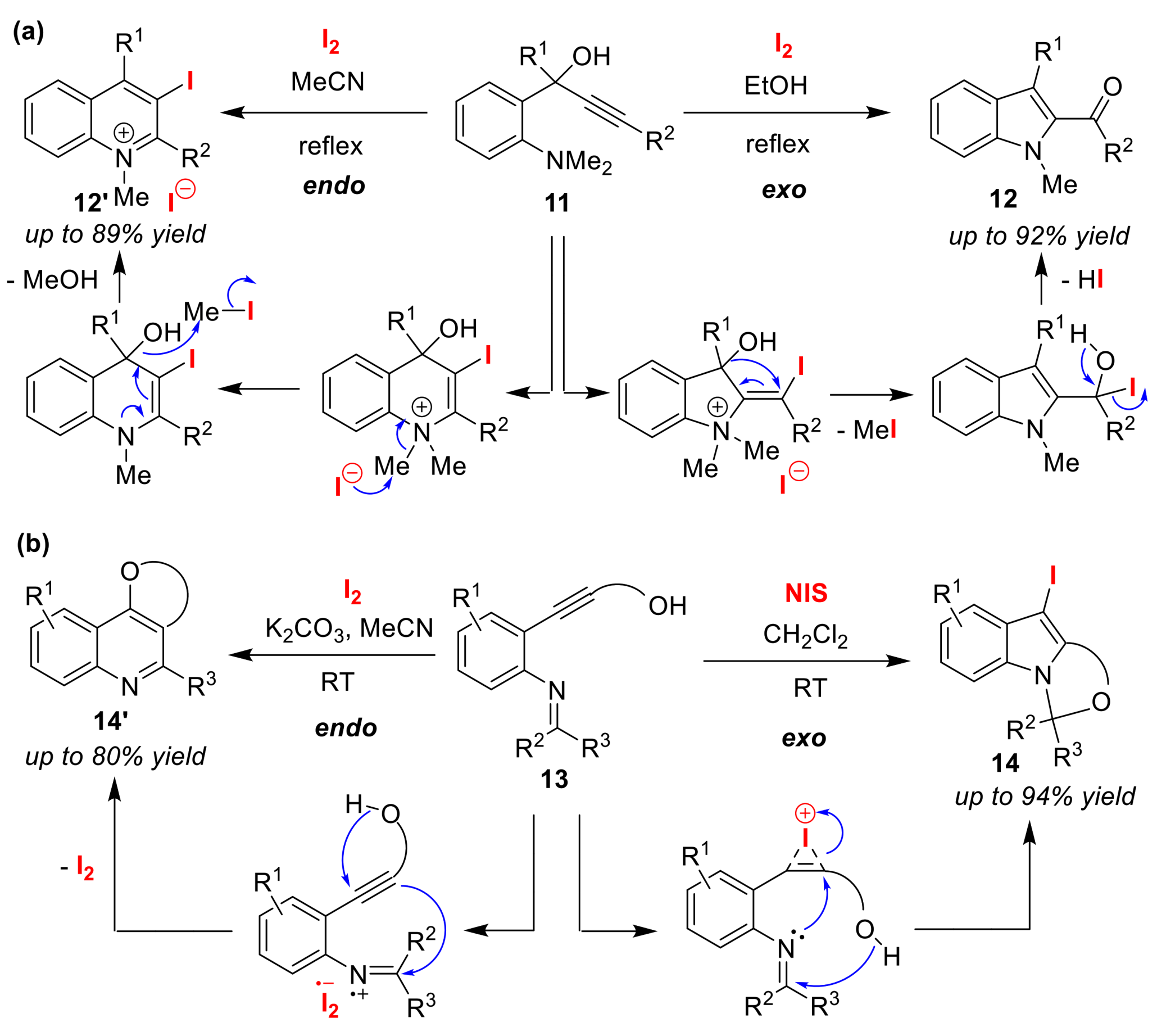
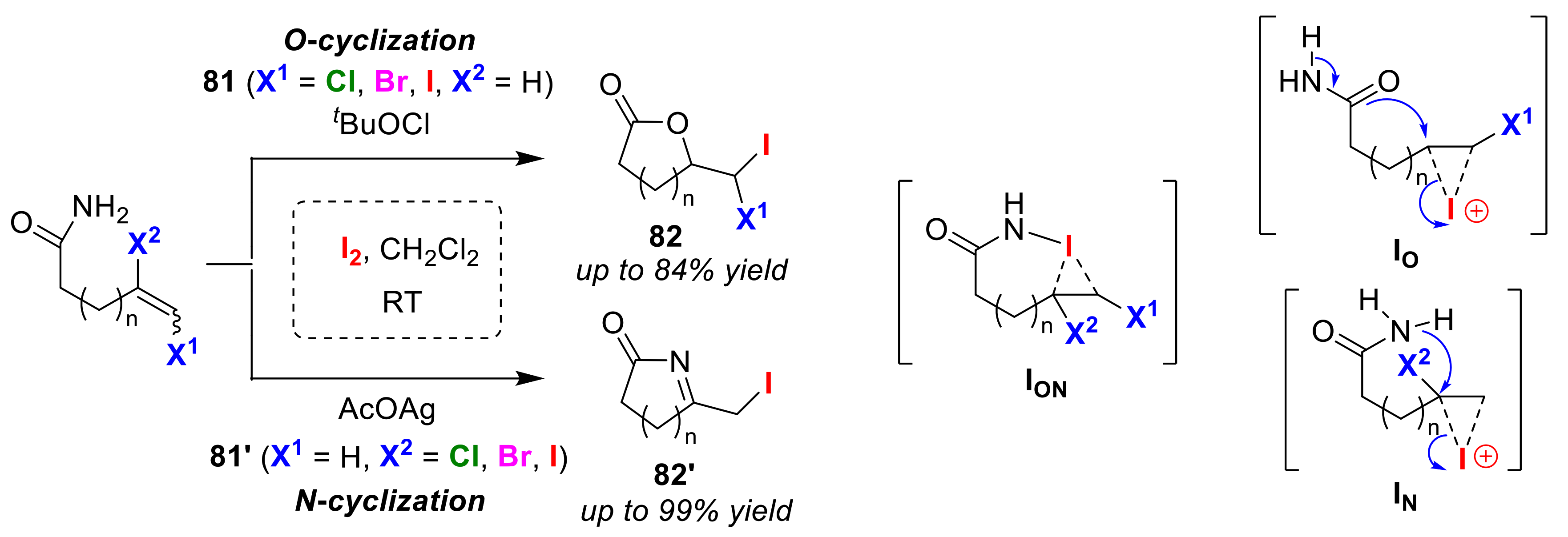
3.2. O/N Atom-Selective Cyclizations
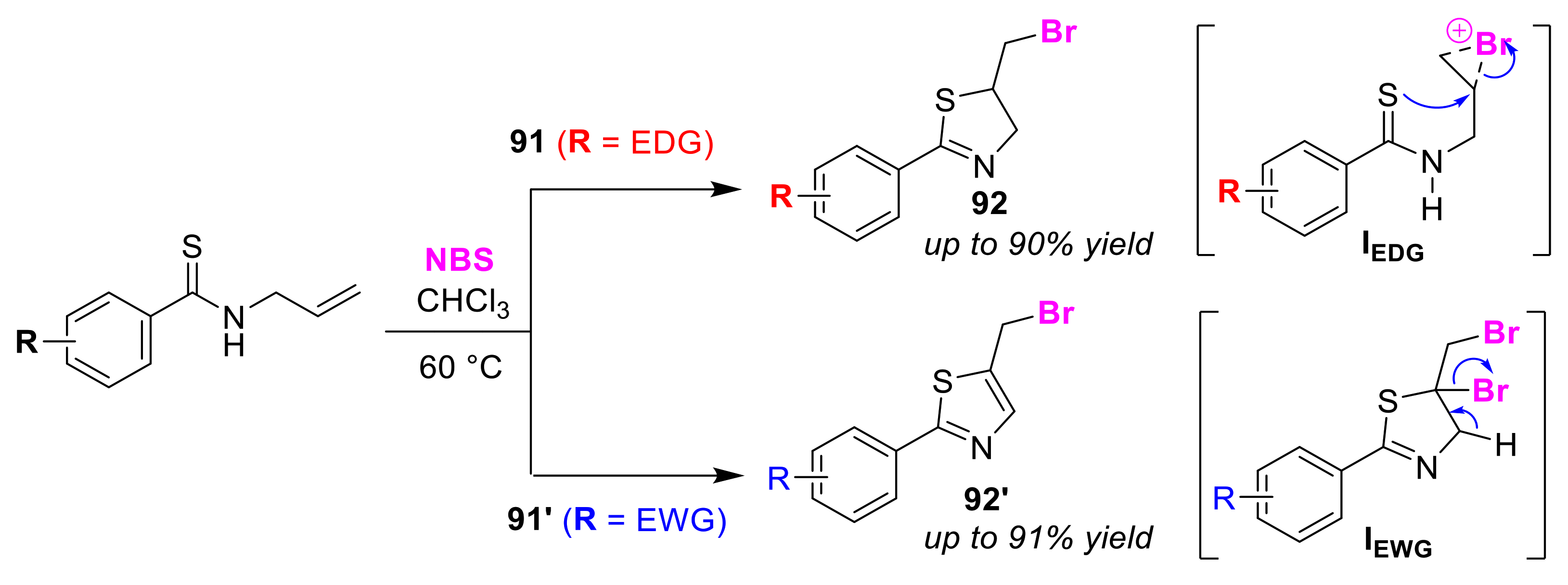
3.3. Ene/Diene Selective Cyclizations
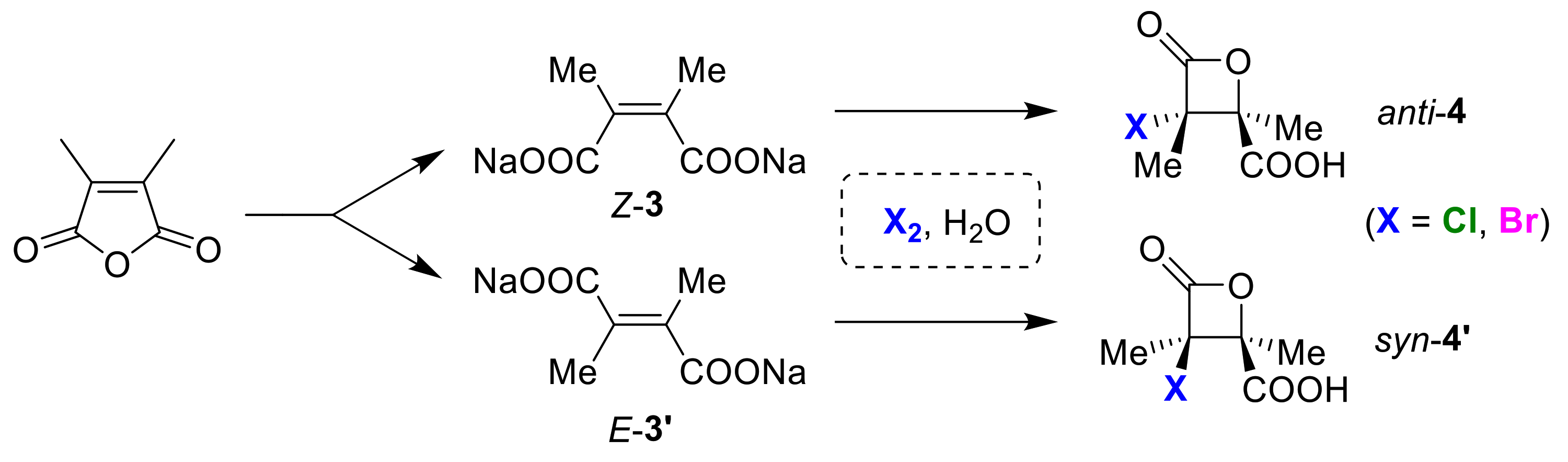
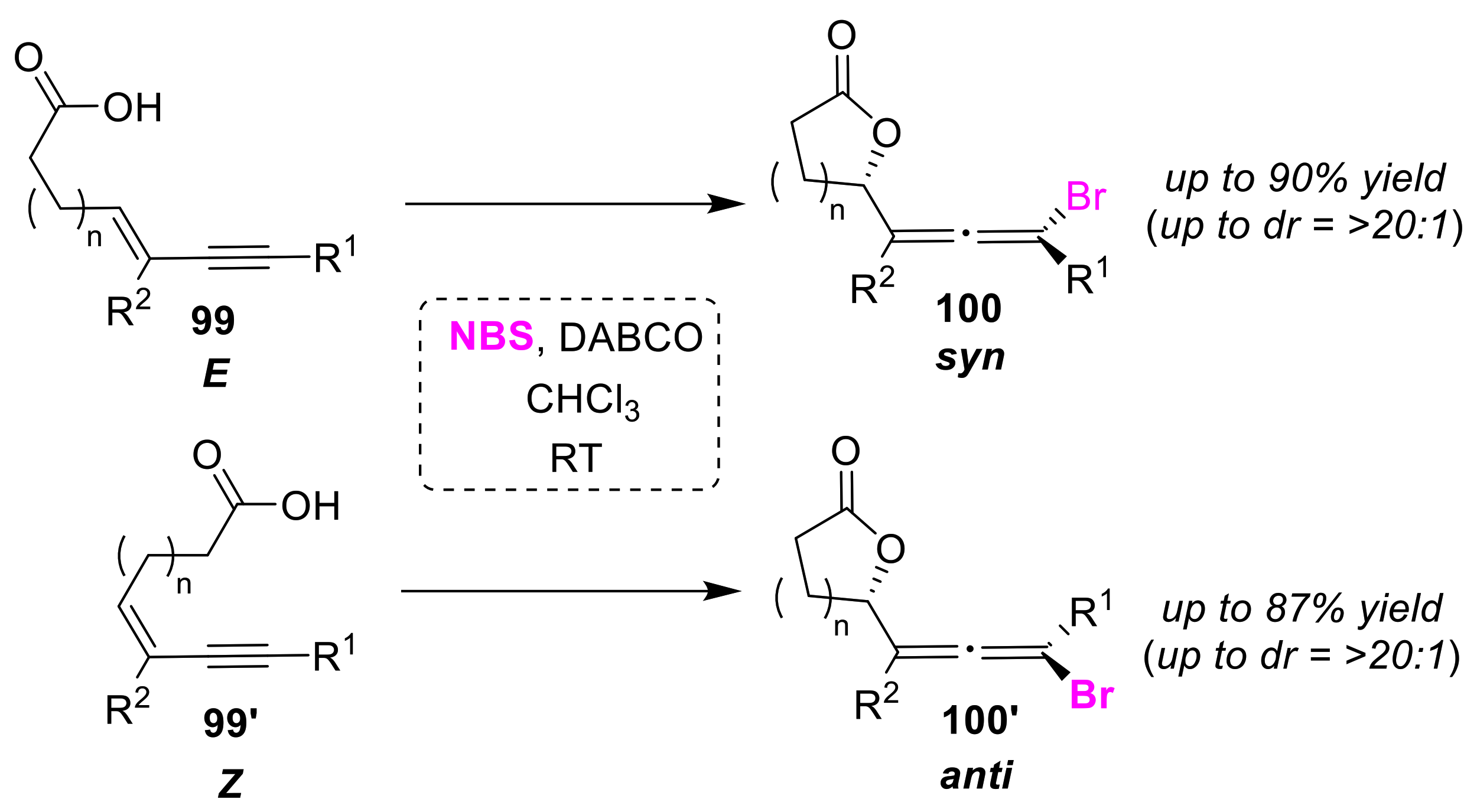
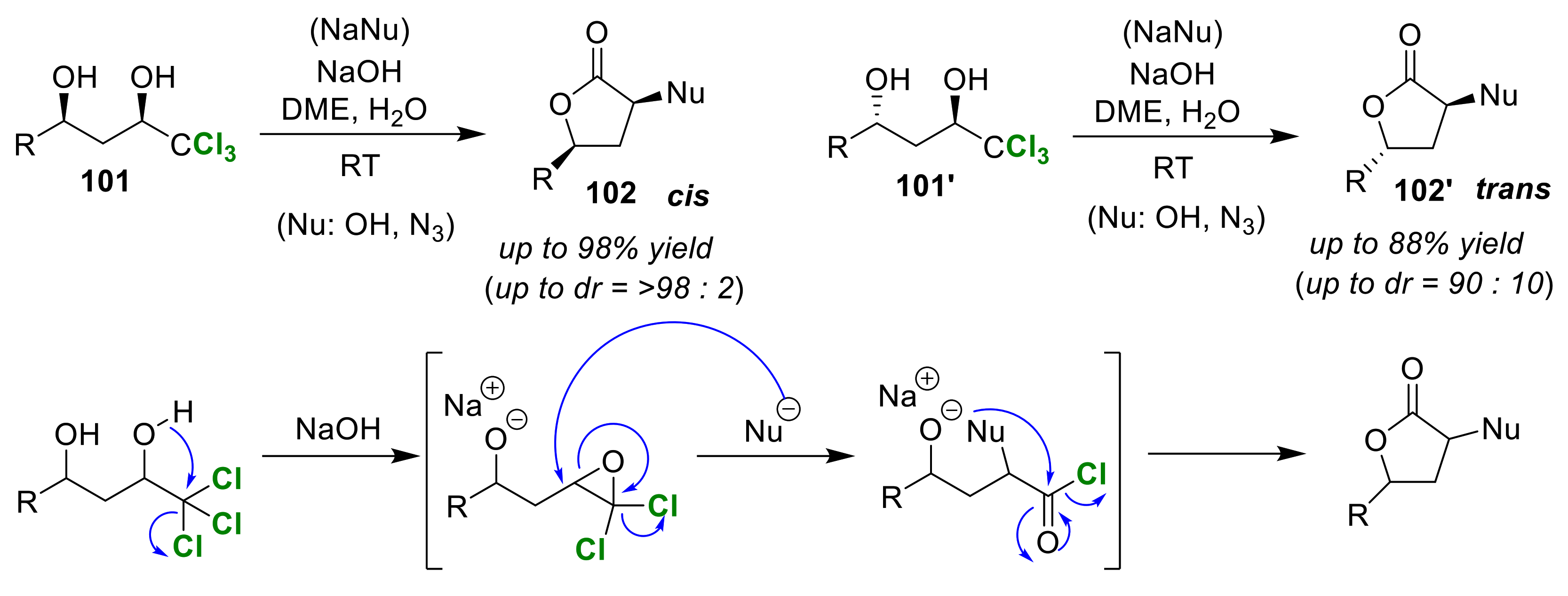
3.4. Syn/Anti (Cis/Trans) Selective Cyclizations
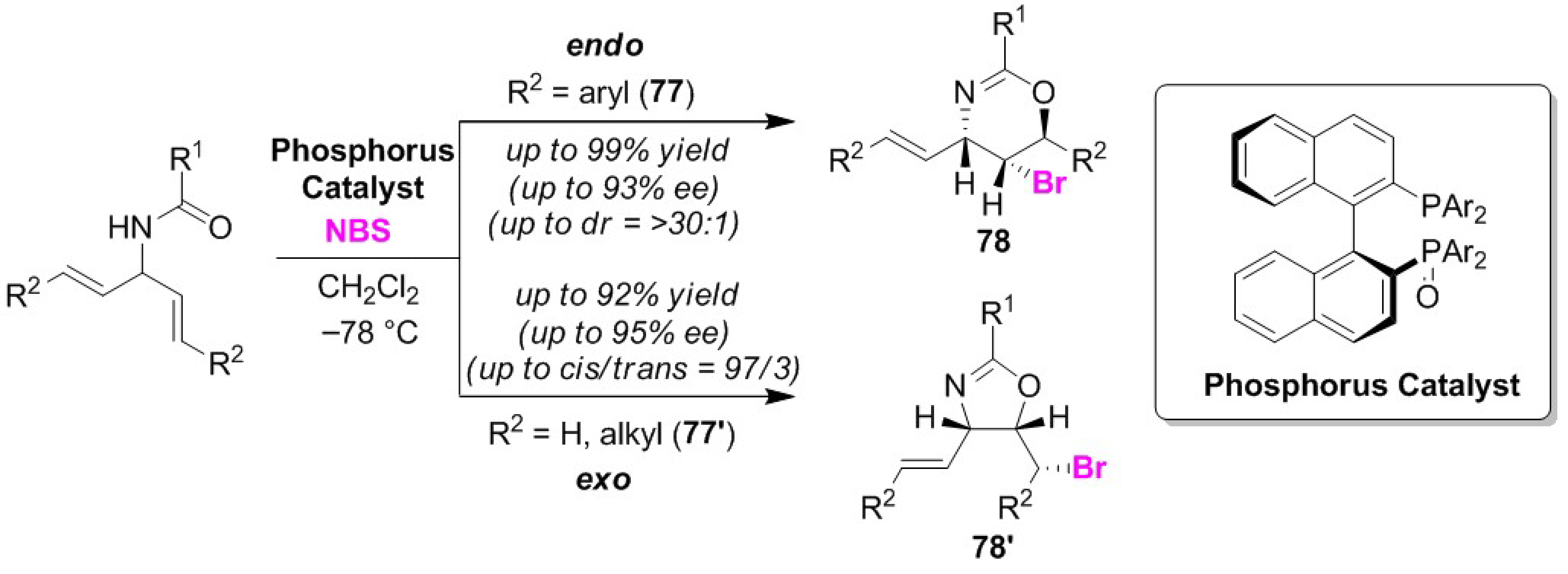
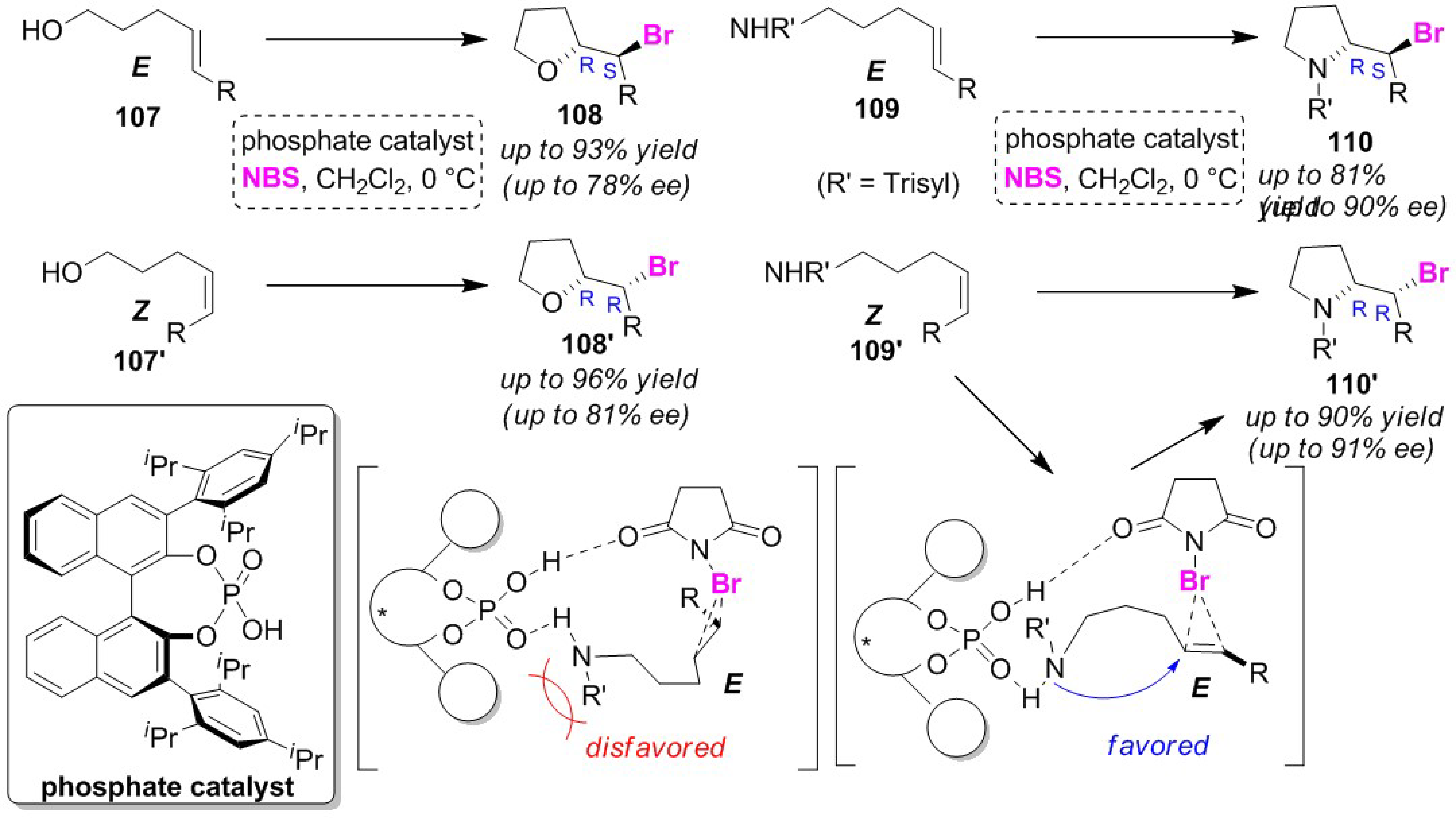
3.5. Enantioselective Cyclizations
3.6. Other Miscellaneous Reactions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nolsøe, J.M.J.; Hansen, T.V. Asymmetric iodolactonization: An evolutionary account. Eur. J. Org. Chem. 2014, 2014, 3051–3065. [Google Scholar] [CrossRef]
- Snyder, S.A.; Treitler, D.S.; Brucks, A.P. Halonium-induced cyclization reactions. Aldrichim. Acta 2011, 44, 27–40. [Google Scholar]
- van Tamelen, E.E.; Shamma, M. Assignment of the olefinic position in unsaturated acids by means of the iodolactonization reaction. J. Am. Chem. Soc. 1954, 76, 2315–2317. [Google Scholar] [CrossRef]
- Robin, S.; Rousseau, G. Formation of four-membered heterocycles through electrophilic heteroatom cyclization. Eur. J. Org. Chem. 2002, 2002, 3099–3114. [Google Scholar] [CrossRef]
- Ranganathan, S.; Muraleed, K.M.; Vaish, N.K.; Jayaraman, N. Halo- and selenolactonisation: The two major strategies for cyclofunctionisation. Tetrahedron 2004, 60, 5273–5308. [Google Scholar] [CrossRef]
- Robin, S.; Rousseau, G. Electrophilic cyclization of unsaturated amides. Tetrahedron 1998, 54, 13681–13736. [Google Scholar] [CrossRef]
- Dowle, M.D.; Davies, D.I. Synthesis and synthetic utility of halolactones. Chem. Soc. Rev. 1979, 8, 171–197. [Google Scholar] [CrossRef]
- Lyubchuk, T.V.; Hordiyenko, O.V. The use of N-halosuccinimides for cyclization with the formation of five-membered heterocyclic compounds. Chem. Heterocycl. Comp. 2020, 56, 1–29. [Google Scholar] [CrossRef]
- Khan, T.; Yaragorla, S. Iodocyclization of propargyl alcohols: Highly facile approach to hetero/carbocyclic iodides. Eur. J. Org. Chem. 2019, 2019, 3989–4012. [Google Scholar] [CrossRef]
- D’hollander, A.; Peilleron, L.; Grayfer, T.; Cariou, K. Halonium-induced polyene cyclizations. Synthesis 2019, 51, 1753–1769. [Google Scholar] [CrossRef]
- de Andrade, V.S.C.; de Mattos, M.C.S. N-Halo reagents: Modern synthetic approaches for heterocyclic synthesis. Synthesis 2019, 51, 1841–1870. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Winska, K.; Maczka, W. An overview of synthetic methods for the preparation of halolactones. Curr. Org. Synth. 2019, 16, 98–111. [Google Scholar] [CrossRef]
- Aggarwal, T.; Kumar, S.; Verma, A.K. Iodine-mediated synthesis of heterocycles via electrophilic cyclization of alkynes. Org. Biomol. Chem. 2016, 14, 7639–7653. [Google Scholar] [CrossRef]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Recent developments in the synthesis of five- and six-membered heterocycles using molecular iodine. Chem. Eur. J. 2012, 18, 5460–5489. [Google Scholar] [CrossRef] [PubMed]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J. Molecular iodine-mediated cyclization of tethered heteroatom-containing alkenyl or alkynyl systems. Molecules 2009, 14, 4814–4837. [Google Scholar] [CrossRef]
