A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders
Abstract
:1. Introduction
2. Phytoalimurgy
3. Obesity and Related Disorders
4. In Vitro and In Vivo Effects on Carbohydrate Metabolism
5. Pancreatic Lipase Inhibition
6. Hypolipidemic Activity
7. Inhibition of Adipogenesis
8. Brown Adipose Tissue Activation
| Plant Species | Study | Activity | Class/Bioactive Compounds | References |
|---|---|---|---|---|
| Allium scorodoprosum L. subsp. rotundum | In vitro | α-Amylase-inhibitory activity | [36] | |
| Amaranthus retroflexus L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Anchusa azurea Mill. | In vitro | Pancreatic lipase inhibition | [50] | |
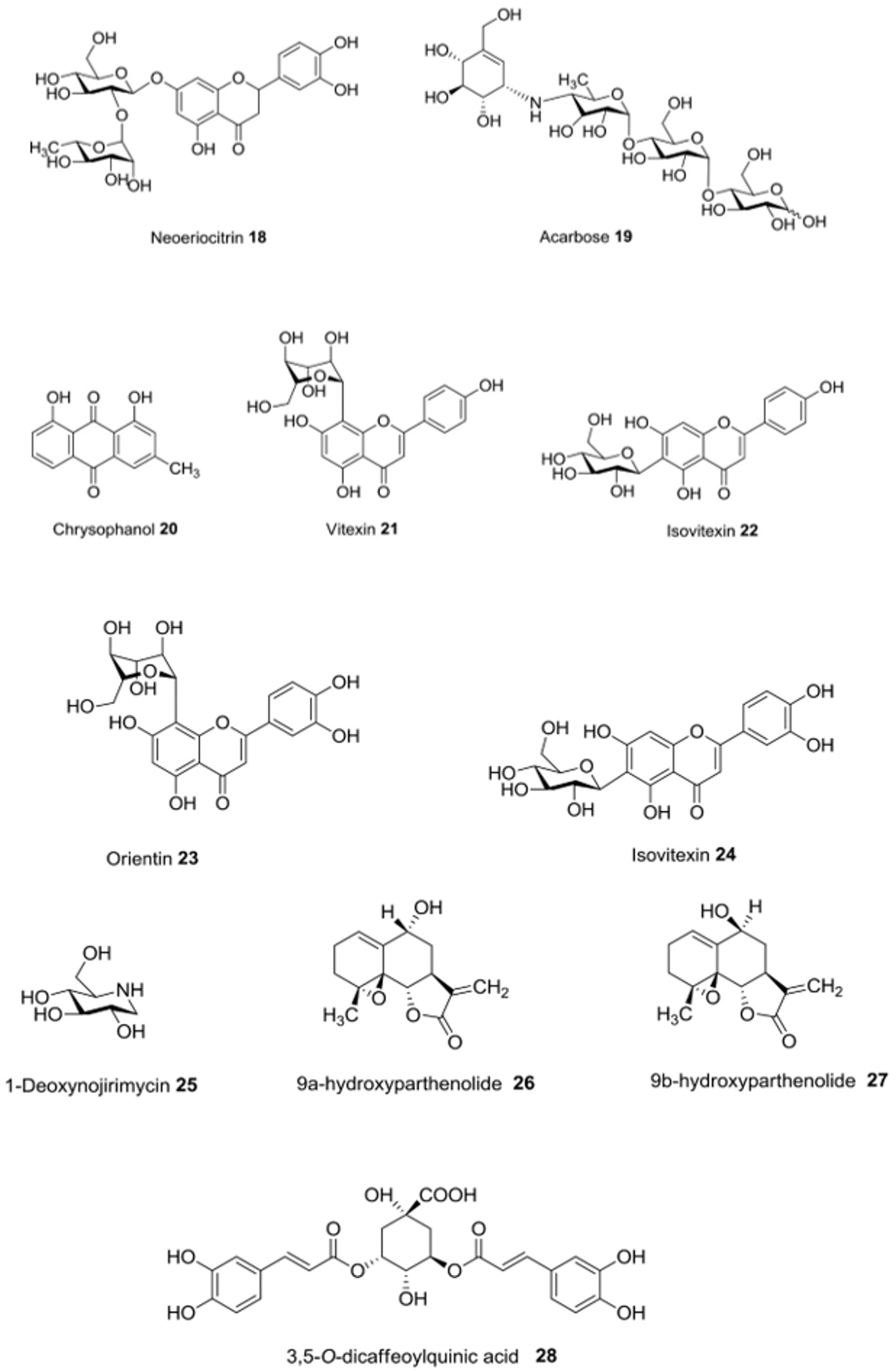
| Anvillea radiata Coss. & Dur. | In vitro | α-Glucosidase-inhibitory activity | 9α-hydroxyparthenolide (26), 9β-hydroxyparthenolide (27) and 3,5-O-dicaffeoylquinic acid (28) | [46] |
| Arum palaestinum Boiss. | In vitro | α-Amylase-inhibitory activity | [37] | |
| Asparagus acutifolius L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Asphodeline lutea Reichenb. | In vitro | α-Glucosidase-inhibitory effects | Chrysophanol (20) | [38,39] |
| Beta vulgaris L. | In vitro | α-Amylase-inhibitory activity; α-glucosidase-inhibitory activity | Vitexin (21), isovitexin (22), orientin (23) and isoorientin (24) | [40] |
| Bituminaria bituminosa (L.) C.H.Stirt | In vitro | α-Amylase-inhibitory activity | [37] | |
| Borago officinalis L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [43] | |
| Capparis orientalis Veill. | In vitro | Pancreatic-lipase-inhibitory activity | Rutin (29) | [54] |
| Capparis sicula Veill. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | Rutin (29) | [43,54] |
| Carduus pycnocephalus L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [44] | |
| Centaurea iberica Trevir. ex Spreng | In vitro | α-Amylase-inhibitory activity | [37] | |
| Cichorium endivia L. | In vitro | α-Amylase-inhibitory activity | [37] | |
| Cichorium intybus L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Citrus bergamia Risso et Poit. (bergamot) | In vivo Clinical | Effectiveness against hyperlipaemia associated or not with hyperglycaemia | [35] | |
| Citrus medica L. cv. Diamante (Diamante citron) | In vitro | Moderate carbohydrate-hydrolyzing enzyme inhibition; | [33] | |
| In vivo | Dose-dependent effect on serum glucose levels in ZDF rats | [34] | ||
| Citrus paradisi Macfad. | In vivo | Rapid blood glucose reduction | [32] | |
| Clematis vitalba L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [44] | |
| Cotoneaster nummularia Fisch. et Mey | In vitro | α-Amylase inhibitory activity; α-glucosidase-inhibitory activity | [41] | |
| Cuscuta pedicellata Ledeb. | In vivo | Reduction of insulin resistance and glucose tolerance | Naringenin (7), kaempferol (8), aromadenderin (9), quercetin (10), aromadenderin-7-O-b-d- glucoside (11), taxifolin 7-O-b-d-glucoside (12) | [26] |
| Diplotaxis tenuifolia (L.) DC. | In vitro | Pancreatic lipase inhibition | [50] | |
| Echium vulgare L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [43] | |
