Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-?-Cyclodextrin Inclusion for Pulmonary Delivery
Abstract
:1. Introduction
2. Results and Discussion
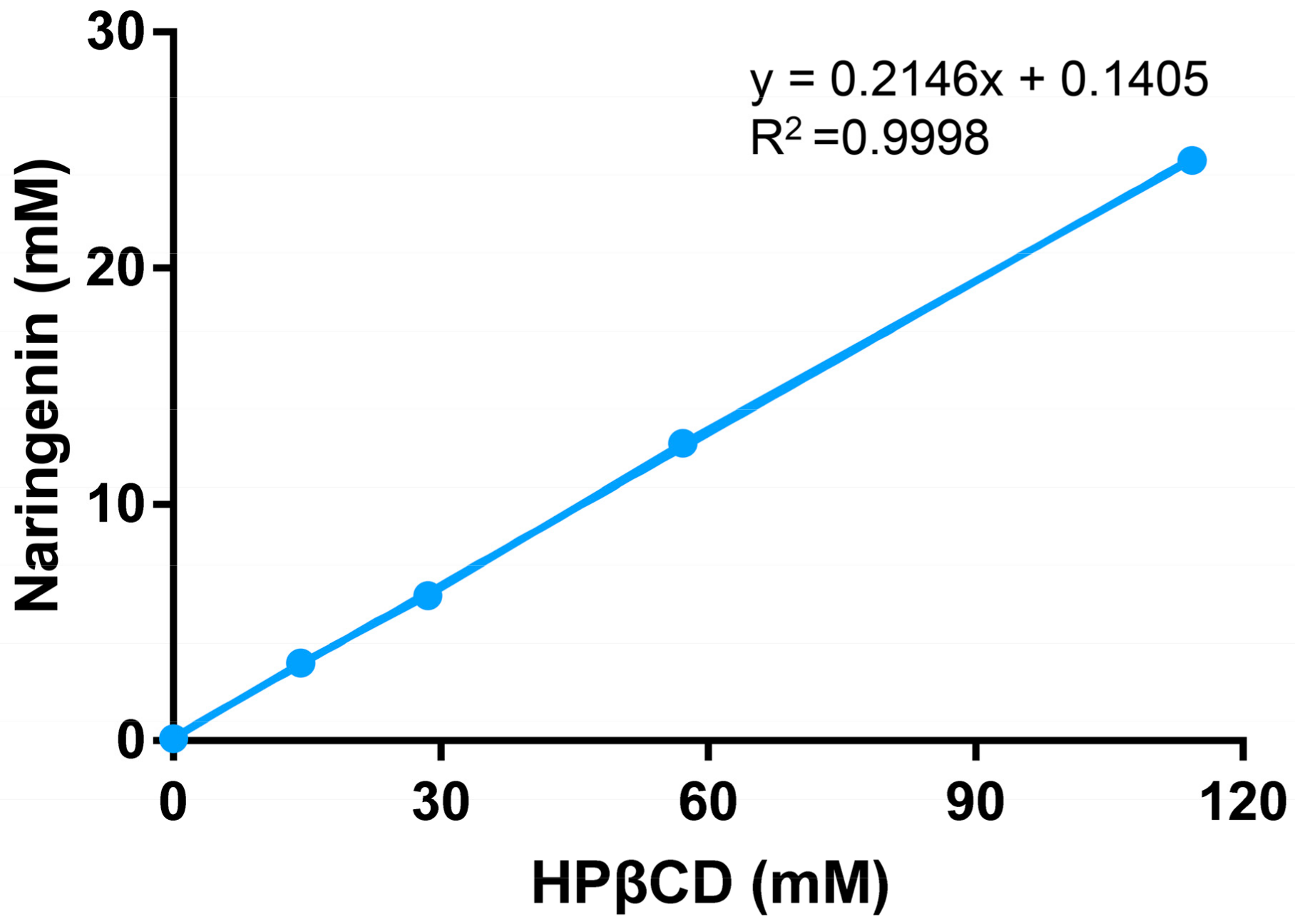
2.1. Phase Solubility Study
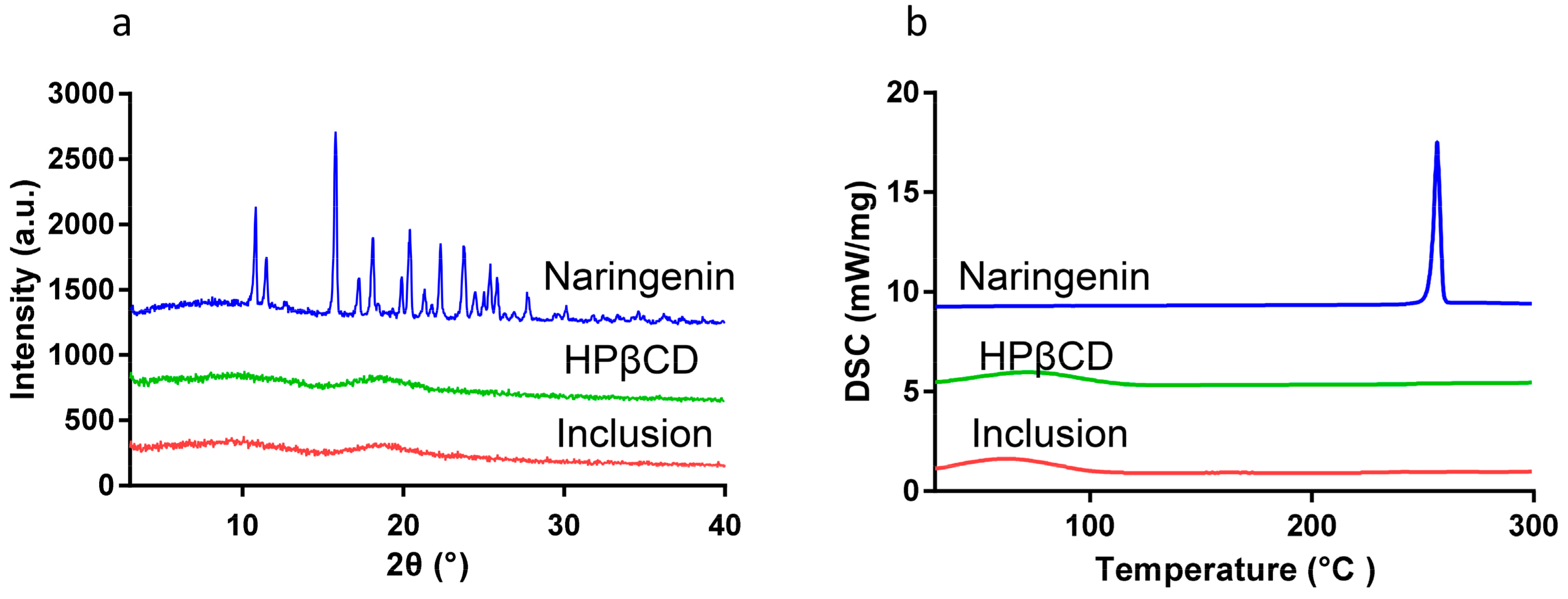
2.2. Powder X-ray Diffraction (XRD)
2.3. Differential Scanning Calorimetry (DSC)
2.4. Proton Nuclear Magnetic Resonance (1HNMR) and Two Dimensional Rotating frame Overhauser Effect Spectroscopy (2D-ROESY) Studies
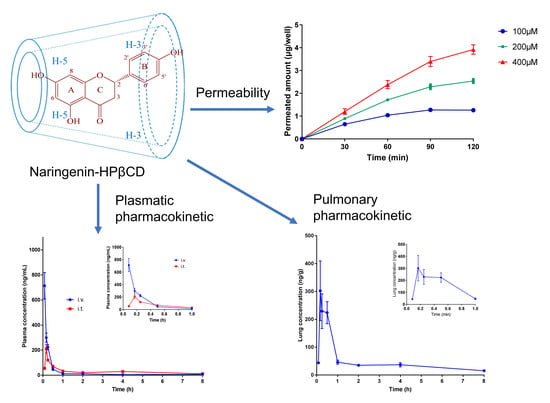
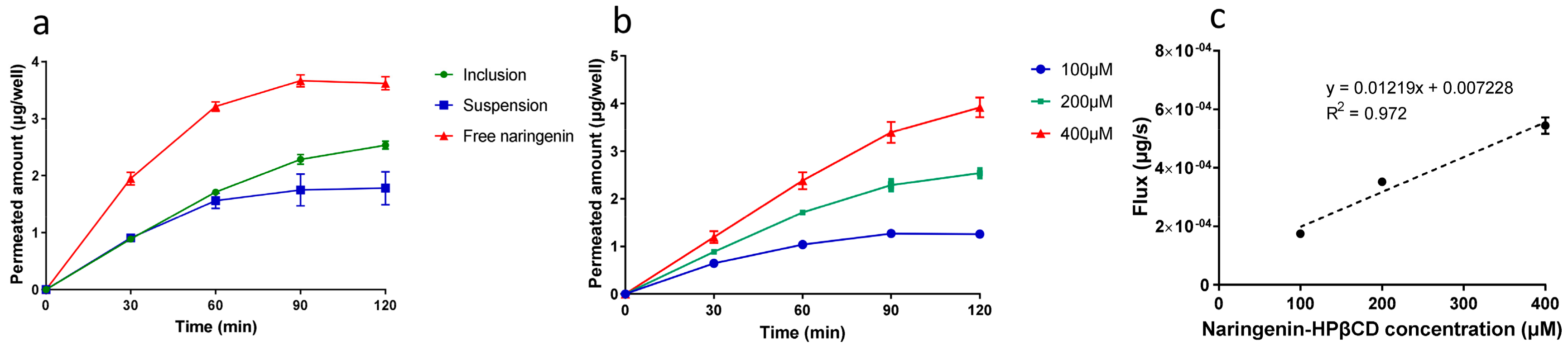
2.5. Permeation Study
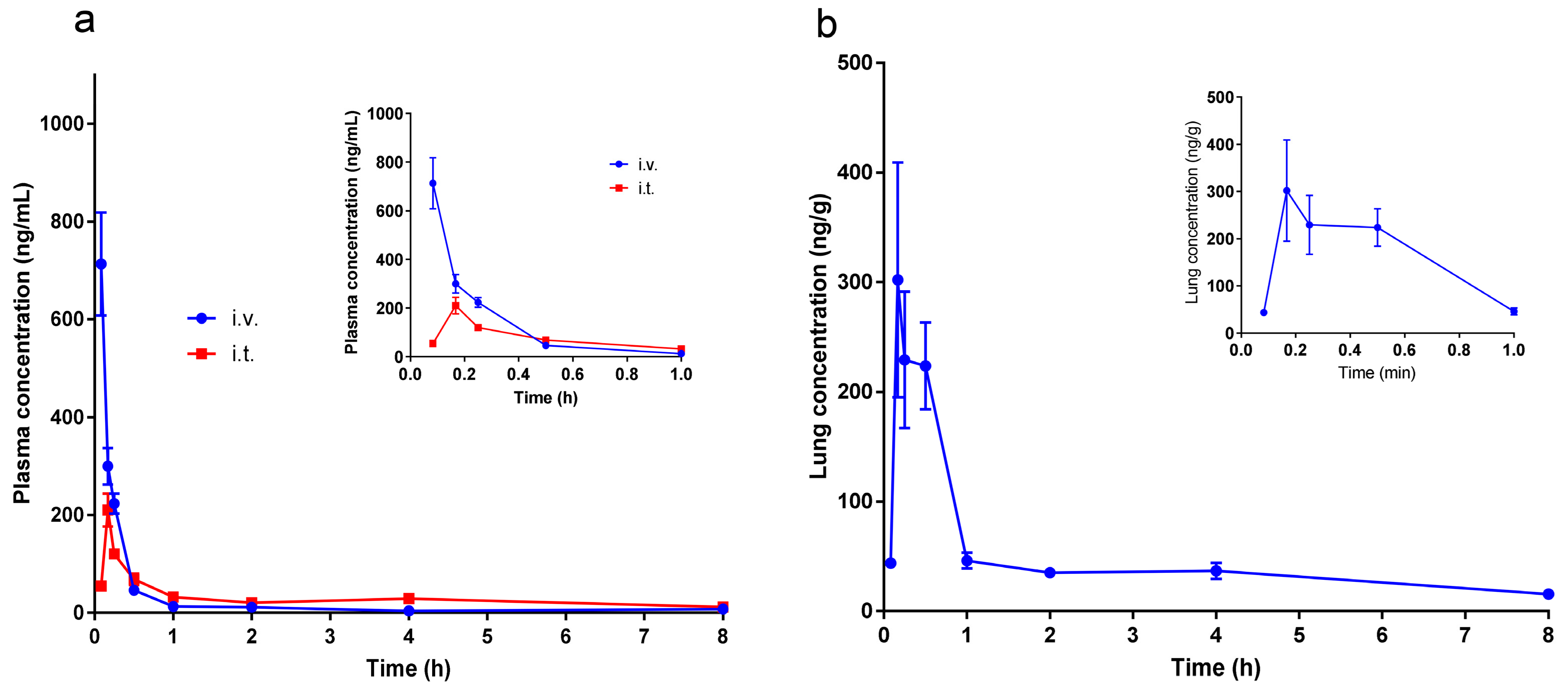
2.6. Pharmacokinetic Studies
3. Materials and Methods
3.1. Materials
3.2. Phase Solubility Study
3.3. Preparation of Naringenin-HPβCD Inclusion
3.4. XRD
3.5. DSC
3.6. HNMR and 2D ROESY
3.7. Permeation Study of Naringenin- HPβCD Inclusion
3.7.1. Cell Culture
3.7.2. TEER Values Measurement
3.7.3. Permeation Study
3.7.4. HPLC Assay
3.8. Pharmacokinetic Study
3.8.1. Animals
3.8.2. Administration Protocol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Delivery 2015, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.Q.; Li, P.B.; Wang, Y.G.; Peng, W.; Wu, Z.; Su, W.W.; Ji, H. The