- Mizar, P.; Wirth, T. Iodoaminations of alkenes. Synthesis 2017, 49, 981–986. [Google Scholar]
- Liang, X.-W.; Zheng, C.; You, S.-L. Dearomatization through halofunctionalization reactions. Chem. Eur. J. 2016, 22, 11918–11933. [Google Scholar]
- Kandimalla, S.R.; Parvathaneni, S.P.; Sabitha, G.; Reddy, B.V.S. Recent advances in intramolecular metal-free oxidative C-H bond aminations using hypervalent iodine (III) reagents. Eur. J. Org. Chem. 2019, 2019, 1687–1714. [Google Scholar] [CrossRef]
- Khalid, M.; Mohammed, S. Recent halocyclization reactions of alkenes—A review. Indian J. Heterocycl. Chem. 2018, 28, 507–527. [Google Scholar]
- Barnett, W.E.; Sohn, W.H. Formation of β-lactones in the iodolactonization reaction. Tetrahedron Lett. 1972, 13, 1777–1780. [Google Scholar] [CrossRef]
- Tarbell, D.S.; Bartlett, P.D. The mechanism of addition reactions. Chloro- and bromo-bata-lactones from dimethylmaleic and dimethylfumaric acids. J. Am. Chem. Soc. 1937, 58, 407–410. [Google Scholar] [CrossRef]
- Robinson, J.J.; Buchanan, J.G.; Charlton, M.H.; Kinsman, R.G.; Mahon, M.F.; William, I.H. Evidence for α-lactone intermediates in addition of aqueous bromine to disodium dimethyl-maleate and -fumarate. Chem. Commun. 2001, 485–486. [Google Scholar] [CrossRef]
- Ke, Z.; Wong, Y.-C.; See, J.Y.; Yeung, Y.-Y. Electrophilic bromolactonization of cyclopropyl carboxylic acids using Lewis basic sulfide catalyst. Adv. Synth. Catal. 2016, 358, 1719–1724. [Google Scholar] [CrossRef]
- China, H.; Okada, Y.; Dohi, T. The multiple reactions in the monochlorodimedone assay: Discovery of unique delaholactonizations under mild conditions. Asian J. Org. Chem. 2015, 4, 1065–1074. [Google Scholar] [CrossRef]
- Kristianslund, R.; Tungen, J.E.; Hansen, T.V. Catalytic enantioselective iodolactonization reactions. Org. Biomol. Chem. 2019, 17, 3079–3092. [Google Scholar] [CrossRef]
- Gieuw, M.H.; Ke, Z.; Yeung, Y.-Y. Lewis base catalyzed stereo- and regioselective bromocyclozation. Chem. Rec. 2017, 17, 287–311. [Google Scholar] [CrossRef]
- Sakakura, A.; Ishihara, K. Stereoselective electrophilic cyclization. Chem. Rec. 2015, 15, 728–742. [Google Scholar] [CrossRef]
- Cheng, Y.A.; Yu, W.Z.; Yeung, Y.-Y. Recent advances in asymmetric intra- and intermolecular halofunctionalizations of alkenes. Org. Biomol. Chem. 2014, 12, 2333–2343. [Google Scholar] [CrossRef]
- Zheng, S.; Schienebeck, C.M.; Zhang, W.; Wang, H.-Y.; Tang, W. Cinchona a alkaloids as organocatalysts in enantioselective halofunctionalization of alkenes and alkynes. Asian J. Org. Chem. 2014, 3, 366–376. [Google Scholar] [CrossRef]
- Tan, C.K.; Yu, W.Z.; Yeung, Y.-Y. Stereoselective bromofunctionalization of alkenes. Chirality 2014, 26, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.B.; Mukherjee, S. Catalytic enantioselective halocyclizations beyond lactones: Emerging routes to enantioenriched nitrogenous heterocycles. Synlett 2014, 25, 163–169. [Google Scholar] [CrossRef]