| Foeniculum vulgare Miller subsp. piperitum (Ucria) Coutinho | In vitro | Pancreatic lipase inhibition | [50] | |
| Leopoldia comosa (L.) Parl. (syn. Muscari comosum (L.) Mill.) | In vitro | Pancreatic lipase inhibition | [55,56,57] | |
| Lepidium sativum L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [44] | |
| Malva sylvestris L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [37,44] | |
| Mentha aquatica L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [43] | |
| Mentha spicata L. ssp glabrata (Lej. et Court.) Lebeau | In vitro | Pancreatic lipase inhibition | [50] | |
| Origanum vulgare L. subsp. viridulum (Martin-Donos) Nyman | In vitro | Pancreatic lipase inhibition | [50] | |
| Oxycoccus quadripetalus Schinz & Thell. (syn. Vaccinium oxycoccos L., wild cranberry) | In vitro | Inhibition of adipogenesis | [70] | |
| Papaver rhoeas L. subsp. rhoeas | In vitro | Pancreatic lipase inhibition | [50] | |
| Picris hieracioides L. | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [44] | |
| Plantago major L. | In vitro | α-Amylase-inhibitory activity | [37] | |
| Poncirus trifoliata (L.) Raf. | In vitro | Inhibition of carbohydrate-hydrolyzing enzymes | Nanirutin (13), poncirin (14), didymin (15), naringin (16), hesperidin (17), neoeriocitrin (18) | [31] |
| Portulaca oleracea L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Raphanus raphanistrum L. subsp. landra (DC.) Bonnier & Layens | In vitro | Pancreatic lipase inhibition | [50] | |
| Raphanus raphanistrum L. subsp. raphanistrum | In vitro | α-Amylase-inhibitory activity; pancreatic-lipase-inhibitory activity | [43] | |
| Rosmarinus officinalis L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Rubus caesius L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Rubus grandifolius L. | In vitro | Glucosidases (α-, β-), α-amylase, and lipase enzyme inhibition | [42] | |
| Rumex conglomeratus Murray | In vitro | Pancreatic lipase inhibition | [50] | |
| Silene vulgaris (Moench) Garcke | In vitro | Pancreatic lipase inhibition | [50] | |
| Sisymbrium irio L. | In vitro | α-Amylase-inhibitory activity | [37] | |
| Smyrnium olusatrum L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Sonchus asper (L.) Hill. | In vitro | Pancreatic lipase inhibition | [50] | |
| Sonchus oleraceus L. | In vitro | Pancreatic lipase inhibition | [50] | |
| Vaccinium angustifolium Ait. (Blueberry) | In vitro | Reduced lipid accumulation in macrophages; | [65] | |
| In vivo | Lowering of plasma HbA1c, retinol-binding protein 4, and resistin; | Antocyanins | [30] | |
| Reduction of blood glucose levels; | [29] | |||
| Lowering of plasma TG, TC, and LDL-C concentrations | [28,59,61] | |||
| - Reduction in glucose, fasting insulin and insulin resistance | [28] |
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter 1 |
| C/EBPα | CCAAT/enhancer binding protein alfa |
| EMEA | European Agency for the Evaluation of Medicinal Products |
| FAO | Food and Agriculture Organization |
| FDA | Food and Drug Administration |
| GLUT4 | Glucose transporter type 4 |
| HbA1c | Glycated hemoglobin A1c |
| HFD | High-fat diet |
| IL-6 | Interleukin 6 |
