Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline
Abstract
1. Introduction
2. Results and Discussion
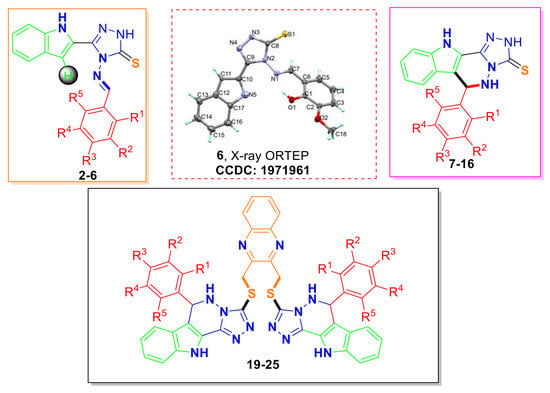
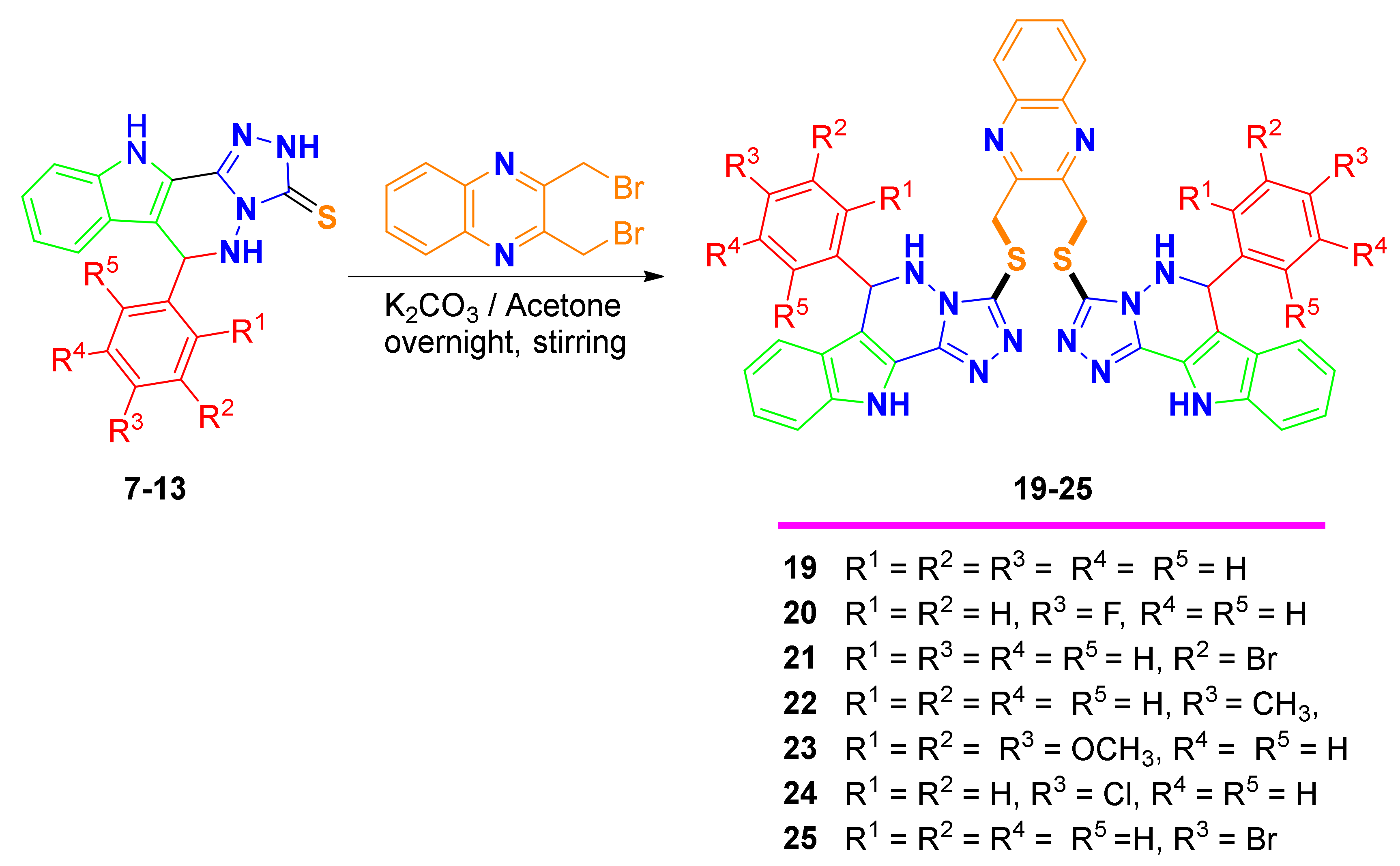
2.1. Synthesis of 2–16 and 19–25
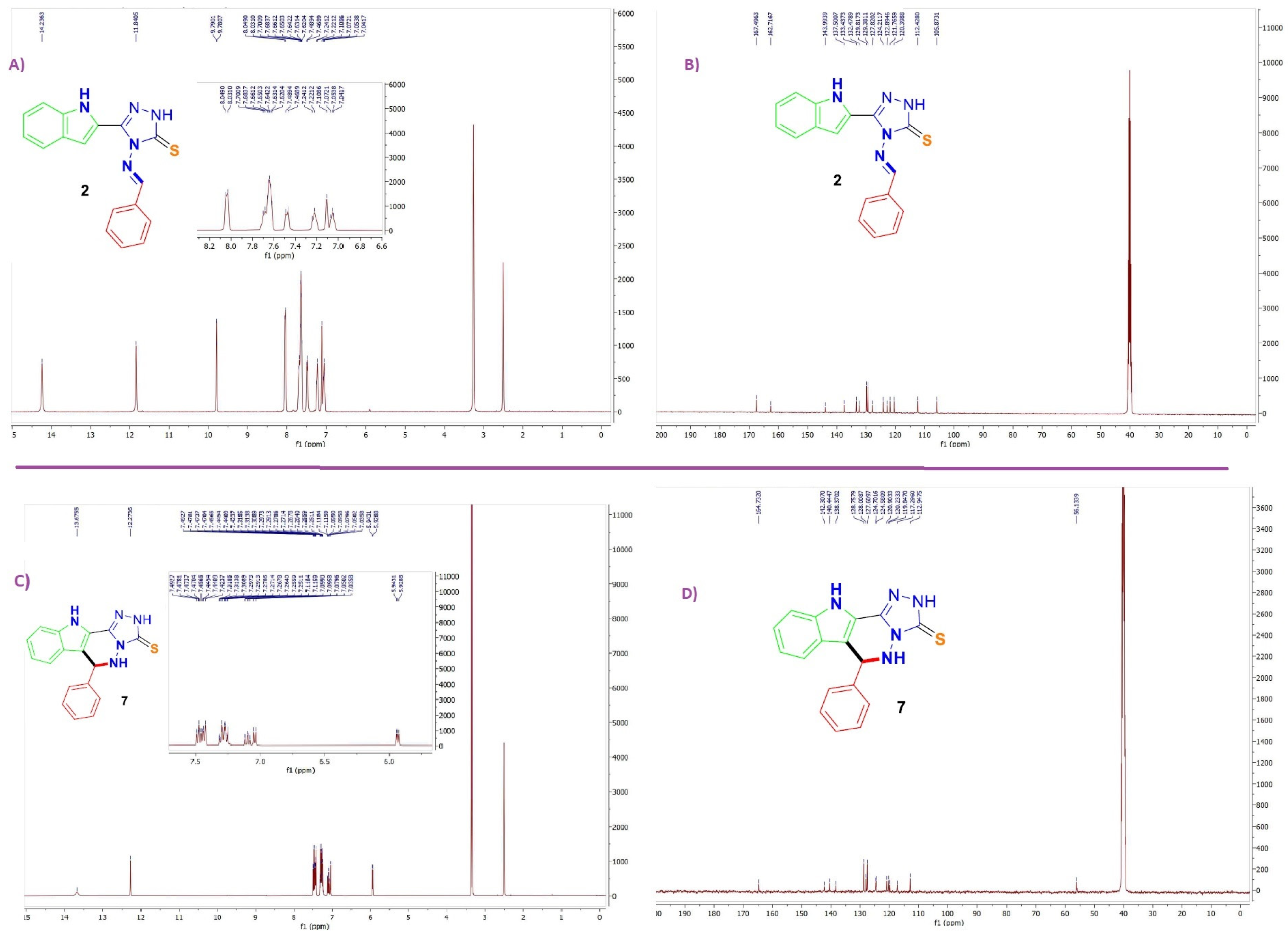
2.2. Structural Assignments
2.3. X-ray Diffraction Analysis of 6
3. Materials and Methods
3.1. Procedure for 2–6
3.2. General Procedure for the Synthesis of Indolo-Triazolo-Pyridazinethiones 7–16
3.3. General Procedure for the Alkylation with Di(bromomethyl)quinoxaline 19–25
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Silakari, O. Triazoles: Multidimensional 5-membered nucleus for designing multitargeting agents. In Key Heterocycle Cores for Designing Multitargeting Molecules; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–342. [Google Scholar]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72, 1185–1199. [Google Scholar] [CrossRef]
- Neda, I.; Kaukorat, T.; Schmutzler, R.; Niemeyer, U.; Kutscher, B.; Pohl, J.; Engel, J. Benzodiaza-, Benzoxaza- and benzodioxaphosphorinones- formation, reactivity, structure and biological activity. Phosphorus Sulfur Silicon 2000, 162, 81–218. [Google Scholar] [CrossRef]
- Simon, M.; Csunderlik, C.; Cotarca, L.; Caproiu, M.T.; Neda, I.; Turoczi, M.C.; Volpicelli, R. Synthesis of new active 0-nitrophenyl carbamates. Synth. Comun. 2005, 35, 1471–1479. [Google Scholar] [CrossRef]
- Mohammad, Y.; Fazili, K.M.; Bhat, K.A.; Ara, T. Synthesis and biological evaluation of novel 3-O-tethered triazoles of diosgenin as potent antiproliferative agents. Steroids 2017, 118, 1–8. [Google Scholar]
- Huang, M.; Deng, Z.; Tian, J.; Liu, T. Synthesis and biological evaluation of salinomycin triazole analogues as anticancer agents. Eur. J. Med. Chem. 2017, 127, 900–908. [Google Scholar] [CrossRef]
- Gujjar, R.; Marwaha, A.; El Mazouni, F.; White, J.; White, K.L.; Creason, S.; Shackleford, D.M.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [CrossRef]
- Chen, M.; Lu, S.; Yuan, G.; Yang, S.; Du, X. Synthesis and antibacterial activity of some heterocyclic β-enamino ester derivatives with 1, 2, 3-triazole. Heterocycl. Commun. 2000, 6, 421–426. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef]