- Hennecke, U.; Wilking, M. Desymmetrization as a strategy in asymmetric halocyclization reactions. Synlett 2014, 25, 1633–1637. [Google Scholar] [CrossRef]
- Chemler, S.R.; Bovino, M. Catalytic aminohalogenation of alkenes and alkynes. ACS Catal. 2013, 3, 1076–1091. [Google Scholar] [CrossRef]
- Murai, K.; Fujioka, H. Recent progress in organocatalytic asymmetric halocyclization. Heterocycles 2013, 87, 763–805. [Google Scholar]
- Mendoza, A.; Fañanás, F.J.; Rodriguez, F. Asymmetric halocyclizations of unsaturated compounds: An overview and recent developments. Curr. Org. Synth. 2013, 10, 384–393. [Google Scholar] [CrossRef]
- Denmark, S.E.; Kuester, W.E.; Burk, M.T. Catalytic, asymmetric halofuncyionalization of alkenes-a critical perspective. Angew. Chem. Int. Ed. 2012, 51, 10938–10953. [Google Scholar] [CrossRef]
- Hennecke, U. New catalytic approaches towards the enantioselective halogenation of alkenes. Chem. Asian J. 2012, 7, 456–465. [Google Scholar] [CrossRef]
- Castellanos, A.; Fletcher, S.P. Current methods for asymmetric halogenation of olefins. Chem. Eur. J. 2011, 17, 5766–5776. [Google Scholar] [CrossRef]
- Tan, C.-K.; Zhou, L.; Yeung, Y.-Y. Organocatalytic enantioselective halolactonizations. Strategies of halogen activation. Synlett 2011, 2011, 1335–1339. [Google Scholar]
- Noack, M.; Kalsow, S.; Gӧttlich, R. Cyclisation of unsaturated N-chloroamines under acidic condition: A polar reaction via chloronium ions. Synlett 2004, 2004, 1110–1112. [Google Scholar]
- Noack, M.; Gӧttlich, R. Iodide-catalysed cyclization of unsaturated N-chloroamines: A new way to synthesise 3-chloropiperidines. Eur. J. Org. Chem. 2002, 2002, 3171–3178. [Google Scholar] [CrossRef]
- Fusaon, R.C.; Zirkle, C.L. Ring enlargement by rearrangement of the 1,2-aminochloroalkyl group; Rearrangement of 1-ethyl-2-chloromethylprrolidine to 1-etyl-3-chloropiperidine. J. Am. Chem. Soc. 1948, 70, 2760–2762. [Google Scholar] [CrossRef]
- Hammer, C.F.; Heller, S.R.; Craig, J.H. Reactions of β-substituted amines-II: Nucleophilic displacement reactions on 3-chloro-1-ethylpiperidine. Tetrahedron 1972, 28, 239–253. [Google Scholar] [CrossRef]
- Hessian, K.O.; Flynn, B.L. Selective endo and exo iodocyclizations in the synthesis of quinolines and indoles. Org. Lett. 2006, 8, 243–246. [Google Scholar] [CrossRef]
- Halim, R.; Scammells, P.J.; Flynn, B.L. Alternating iodonium-mediated reaction cascades giving indole- and quinoline-containing polycycles. Org. Lett. 2008, 10, 1967–1970. [Google Scholar] [CrossRef]
- Halim, R.; Aurelio, L.; Scammells, P.J.; Flynn, B.L. Scafford-divergent synthesis of ring-fused indoles, quinolines, and quinolones via iodonium-induced reaction cascades. J. Org. Chem. 2013, 78, 4708–4718. [Google Scholar] [CrossRef]
- Mizar, P.; Burrelli, A.; Günther, E.; Softje, M.; Farooq, U.; Wirth, T. Organocatalytic stereoselective iodoamination of alkenes. Chem. Eur. J. 2014, 20, 13113–13116. [Google Scholar] [CrossRef]