expectorant activity of naringenin. Pulm. Pharmacol. Ther. 2008, 21, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-kappaB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Zhang, C.C.; Li, P.B.; Nie, Y.C.; Wu, H.; Shen, J.G.; Su, W.W. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int. Immunopharmacol. 2012, 13, 301–307. [Google Scholar] [CrossRef]
- Nie, Y.C.; Wu, H.; Li, P.B.; Luo, Y.L.; Long, K.; Xie, L.M.; Shen, J.G.; Su, W.W. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J. Med. Food 2012, 15, 894–900. [Google Scholar] [CrossRef]
- Nie, Y.C.; Wu, H.; Li, P.B.; Xie, L.M.; Luo, Y.L.; Shen, J.G.; Su, W.W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways. Eur. J. Pharmacol. 2012, 690, 207–213. [Google Scholar] [CrossRef]
- Jiao, H.Y.; Su, W.W.; Li, P.B.; Liao, Y.; Zhou, Q.; Zhu, N.; He, L.L. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm. Pharmacol. Ther. 2015, 33, 59–65. [Google Scholar] [CrossRef]
- Shi, R.; Xiao, Z.T.; Zheng, Y.J.; Zhang, Y.L.; Xu, J.W.; Huang, J.H.; Zhou, W.L.; Li, P.B.; Su, W.W. Naringenin Regulates CFTR Activation and Expression in Airway Epithelial Cells. Cell. Physiol. Biochem. 2017, 44, 1146–1160. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Su, W.W.; Zhu, Z.T.; Guan, M.Y.; Cheng, K.L.; Fan, W.Y.; Wei, G.Y.; Li, P.B.; Yang, Z.Y.; Yao, H.L. Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine 2019, 63, 153004. [Google Scholar] [CrossRef]
- Shi, R.; Xu, J.W.; Xiao, Z.T.; Chen, R.F.; Zhang, Y.L.; Lin, J.B.; Cheng, K.L.; Wei, G.Y.; Li, P.B.; Zhou, W.L.; et al. Naringin and Naringenin Relax Rat Tracheal Smooth by Regulating BKCa Activation. J. Med. Food 2019. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropyl-β-cyclodextrin. [corrected]. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev. Ind. Pharm. 2015, 41, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-beta-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Rice-Evans, C.; Moore, K.; Gibson, G.; Debnam, E.; Srai, S.K. The differential tissue distribution of the citrus flavanone naringenin following gastric instillation. Free Radic. Res. 2004, 38, 1329–1340. [Google Scholar] [CrossRef]
- Zou, W.; Yang, C.; Liu, M.; Su, W. Tissue distribution study of naringin in rats by liquid chromatography-tandem mass spectrometry. Arzneimittel-Forschung 2012, 62, 181–186. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Peng, W.; Yao, H. Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Naringin in Aged Rats. Front. Pharmacol. 2019, 10, 34. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Bur, M.; Huwer, H.; Muys, L.; Lehr, C.-M. Drug Transport Across Pulmonary Epithelial Cell Monolayers: Effects of Particle Size, Apical Liquid Volume, and Deposition Technique. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 119–127. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.M.; Cataldo, D. Cyclodextrins as a potential carrier in drug nebulization. J. Controll. Release 2004, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Brillault, J.; Couet, W.; Olivier, J.-C. Formulation of rifampicin–cyclodextrin complexes for lung nebulization. J. Controll. Release 2008, 129, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.H.; Azaroual, N.; Flament, M.P. Characterization and in vitro evaluation of the formoterol/cyclodextrin complex for pulmonary administration by nebulization. Eur. J. Pharm. Biopharm. 2009, 72, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, N.; Taylor, K.M.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-beta-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Zheng, Z.P.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation Mechanism of Oxyresveratrol by β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin and Computational Analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Xu, R.; Ge, X.; Cheng, J. Complexation of capsaicin with hydroxypropyl-beta-cyclodextrin and its analytical application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117278. [Google Scholar] [CrossRef]
- Adhikari, S.; Daftardar, S.; Fratev, F.; Rivera, M.; Sirimulla, S.; Alexander, K.; Boddu, S.H.S. Elucidation of the orientation of selected drugs with 2-hydroxylpropyl-beta-cyclodextrin using 2D-NMR spectroscopy and molecular modeling. Int. J. Pharm. 2018, 545, 357–365. [Google Scholar] [CrossRef]
- Asai, A.; Okuda, T.; Sonoda, E.; Yamauchi, T.; Kato, S.; Okamoto, H. Drug Permeation Characterization of Inhaled Dry Powder Formulations in Air-Liquid Interfaced Cell Layer Using an Improved, Simple Apparatus for Dispersion. Pharma. Res. 2016, 33, 487–497. [Google Scholar] [CrossRef]
- Bur, M.; Rothen-Rutishauser, B.; Huwer, H.; Lehr, C.M. A novel cell compatible impingement system to study in vitro drug absorption from dry powder aerosol formulations. Eur. J. Pharm. Biopharm. 