- Akhtar, T.; Hameed, S.; Khan, K.M.; Choudhary, M.I. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H, 4H-1,2,4-triazoles. Med. Chem. 2008, 4, 539–543. [Google Scholar] [CrossRef]
- Sevaille, L.; Gavara, L.; Bebrone, C.; De Luca, F.; Nauton, L.; Achard, M.; Mercuri, P.; Tanfoni, S.; Borgianni, L.; Guyon, C.; et al. 1,2,4-Triazole-3-thione compounds as inhibitors of dizinc metallo-β-lactamases. ChemMedChem 2017, 12, 972–985. [Google Scholar] [CrossRef]
- Gilmore, J.L.; King, B.W.; Asakawa, N.; Harrison, K.; Tebben, A.; Sheppeck, J.E., II; Liu, R.Q.; Covington, M.; Duan, J.J.W. Synthesis and structure–activity relationship of a novel, non-hydroxamate series of TNF-α converting enzyme inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4678–4682. [Google Scholar] [CrossRef] [PubMed]
- Maingot, L.; Leroux, F.; Landry, V.; Dumont, J.; Nagase, H.; Villoutreix, B.; Sperandio, O.; Deprez-Poulain, R.; Deprez, B. New non-hydroxamic ADAMTS-5 inhibitors based on the 1,2,4-triazole-3-thiol scaffold. Bioorg. Med. Chem. Lett. 2010, 20, 6213–6216. [Google Scholar] [CrossRef] [PubMed]
- Kruse, L.I.; Kaiser, C.; DeWolf, W.E.; Finkelstein, J.A.; Frazee, J.S.; Hilbert, E.L.; Ross, S.T.; Flaim, K.E.; Sawyer, J.L. Some benzyl-substituted imidazoles, triazoles, tetrazoles, pyridinethiones, and structural relatives as multisubstrate inhibitors of dopamine. β-hydroxylase. 4. Structure-activity relationships at the copper binding site. J. Med. Chem. 1990, 33, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Timur, İ.; Kocyigit, Ü.M.; Dastan, T.; Sandal, S.; Ceribası, A.O.; Taslimi, P.; Gulcin, İ.; Koparir, M.; Karatepe, M.; Çiftçi, M. In vitro cytotoxic and in vivo antitumoral activities of some aminomethyl derivatives of 2, 4-dihydro-3H-1,2,4-triazole-3-thiones—Evaluation of their acetylcholinesterase and carbonic anhydrase enzymes inhibition profiles. J. Biochem. Mol. Toxic. 2019, 33, e22239. [Google Scholar] [CrossRef]
- Hamdy, R.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef]
