A Quantitative Analysis Model Established to Determine the Concentration of Each Source in Mixed Astaxanthin from Different Sources
Abstract
:1. Introduction
2. Results and Discussion
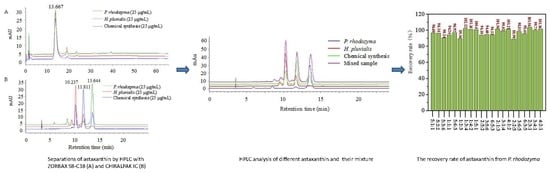
2.1. Identification of Astaxanthin from Different Sources Using High-Performance Liquid Chromatography (HPLC)
2.2. Establishment of the Quantitative Analysis Model
2.3. Detection of Astaxanthin with the Quantitative Analysis Model
2.4. Verification of the Quantitative Analysis Model
3. Experimental
3.1. Materials and Reagents
3.2. Instrumentation
3.3. HPLC Analysis
3.4. Sample Preparation
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayan, R.G. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratpalin, M.; Thongrod, S.; Supamattaya, K.; Britton, G.; Schlipalius, L.E. Effects of β-carotene source, Dunaliella salina, and astaxanthin on pigmentation, growth, survival and health of Penaeus monodon. Aquac. Res. 2001, 32, 182–190. [Google Scholar] [CrossRef]
- Liu, X.J.; Luo, Q.X.; Rakariyatham, K.; Cao, Y. Antioxidation and anti-ageing activities of different stereoisomeric astaxanthin in vitro and in vivo. J. Funct. Foods 2016, 25, 50–61. [Google Scholar] [CrossRef]
- Kindlund, P.J. Astaxanthin. Nutrafoods 2011, 10, 27–31. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Goswami, G.; Chaudhuri, S.; Dutta, D. The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microb. Biot. 2010, 26, 1925–1939. [Google Scholar] [CrossRef]
- Du, X.P.; Bai, M.L.; Huang, Y.; Jiang, Z.D.; Chen, F.; Ni, H.; Li, Q.B. Inhibitory effect of astaxanthin on pancreatic lipase with inhibition kinetics integrating molecular docking simulation. J. Funct. Foods 2018, 48, 551–557. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.D. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [Green Version]
- Ying, C.J.; Zhang, F.; Zhou, X.Y.; Hu, X.T.; Chen, J.; Wen, X.R.; Sun, Y.; Zheng, K.Y.; Tang, R.X.; Song, Y.J. Anti-inflammatory effect of astaxanthin on the sickness behavior induced by diabetes mellitus. Cell Mol. Neurobiol. 2015, 35, 1–11. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Ye, C.X.; Wang, A.L. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifuguobscurus) under high temperature stress. Fish Physiol. Biochem. 2017, 44, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Nedbalová, L.; Kolouchová, I.; Sigler, K. LC–MS/APCI identification of glucoside esters and diesters of astaxanthin from the snow alga Chlamydomonas nivalis including their optical stereoisomers. Phytochemistry 2013, 88, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Song, M.Y.; Gao, Z.L.; Cai, X.K.; Dixon, W.; Chen, X.F.; Cao, Y.; Xiao, H. Stereoisomers of astaxanthin inhibit human colon cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. J. Agric. Food Chem. 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Hong, L.; Zhai, Y.X.; Gao, L.M.; Leng, K.L.; Xing, L.H. Separation, purification, and identification of (3S, 3’S)-trans-astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2015, 50, 1377–1383. [Google Scholar] [CrossRef]
- Wang, C.L.; Armstrong, D.W.; Chang, C.D. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J. Chromatogr. A 2008, 1194, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Chen, X.F.; Liu, H.; Cao, Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J. Funct. Foods 2018, 44, 127–136. [Google Scholar] [CrossRef]
- Moretti, V.M.; Mentasti, T.; Bellagamba, F.; Luzzana, U.; Caprino, F.; Turchini, G.M.; Giani, I.; Valfrè, F. Determination of astaxanthin stereoisomers and colour attributes in flesh of rainbow trout (Oncorhynchus mykiss) as a tool to distinguish the dietary pigmentation source. Food Addit. Contam. 2006, 23, 1056–1063. [Google Scholar] [CrossRef]
- Grewe, C.; Menge, S.; Griehl, C. Enantioselective separation of all- E -astaxanthin and its determination in microbial sources. J. Chromatogr. A 2007, 1166, 97–100. [Google Scholar] [CrossRef]