2009, 72, 350–357. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Young, P.M. Pharmaceutical applications of the Calu-3 lung epithelia cell line. Expert Opin. Drug Deliv. 2013, 10, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006, 58, 1030–1060. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Frohlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In Vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol–Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Matilainen, L.; Toropainen, T.; Vihola, H.; Hirvonen, J.; Jarvinen, T.; Jarho, P.; Jarvinen, K. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J. Controll. Release 2008, 126, 10–16. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Chen, D.; Fang, T.; Li, H.; Su, W. LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int. J. Pharm. 2006, 307, 292–299. [Google Scholar] [CrossRef]
- Fang, T.Z. Studies on Pharmacodynamics and Pharmacokinetics of Naringenin. Ph.D Thesis, Sun Yat-Sen University, Guangzhou, China, 2005. [Google Scholar]
- Zeng, X.; Su, W.; Liu, H.; Zheng, Y.; Chen, T.; Zhang, W.; Yan, Z.; Bai, Y.; Yao, H. Simultaneous determination of rosuvastatin, naringin and naringenin in rat plasma by RRLC-MS/MS and its application to a pharmacokinetic drug interaction study. J. Chromatogr. Sci. 2018, 56, 611–618. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |
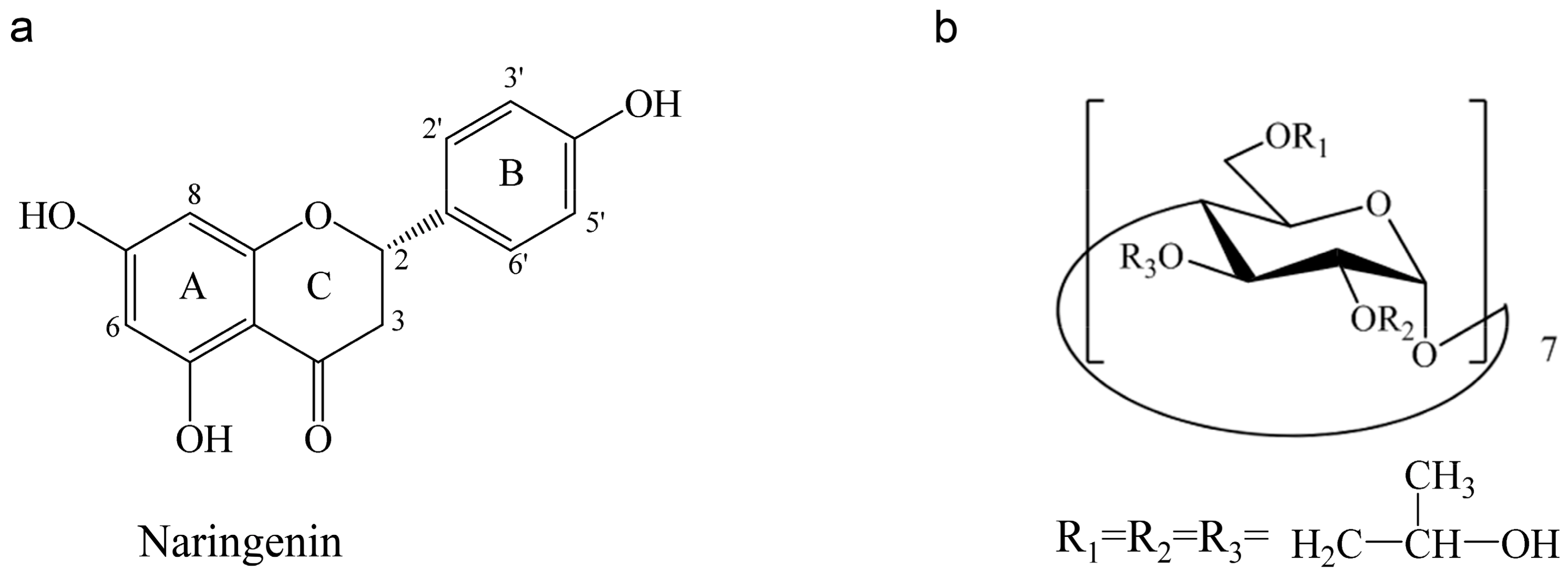
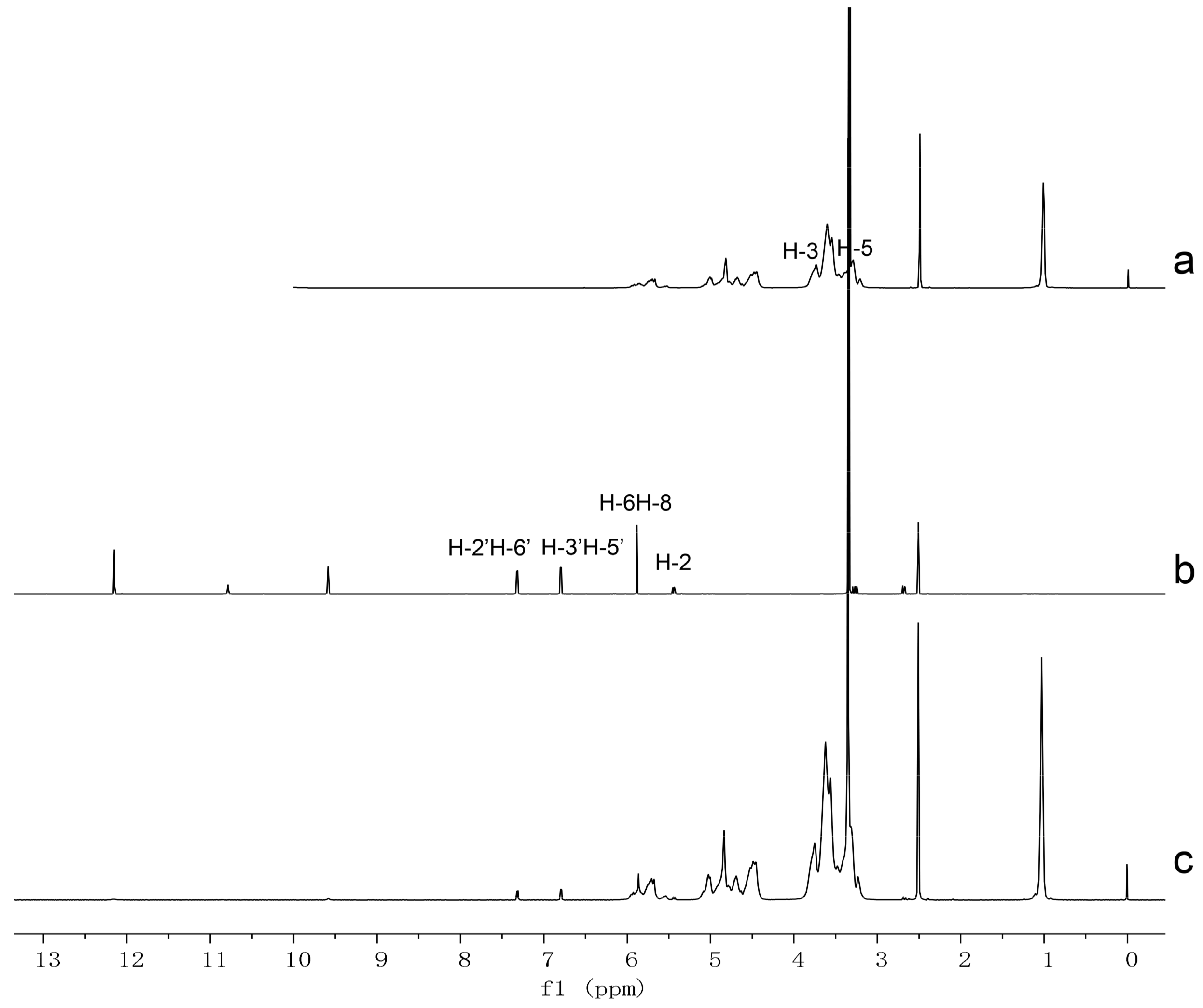

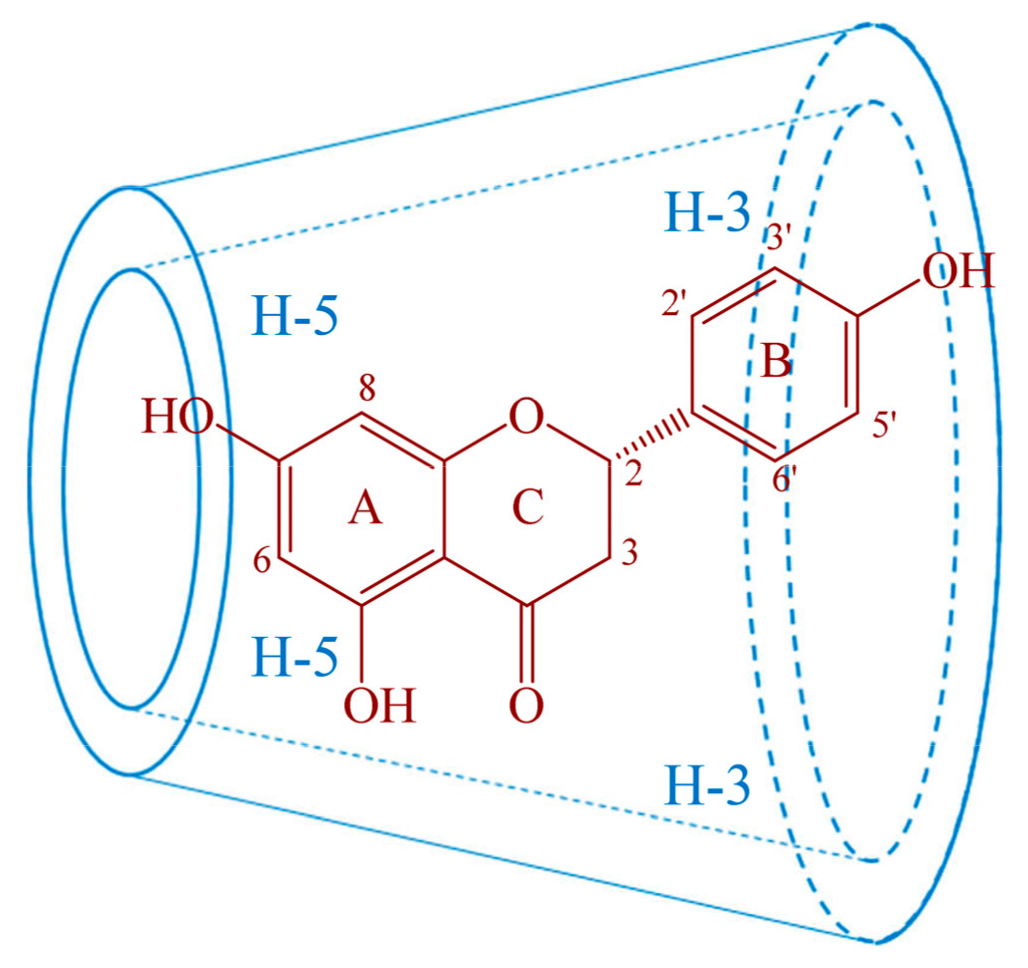
| Compound | Proton | Chemical Shift δ (ppm) | Δδ (ppm) | |
|---|---|---|---|---|
| δ (Free) | δ (Inclusion) | |||
| Naringenin | 2 | 5.438 | 5.427 | −0.011 |
| 6 or 8 | 5.878 | 5.857 | −0.021 | |
| 3′ or 5′ | 6.789 | 6.785 | −0.004 | |
| 2′ or 6′ | 7.312 | 7.308 | −0.004 | |
| HPβCD | H-3 | 3.736 | 3.755 | 0.019 |
| H-5 | 3.468 | 3.470 | 0.002 | |
| Day | 4 | 8 | 10 | 12 | 14 |
|---|---|---|---|---|---|
| TEER (Ω) | 212 ± 25 | 462 ± 33 | 510 ± 70 | 576 ± 30 | 581 ± 31 |
| Form | Permeated Amount (μg/well) | Flux (μg/s × 10−4) |
|---|---|---|
| Naringenin solution | 3.61 ± 0.11 | 5.03 ± 0.16 |
| Naringenin inclusion | 2.53 ± 0.06 | 3.52 ± 0.09 |
| Naringenin suspension | 1.78 ± 0.30 | 2.47 ± 0.40 |
| Parameter | IV | IT |
|---|---|---|
| AUC0-t (μg/L∗h) | 268.20 ± 85.62 | 233.60 ± 37.47 |
| AUC(0-∞) (μg/L∗h) | 273.00 ± 84.46 | 291.15 ± 77.18 |
| Tmax (h) | 0.083 | 0.181 ± 0.034 |