- Ziedan, N.I.; Hamdy, R.; Cavaliere, A.; Kourti, M.; Prencipe, F.; Brancale, A.; Jones, A.T.; Westwell, A.D. Virtual screening, SAR, and discovery of 5-(indole-3-yl)-2-[(2-nitrophenyl) amino][1,3,4]-oxadiazole as a novel Bcl-2 inhibitor. Chem. Biol. Drug Des. 2017, 90, 147–155. [Google Scholar] [CrossRef]
- Boraei, A.T.; Ghabbour, H.A.; Gomaa, M.S.; El Ashry, E.S.H.; Barakat, A. Synthesis and anti-proliferative assessment of triazolo-thiadiazepine and triazolo-thiadiazine scaffolds. Molecules 2019, 24, 4471. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Gomaa, M.S.; El Sayed, E.S.H.; Duerkop, A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef]
- Darestani-Farahani, M.; Faridbod, F.; Ganjali, M.R. A sensitive fluorometric DNA nanobiosensor based on a new fluorophore for tumor suppressor gene detection. Talanta 2018, 190, 140–146. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, Z. Microwave irradiation synthesis of novel indole triazole Schiff base fluorescent probe for Al3+ ion. Inorganica Chim. Acta 2019, 498, 119135. [Google Scholar] [CrossRef]
- Boraei, A.T.; Singh, P.K.; Sechi, M.; Satta, S. Discovery of novel functionalized 1, 2, 4-triazoles as PARP-1 inhibitors in breast cancer: Design, synthesis and antitumor activity evaluation. Eur. J. Med. Chem. 2019, 182, 111621. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.; Ashour, H.K.; El Sayed, H.; Abdelmoaty, N.; El-Falouji, A.I.; Gomaa, M.S. Design and synthesis of new phthalazine-based derivatives as potential EGFR inhibitors for the treatment of hepatocellular carcinoma. Bioorg. Chem. 2019, 85, 293–307. [Google Scholar] [CrossRef]
- Chehrouri, M.; Othman, A.A.; Jiménez-Cecilia, S.; Moreno-Cabrerizo, C.; Sansano, J.M. 4-Amino-3-pentadecyl-3H-1,2,4-triazole-3-thiones and 3-pentadecyl-1,3,4-oxadiazole-2 (3H)-thione for the preparation of dimeric palladium (II) complexes and their applications in Tsuji–Trost and Mizoroki–Heck reactions. Synth. Commun. 2019, 49, 1301–1307. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and biological activities of 7-arylazo-7H-pyrazolo [5,1-c][1,2,4] triazol-6 (5H)-ones and 7-arylhydrazono-7H-[1,2,4] triazolo [3,4-b][1,3,4] thiadiazines. J. Chin. Chem. Soc. 2005, 52, 987–994. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4] triazolo [3,4-b][1,3,4] thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Z.; Liu, X.; Li, G. Microwave-assisted synthesis and biological activity of new Schiff bases derived from dimers of 4-amino-3-[3-(1-benzyl)indole]-5-thiomethyl-1,2,4-triazole. Res. Chem. Intermediat. 2013, 39, 1897–1905. [Google Scholar] [CrossRef]
- Peng, Y.L.; Liu, X.L.; Wang, X.H.; Zhao, Z.G. Microwave-assisted synthesis and antibacterial activity of derivatives of 3-[1-(4-fluorobenzyl)-1H-indol-3-yl]-5-(4-fluorobenzylthio)-4H-1, 2, 4-triazol-4-amine. Chem. Pap. 2014, 68, 401–408. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, Z.; Huang, M.; Fu, X. Ultrasound-assisted, one-pot, three-component synthesis and antibacterial activities of novel indole derivatives containing 1,3,4-oxadiazole and 1,2,4-triazole moieties. C. R. Chim. 2015, 18, 1320–1327. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Li Petri, G.; Cusumano, M.G.; Schillaci, D.; Sarno, V.D.; Musella, S.; Giovannetti, E.; Cirrincione, G.; Diana, P. 2,6-Disubstituted imidazo[2,1-b][1,3,4]thiadiazole derivatives as potent staphylococcal biofilm inhibitors. Eur. J. Med. Chem. 2019, 167, 200–210. [Google Scholar] [CrossRef]
- Ishikawa, H.; Sugiyama, T.; Kurita, K.; Yokoyama, A. Synthesis and antimicrobial activity of 2, 3-bis (bromomethyl) quinoxaline derivatives. Bioorg. Chem. 2012, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Sissi, C.; Palumbo, M.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Bruker, A. SAINT Software Reference Manual; Bruker AXS, Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Spek, L.A. Single-crystal structure validation with the program PLATON. J. Appl. Chem. 2002, 36, 7–13. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–16 and 19–25 are available from the authors. |




| Chemical formula | C38H26N10O4S2 |
| Formula weight | 726.79 g/mol |
| Temperature | 104(2) K |
| Wavelength | 1.54178 Å |
| Crystal size | 0.030 × 0.070 × 0.130 mm |
| Crystal habit | Yellow plate |
| Crystal system | Triclinic |
| Space group | P -1 |
| Unit cell dimensions | a = 10.1105(6) Å |