- Hirama, M.; Iwashita, M.; Yamasaki, Y.; Ito, S. Carbamate mediated functionalization of unsaturated alcohols II. Regio- and stereo-selective synthesis of 1,3-syn and 1,2-anti amino-alcohol derivatives via iodocarbamation. Tetrahedron Lett. 1984, 25, 4963–4964. [Google Scholar] [CrossRef]
- Kitagawa, O.; Fujita, M.; Li, H.; Taguchi, T. Regio-controlled iodoaminocyclization reaction of an ambident nucleophile mediated by LiAl(OtBu)4. Tetrahedron Lett. 1997, 38, 615–618. [Google Scholar]
- Fujita, M.; Kitagawa, O.; Suzuki, T.; Taguchi, T. Regiocontrolled iodoaminocyclization reaction of an ambident nucleophile mediated by basic metallic reagent. J. Org. Chem. 1997, 62, 7330–7335. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-T.; Sun, M.-H.; Gao, G.; Chen, J.; Zhou, L. Regioselective bromocyclization of unsaturated N-tosylcarbamates promoted by N,N-dibromosulfonamides. Synlett 2014, 25, 1921–1925. [Google Scholar]
- Peilleron, L.; Retailleau, P.; Cariou, K. Synthesis of cyclic N-hydroxylated ureas and oxazolidinone oximes enabled by chemoselective iodine(III)-mediated radical or cationic cyclizations of unsaturated N-alkoxyureas. Adv. Synth. Catal. 2019, 361, 5160–5169. [Google Scholar] [CrossRef]
- Rao, W.-H.; Yin, X.-S.; Shi, B.-F. Catalyst-controlled amino- versus oxy-acetoxylation of urea-tethered alkenes: Efficient synthesis of cyclic ureas and isoureas. Org. Lett. 2015, 17, 3758–3761. [Google Scholar] [CrossRef] [PubMed]
- Cochran, B.M.; Michael, F.E. Metal-free oxidative cyclization of urea-tethered alkenes with hypervalent iodine. Org. Lett. 2008, 10, 5039–5042. [Google Scholar] [CrossRef]
- Muñiz, K.; Hövelmann, C.H.; Campos-Gómez, E.; Barluenga, J.; González, J.M.; Streuff, J.; Nieger, M. Intramolecular diamination of alkenes with palladium(II)/copper(II) bromide and IPy2BF4: The role of halogenated intermediates. Chem. Asian. J. 2008, 3, 776–788. [Google Scholar]
- Broggini, G.; Barbera, V.; Beccalli, E.M.; Chiacchio, U.; Fasana, A.; Galli, S.; Gazzola, S. Selective intramolecular palladium(II)-catalyzed aminooxygenation vs. deamination of alkenylureas: Efficient microwave-assisted reactions to bicyclic piperazinones. Adv. Synth. Catal. 2013, 355, 1640–1648. [Google Scholar] [CrossRef]
- Li, H.; Widenhoefer, R.A. Intramolecular diamination and alkoxyamination of alkenes with N-sulfonyl ureas employing N-iodosuccinimide. Tetrahedron 2010, 66, 4827–4831. [Google Scholar] [CrossRef]
- Alanine, A.I.D.; Fishwick, C.W.G.; Szantay, C., Jr. Facile proparation of 2-imino tetrahydrofurans, pyrans and oxepans. Tetrahedron Lett. 1989, 30, 6571–6572. [Google Scholar] [CrossRef]
- Okitsu, T.; Sato, K.; Potewar, T.M.; Wada, A. Reagent-controlled oxidative aromatization in iodocyclization: Switchable access to dihydropyrazoles and pyrazoles. Org. Lett. 2010, 12, 3506–3509. [Google Scholar] [CrossRef]