| Cmax (μg/L) | 713.12 ± 258.564 | 217.86 ± 66.35 |
| t1/2 (h) | 2.25 ± 0.90 | 4.15 ± 2.13 |
| Clearance (L/h/kg) | 1.59 ± 0.51 | 1.46 ± 0.38 |
| Bioavailability (%) | 87 1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, M.; Shi, R.; Zheng, Y.; Zeng, X.; Fan, W.; Wang, Y.; Su, W. Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-?-Cyclodextrin Inclusion for Pulmonary Delivery. Molecules 2020, 25, 554. https://doi.org/10.3390/molecules25030554
Guan M, Shi R, Zheng Y, Zeng X, Fan W, Wang Y, Su W. Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-?-Cyclodextrin Inclusion for Pulmonary Delivery. Molecules. 2020; 25(3):554. https://doi.org/10.3390/molecules25030554
Chicago/Turabian StyleGuan, Minyi, Rui Shi, Yuying Zheng, Xuan Zeng, Weiyang Fan, Yonggang Wang, and Weiwei Su. 2020. "Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-?-Cyclodextrin Inclusion for Pulmonary Delivery" Molecules 25, no. 3: 554. https://doi.org/10.3390/molecules25030554
APA StyleGuan, M., Shi, R., Zheng, Y., Zeng, X., Fan, W., Wang, Y., & Su, W. (2020). Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-?-Cyclodextrin Inclusion for Pulmonary Delivery. Molecules, 25(3), 554. https://doi.org/10.3390/molecules25030554