| b = 13.1764(6) Å | |
| c = 14.2858(6) Å | |
| Volume | 1705.44(15) Å3 |
| Z | 2 |
| Density (calculated) | 1.415 g/cm3 |
| Absorption coefficient | 1.893 mm−1 |
| F(000) | 752 |
| Theta range for data collection | 3.38 to 68.23° |
| Index ranges | −12 ≤ h ≤ 12, −15 ≤ k ≤ 15, −17 ≤ l ≤ 17 |
| Reflections collected | 54525 |
| Independent reflections | 6232 [R(int) = 0.1173] |
| Coverage of independent reflections | 99.9% |
| Absorption correction | Multi-scan |
| Max. and min. transmission | 0.9470 and 0.7960 |
| Structure solution technique | direct methods |
| Structure solution program | SHELXT 2014/5 (Sheldrick, 2014) |
| Refinement method | Full-matrix least-squares on F2 |
| Refinement program | SHELXL-2017/1 (Sheldrick, 2017) |
| Function minimized | Σ w(Fo2 − Fc2)2 |
| Data/restraints/parameters | 6232/0/471 |
| Goodness-of-fit on F2 | 1.085 |
| Final R indices | 4104 data; I > 2σ(I) |
| all data | |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.1000P)2] where P = (Fo2 + 2Fc2)/3 |
| Largest diff. peak and hole | 1.57 and −0.52 eÅ−3 |
| RMS deviation from mean | 0.110 eÅ−3 |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| O1-H1…N1 | 0.84 | 1.94 | 2.659(6) | 143 |
| O3-H3A…N6 | 0.84 | 1.95 | 2.664(6) | 143 |
| C11-H11…S2 | 0.95 | 2.51 | 3.391(5) | 154 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraei, A.T.A.; Sarhan, A.A.M.; Yousuf, S.; Barakat, A. Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline. Molecules 2020, 25, 450. https://doi.org/10.3390/molecules25030450
Boraei ATA, Sarhan AAM, Yousuf S, Barakat A. Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline. Molecules. 2020; 25(3):450. https://doi.org/10.3390/molecules25030450
Chicago/Turabian StyleBoraei, Ahmed T. A., Ahmed A. M. Sarhan, Sammer Yousuf, and Assem Barakat. 2020. "Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline" Molecules 25, no. 3: 450. https://doi.org/10.3390/molecules25030450
APA StyleBoraei, A. T. A., Sarhan, A. A. M., Yousuf, S., & Barakat, A. (2020). Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline. Molecules, 25(3), 450. https://doi.org/10.3390/molecules25030450