- Okitsu, T.; Sato, K.; Potewar, T.M.; Wada, A. Iodocyclization of hydroxylamine derivatives based on the control of oxidative aromatization leading to 2,5-dihydroisoxazoles. J. Org. Chem. 2011, 76, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, T.; Yumitate, S.; Sato, K.; In, Y.; Wada, A. Substituent effect of bis(pyridines)iodonium complexes as iodinating reagents: Control of the iodocyclization/oxidation process. Chem. Eur. J. 2013, 19, 4992–4996. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-C.; Hu, F.; Huo, Y.-M.; Chang, H.-H.; Li, X.; Wei, W.-L. I2-catalyzed C-O bond formation and dehydrogenation: Facile synthesis of oxazolines and oxazoles controlled by bases. Org. Lett. 2015, 17, 3914–3917. [Google Scholar] [CrossRef]
- Li, J.; Jiao, J.; Zhang, C.; Shi, M.; Zhang, J. Facile syntheses of N-heterocyclic carbene precursors through I2- or NIS-promoted amidiniumation of N-alkenyl formamidines. Chem. Asian J. 2016, 11, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Gieuw, M.H.; Leung, V.M.-Y.; Ke, Z.; Yeung, Y.-Y. Electrophilic bromolactonization of cyclopropyl diesters using Lewis basic chalcogenide catalysts. Adv. Synth. Catal. 2018, 360, 4306–4311. [Google Scholar] [CrossRef]
- Takano, S.; Sato, N.; Akiyama, M.; Ogasawara, K. A synthesis of trans- and cis-caronaldehydes. Heterocycles 1985, 23, 2859–2872. [Google Scholar] [CrossRef]
- Garzan, A.; Jaganathan, A.; Marzijarani, N.S.; Yousefi, R.Y.; Whitehead, D.C.; Jackson, J.E.; Borthan, B. Solvent-dependent enantiodivergence in the chlorocyclization of unsaturated carbamates. Chem. Eur. J. 2013, 19, 9015–9021. [Google Scholar] [CrossRef]
- Cao, Q.; Luo, J.; Zhao, X. Chiral sulfide catalysis for desymmetrizing enantioselective chlorination. Angew. Chem. Int. Ed. 2019, 58, 1315–1319. [Google Scholar]
- Zhou, L.; Chen, J.; Tan, C.K.; Yeung, Y.-Y. Enantioselective bromoaminocyclization using amino-thiocarbamate catalysts. J. Am. Chem. Soc. 2011, 133, 9164–9167. [Google Scholar] [CrossRef]
- Lu, Y.; Nakatsuji, H.; Okamura, Y.; Yao, L.; Ishihara, K. Enantioselective halo-oxy- and halo-azacyclizations induced by chiral amidophosphate catalysts and halo-lewis acids. J. Am. Chem. Soc. 2018, 140, 6039–6043. [Google Scholar] [CrossRef]
- Ranganathan, S.; Ranganathan, D.; Mehrotra, A.K. A novel facet of the halolactonisation reaction. Tetrahedron 1977, 33, 807–812. [Google Scholar] [CrossRef]
- Askani, R.; Keller, U. Halogenlactonisierung zur stereokontrollierten synthese von rac-isolineatin. Justus Liebigs Ann. Chem. 1988, 1988, 61–68. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, J. Access to functionalized isoquinoline N-oxides via sequential electrophilic cyclization/cross-coupling reactions. Adv. Synth. Catal. 2008, 350, 1850–1854. [Google Scholar] [CrossRef]
- Singha, R.; Ghosh, M.; Das, S.; Das, D.; Ray, J.K. Synthesis of 1,3-dibromo-2-aryl-1H-indenes via NBS mediated unusual bromination of 2-alkynylbenzaldoximes. New. J. Chem. 2016, 40, 7269–7272. [Google Scholar] [CrossRef]