- Subramanian, B.; Tchoukanova, N.; Djaoued, Y.; Pelletier, C.; Ferron, M.; Robichaud, J. Investigations on the geometrical isomers of astaxanthin: Raman spectroscopy of conjugated polyene chain with electronic and mechanical confinement. J. Raman Spectrosc. 2014, 45, 299–304. [Google Scholar] [CrossRef]
- Turujman, S.A.; Wamer, W.G.; Wei, R.R.; Albert, R.H. Rapid liquid chromatographic method to distinguish wild salmon from aquacultured salmon fed synthetic astaxanthin. J. AOAC Int. 1997, 80, 622–632. [Google Scholar] [PubMed]
- Dissing, B.S.; Nielsen, M.E.; Ersbøll, B.K.; Frosch, S. Multispectral imaging for determination of astaxanthin concentration in salmonids. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, M.L.; Chen, Y.H.; Huang, Y.; Huang, G.L.; Yang, Y.F.; Du, X.P. Separation of carotenoids from Phaffia rhodozyma by HSCCC and silica gel column chromatography. Food Industry 2019, 40, 143–147. [Google Scholar]
- Bai, M.L.; Wang, H.Q.; Wu, L.; Chen, Y.H.; Huang, G.L.; Du, X.P. Optimization and separation of astaxanthin from Haematococcus pluvialis by High-Speed Counter-Current Chromatography. Acta Laser Biology Sinica 2018, 27, 460–466. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |



| Isomer | P. rhodozyma (mAU*S) | H. pluvialis (mAU*S) | Chemical Synthesis (mAU*S) |
|---|---|---|---|
| 3S,3′S | -- | 640.53 | 405.19 |
| 3S,3′R | 55.87 | 222.55 | 828.27 |
| 3R,3′R | 789.90 | -- | 415.65 |
| S | Calibration Curve | a | b | k |
|---|---|---|---|---|
| S1 = y11 + y12 | y11 = 26.12x2 − 5.6712 | 26.12 | −5.6712 | k1 = −4.0403 |
| y12 = 16.595x3 + 1.6309 | 16.595 | 1.6309 | ||
| S2 = y21 + y22 + y23 | y21 = 2.3151x1 − 0.4045 | 2.3151 | −0.4045 | k2 = −2.3492 |
| y22 = 9.4757x2 − 1.489 | 9.4757 | −1.489 | ||
| y23 = 34.198x3 − 0.4557 | 34.198 | −0.4557 | ||
| S3 = y31 + y32 | y31 = 32.543x1 − 7.7618 | 32.543 | −7.7618 | k3 = −10.6925 |
| y32 = 17.259x3 − 2.9307 | 17.259 | −2.9307 |
| Actual Concentration (µg/mL) | S1 (mAU*S) | S2 (mAU*S) | S3 (mAU*S) | Calculated Concentration (µg/mL) | Recovery Rate (%) |
|---|---|---|---|---|---|
| 25x1 + 5x2 + 5x3 | 215.80 ± 11.16 | 272.68 ± 9.21 | 861.04 ± 69.41 | 24.15x1 + 5.23x2 + 4.96x3 | 96.6 |
| 25x1 + 25x2 + 25x3 | 1084.81 ± 12.72 | 1189.62 ± 32.78 | 1211.06 ± 41.44 | 23.56x1 + 24.92x2 + 26.35x3 | 94.2 |
| 25x1 + 75 x2 + 100x3 | 3706.90 ± 111.08 | 4376.76 ± 11.01 | 2641.15 ± 110.07 | 25.53x1 + 75.03x2 + 105.52x3 | 102.1 |
| 10x1 + 25x2 + 10x3 | 804.46 ± 16.58 | 601.49 ± 15.88 | 472.68 ± 15.82 | 9.41x1 + 24.41x2 + 10.25x3 | 94.1 |
| 30x1 +25 x2 + 15x3 | 879.53 ± 15.09 | 818.29 ± 15.26 | 1174.93 ± 43.63 | 28.24x1 + 23.99x2 + 15.44x3 | 94.1 |
| 50x1 + 75x2 + 25x3 | 2429.42 ± 49.13 | 1825.07 ± 7.79 | 2179.89 ± 19.12 | 51.78x1 + 74.55x2 + 29.27x3 | 103.6 |
| 100x1 +50x2 + 25x3 | 1846.67 ± 29.74 | 1787.07 ± 5.19 | 3829.39 ± 18.43 | 101.36x1 +50.91x2 + 31.36x3 | 101.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Wang, X.; Bai, M.; Liu, S.; Huang, G.; Zhang, Q.; Ni, H.; Chen, F. A Quantitative Analysis Model Established to Determine the Concentration of Each Source in Mixed Astaxanthin from Different Sources. Molecules 2020, 25, 628. https://doi.org/10.3390/molecules25030628
Du X, Wang X, Bai M, Liu S, Huang G, Zhang Q, Ni H, Chen F. A Quantitative Analysis Model Established to Determine the Concentration of Each Source in Mixed Astaxanthin from Different Sources. Molecules. 2020; 25(3):628. https://doi.org/10.3390/molecules25030628
Chicago/Turabian StyleDu, Xiping, Xin Wang, Manli Bai, Shaosong Liu, Gaoling Huang, Qin Zhang, Hui Ni, and Feng Chen. 2020. "A Quantitative Analysis Model Established to Determine the Concentration of Each Source in Mixed Astaxanthin from Different Sources" Molecules 25, no. 3: 628. https://doi.org/10.3390/molecules25030628
APA StyleDu, X., Wang, X., Bai, M., Liu, S., Huang, G., Zhang, Q., Ni, H., & Chen, F. (2020). A Quantitative Analysis Model Established to Determine the Concentration of Each Source in Mixed Astaxanthin from Different Sources. Molecules, 25(3), 628. https://doi.org/10.3390/molecules25030628