- Janreddy, D.; Kavata, V.; Kotipalli, T.; Rajawinslin, R.R.; Kuo, C.-W.; Huang, W.-C.; Yao, C.-F. Molecular iodine-mediated reaction of 2-(2-phenylethynyl)-Morita-Baylis-Hillman adducts: An easy route to naphthyl ketones and iodo-substituted isochromenes. Org. Biomol. Chem. 2014, 12, 8247–8256. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Burk, M.T. Lewis base catalysis of bromo- and iodolactonization, and cycloetherification. Proc. Natl. Acad. Sci. USA 2010, 107, 20655–20660. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Le, C.; Yeung, Y.-Y. Aminothiocarbamate-catalyzed asymmetric bromolactonization of 1,2-disubstituted olefinic acids. Org. Lett. 2011, 13, 2738–2741. [Google Scholar] [CrossRef]
- Tan, C.K.; Le, C.; Yeung, Y.-Y. Enantioselective bromolactonization of cis-1,2-disubstituted olefinic acids using an amino-thiocarbamate catalyst. Chem. Commun. 2012, 48, 5793–5795. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Hamamoto, Y.; Satoh, R.; Akada, N.; Kajita, S.; Nomoto, M.; Miyato, M.; Nakamura, M.; Matsubara, C.; Hara, O. Enantioselective bromolactonization of trisubstituted olefinic acids catalyzed by chiral pyridyl phosphoramides. Chem. Eur. J. 2018, 24, 18880–18885. [Google Scholar] [CrossRef]
- Nishiyori, R.; Tsuchihashi, A.; Mochizuki, A.; Kaneko, K.; Yamanaka, M.; Shirakawa, S. Design of chiral bifunctional dialkyl sulfide catalysts for regio-, diastereo-, and enantioselective bromolactonization. Chem. Eur. J. 2018, 24, 16747–16752. [Google Scholar] [CrossRef]
- Tsuchihashi, A.; Shirakawa, S. Catalyst-controlled regio- and stereoselective bromolactonization with chiral bifunctional sulfides. Synlett 2019, 30, 1662–1666. [Google Scholar] [CrossRef]
- Hu, T.; Shen, M.; Chen, Q.; Li, C. Pushing radical cyclization from regioselective to regiospecific: Cyclization of amidyl radicals controlled by vinylic halogen substitution. Org. Lett. 2006, 8, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, Q.; Li, C. Control of the regioselectivity of sulfonamidyl radical cyclization by vinylic halogen substitution. J. Org. Chem. 2007, 72, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Q.; Li, Y.-M. Regioselective (diacetoxyiodo)benzene-promoted halocyclization of unfunctionalized olefins. J. Org. Chem. 2014, 79, 10094–10109. [Google Scholar] [CrossRef]
- Jaganathan, A.; Garzan, A.; Whitehead, D.C.; Staples, R.J.; Borhan, B. A catalytic asymmetric chlorocyclization of unsaturated amides. Angew. Chem. Int. Ed. 2011, 50, 2593–2596. [Google Scholar] [CrossRef]
- Jaganathan, A.; Borhan, B. Chlorosulfonamide salts are superior electrophilic chlorine precursors for the organocatalytic asymmetric chlorocyclization of unsaturated amides. Org. Lett. 2014, 16, 3616–3619. [Google Scholar] [CrossRef]
- Wong, Y.-C.; Ke, Z.; Yeung, Y.-Y. Lewis basic sulfide catalyzed electrophilic bromocyclization of cyclopropylmethyl amide. Org. Lett. 2015, 17, 4944–4947. [Google Scholar] [CrossRef]
- Kawato, Y.; Hamashima, Y. Enantioselective bromocyclization of allylic amides mediated by phosphorus catalysis. Synlett 2018, 29, 1257–1271. [Google Scholar]
- Balko, T.W.; Brinkmeyer, R.S.; Terando, N.H. Halocyclizations: The cyclization of heterocyclic olefinic amides and urea. Tetrahedron Lett. 1989, 30, 2045–2048. [Google Scholar] [CrossRef]
- Hu, T.; Liu, K.; Shen, M.; Yuan, X.; Tang, Y.; Li, C. O-Attack versus N-attack: Electrophilic halocyclization ofunsaturated amides with vinylic halogen substitution. J. Org. Chem. 2007, 72, 8555–8558. [Google Scholar] [CrossRef]
- Minakata, S.; Morino, Y.; Oderaotoshi, Y.; Komatsu, M. Practical and convenient synthesis of N-heterocycles: Stereoselective cyclization of N-alkenylamides with t-BuOI under neutral conditions. Org. Lett. 2006, 8, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Hidaka, I.; Oderaotoshi, Y.; Komatsu, M.; Minakata, S. Electrophilic cyclization of N-alkenylamides using a chloramine-T/I2 system. Tetrahedron 2006, 62, 12247–12251. [Google Scholar] [CrossRef]
- Yi, W.; Wang, P.-F.; Lu, M.; Liu, Q.-Q.; Bai, X.; Chen, K.-D.; Zhang, J.-W.; Liu, G.-Q. Environmentally friendly protocol for the oxidative iodofunctionalization of olefins in a green solvent. ACS Sustain. Chem. Eng. 2019, 7, 16777–16785. [Google Scholar] [CrossRef]
- Okitsu, T.; Nakahigashi, H.; Sugihara, R.; Fukuda, I.; Tsuji, S.; In, Y.; Wada, A. Silyl group-directed 6-exo-dig iodocyckization of homopropargylic carbamates and amides. Chem. Eur. J. 2018, 24, 18638–18642. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-C.; Jiang, S.; Wang, R.-L.; Zhang, C. Iodine-mediated intramolecular amination of ketones: The synthesis of 2-acylindoles and 2-acylindolines by tuning N-protecting groups. Chem. Commun. 2013, 49, 4890–4892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Cyclization reaction of N-allylbenzothioamide for direct construction of thiazole and thiazoline. Tetrahedron Lett. 2015, 56, 4128–4130. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Gurjar, M.K.; Nallaganchu, B.R.; Bkandari, A. Studies on cyclodepsipeptides-part I: A stereoselective synthesis of C12 polyketide unit (C1-C8) present in Jaspamide and Geodiamolide A-F. Tetrahedron Lett. 1993, 34, 7081–7084. [Google Scholar] [CrossRef]
- Trova, M.P.; Wissner, A.; Casscles, W.T.; Hsu, G.C. Asymmetric synthesis of cis- and trans-γ-lactones useful in HIV-1 protease inhibitor synthesis. Bioorg. Med. Chem. Lett. 1994, 4, 903–906. [Google Scholar] [CrossRef]
- Moriyama, K.; Sugiue, T.; Nishinohara, C.; Togo, H. Divergent Synthesis of α,γ-Disubstituted γ-Butyrolactones through Diastereoselective Bromolactonization with Alkali Metal Bromide: Asymmetric Total Synthesis of (+)-Dubiusamine C. J. Org. Chem. 2015, 80, 9132–9140. [Google Scholar] [CrossRef]
- Moriyama, K.; Nishinohara, C.; Sugiue, T.; Togo, H. Oxidative oxygen-nucleophilic bromo-cyclization of alkenyl carbonyl compounds without organic wastes using alkali metal reagents in green solvent. RSC Adv. 2015, 5, 85872–85878. [Google Scholar] [CrossRef]
- Moon, H.-S.; Eisenberg, W.E.; Wilson, M.E.; Schore, N.E.; Kurth, M.J. Preparation of nonracemic 3,5-disubstituted-γ-butyrolactones: An effective sequent auxiliary for amide alkylation and iodolactonization. J. Org. Chem. 1994, 59, 6504–6505. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xu, H.; Tang, W. DABCO-catalyzed 1,4-bromolactonization of conjugated enynes: Highly stereoselective formation of a stereogenic center and an axially chiral allene. J. Am. Chem. Soc. 2009, 131, 3832–3833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, N.; Schienebeck, C.M.; Decloux, K.; Zheng, S.; Werness, J.B.; Tang, W. Catalytic enantioselective halolactonization of enynes and alkenes. Chem. Eur. J. 2012, 18, 7296–7305. [Google Scholar] [CrossRef] [PubMed]
- Ganta, A.; Shamshina, J.L.; Cafiero, L.R.; Snowden, T.S. Stereoselective synthesis of cis- or trans-2,4-disubstituted butyrolactones from Wynberg lactone. Tetrahedron 2012, 68, 5396–5405. [Google Scholar] [CrossRef]
- Rofoo, M.; Roux, M.C.; Rousseau, G. Preparation of oxetanes by silicon-directed 4-exo-trig electrophilic cyclisations of homoallylic alcohols. Tetrahedron Lett. 2001, 42, 2481–2484. [Google Scholar] [CrossRef]
- Braddock, D.C.; Cansell, G.; Hermitage, S.A. Ortho-substituted iodobenzenes as novel organocatalyses for bromination of alkenes. Chem. Commun. 2006, 2483–2485. [Google Scholar] [CrossRef]
- Huang, D.; Wang, H.; Xue, F.; Guan, H.; Li, L.; Peng, X.; Shi, Y. Enantioselective bromocyclization of olefins catalyzed by chiral phosphoric acid. Org. Lett. 2011, 13, 6350–6353. [Google Scholar] [CrossRef]
- Denmark, S.E.; Burk, M.T. Enantioselective bromocycloetherification by Lewis base / chira Brønsted acid cooperative catalysis. Org. Lett. 2012, 14, 256–259. [Google Scholar] [CrossRef]
- Nigatimin, M.; Frey, R.; Levens, A.; Nakano, Y.; Kowalczyk, M.; Konstas, K.; Hutt, O.E.; Lupton, D.W. Iodobenzene-catalyzed oxabicyclo[3.2.1]octane and [4.2.1]nonane synthesis via cascade C-O/C-C formation. Org. Lett. 2013, 15, 5858–5861. [Google Scholar] [CrossRef]
- Yi, W.; Fang, X.-X.; Liu, Q.-Y.; Liu, G.-Q. Metal-free synthesis of oxazolidine-2,4-diones and 3,3-disubstituted oxindoles via ICl-induced cyclization. Eur. J. Org. Chem. 2018, 2018, 6671–6681. [Google Scholar] [CrossRef]

















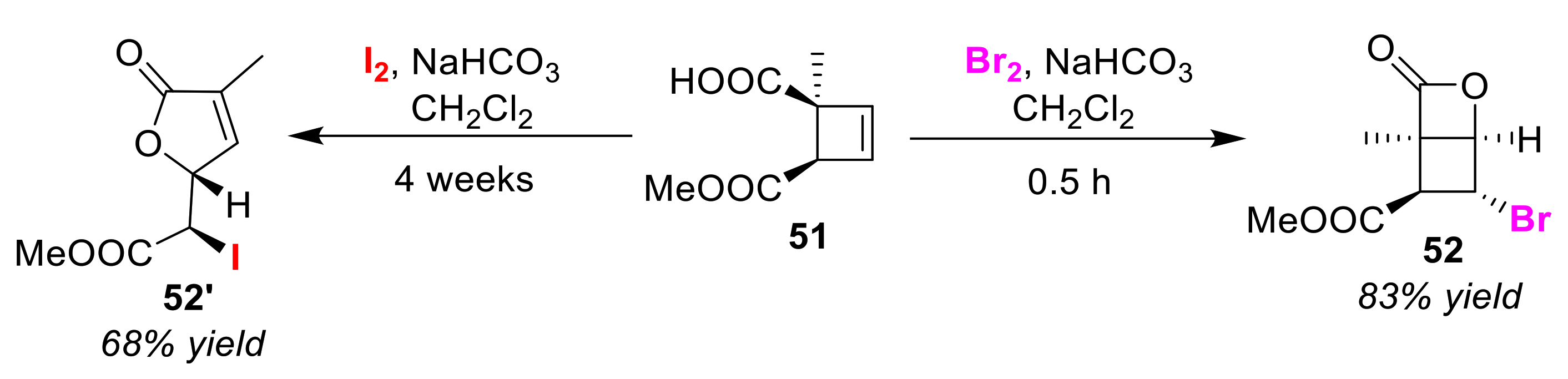
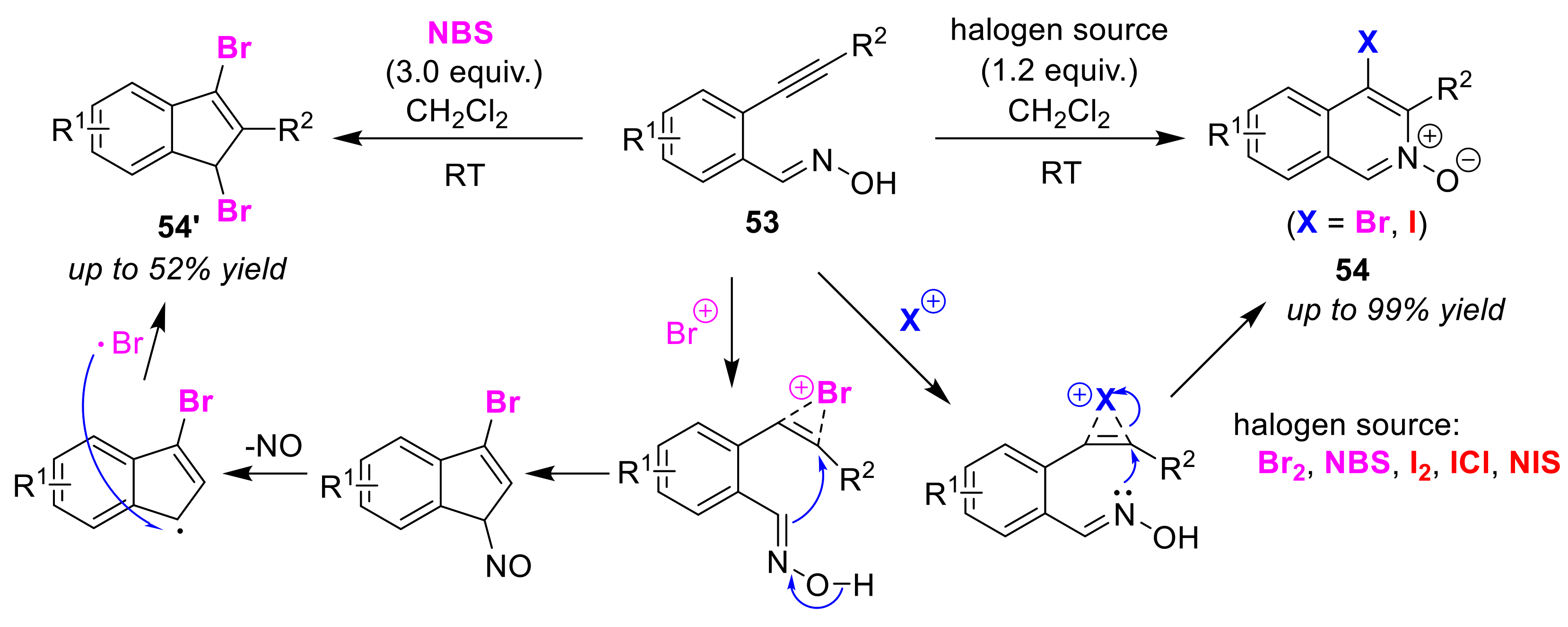



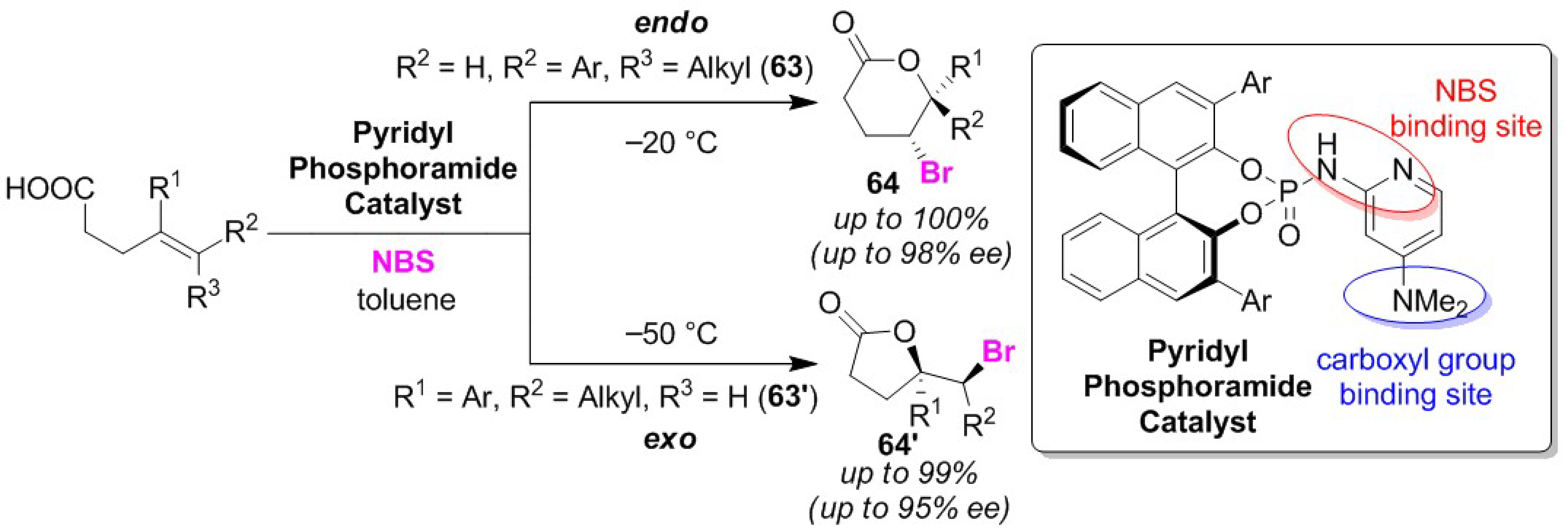



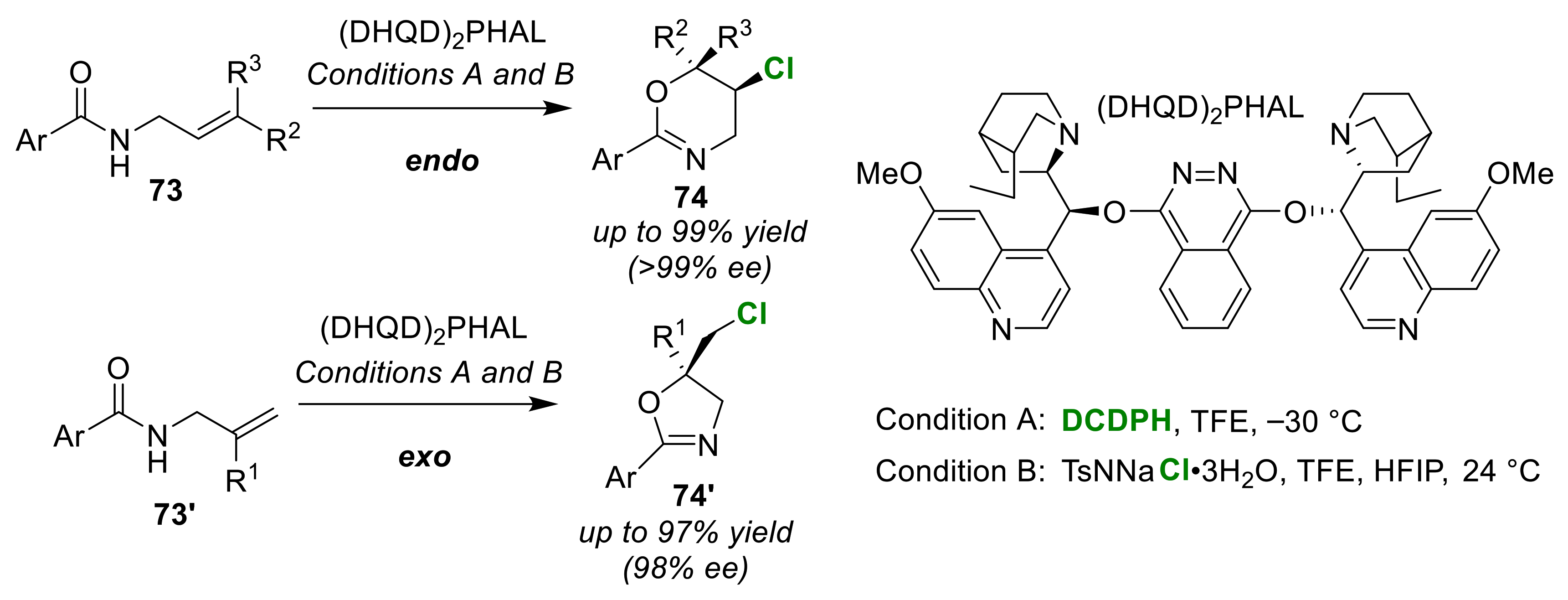











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
China, H.; Kumar, R.; Kikushima, K.; Dohi, T. Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy. Molecules 2020, 25, 6007. https://doi.org/10.3390/molecules25246007
China H, Kumar R, Kikushima K, Dohi T. Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy. Molecules. 2020; 25(24):6007. https://doi.org/10.3390/molecules25246007
Chicago/Turabian StyleChina, Hideyasu, Ravi Kumar, Kotaro Kikushima, and Toshifumi Dohi. 2020. "Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy" Molecules 25, no. 24: 6007. https://doi.org/10.3390/molecules25246007
APA StyleChina, H., Kumar, R., Kikushima, K., & Dohi, T. (2020). Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy. Molecules, 25(24), 6007. https://doi.org/10.3390/molecules25246007