| LDL-C | Low density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| PPARγ | Peroxisome proliferator activated receptor gamma |
| SREBP1 | Sterol regulatory element binding protein 1 |
| TC | Total cholesterol |
| TG | Plasma triglycerides |
| TNF-α | Tumor necrosis factor α |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| ZDF | Zucker diabetic fatty |
References
- Garn, S.M.; Leonard, W.R. What did our ancestors eat? Nutr Rev. 1989, 47, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, A.; Chandler, D.; Grant, W.P.; Greaves, J.; Prince, G.; Tatchell, M. Biopesticides: Pest Management and Regulation; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T. A manifesto for the valorization of wild edible plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sõukand, R. Perceived reasons for changes in the use of wild food plants in Saaremaa, Estonia. Appetite 2016, 107, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Baceta, G.; Pardo-de-Santayana, M.; Aceituno-Mata, L.; Tardío, J.; Reyes-García, V. Trends in wild food plants uses in Gorbeialdea (Basque Country). Appetite 2017, 112, 9–16. [Google Scholar] [CrossRef]
- Pieroni, A.; Soukand, R.; Quave, C.L.; Hajdari, A.; Mustafa, B. Traditional food uses of wild plants among the Gorani of South Kosovo. Appetite 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [Green Version]
- Kristanc, L.; Kreft, S. European medicinal and edible plants associated with subacute and chronic toxicity part II: Plants with hepato-, neuro-, nephro-and immunotoxic effects. Food Chem. Toxicol. 2016, 92, 38–49. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the alimurgic plants Taraxacum officinale, Papaver rhoeas and Urtica dioica by combined NMR and GC–MS analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mattirolo, O.; Gallino, B.; Pallavicini, G. Phytoalimurgia pedemontana. Come Alimentarsi con le Piante Selvatiche; Blu Edizioni: Torino, Italy, 2011. [Google Scholar]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, people and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighbouring areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Nebel, S.; Pieroni, A.; Heinrich, M. Ta chòrta: Wild edible greens used in the Graecanic area in Calabria, Southern Italy. Appetite 2006, 47, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Batal, M.; Hunter, E. Traditional Lebanese recipes based on wild plants: An answer to diet simplification? Food Nutr. Bull. 2007, 28, S303–S311. [Google Scholar] [CrossRef] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypert. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of saponins on lipid metabolism: A review of potential health benefits in the treatment of obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef] [Green Version]
- Al-Suwailem, A.; Al-Tamimi, A.; Al-Omar, M.; Al-Suhibani, M. Safety and mechanism of action of orlistat (tetrahydrolipstatin) as the first local antiobesity drug. JASR 2006, 2, 205–208. [Google Scholar]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehanna, E.T.; El-sayed, N.M.; Ibrahim, A.K.; Ahmed, S.A.; Abo-Elmatty, D.M. Isolated compounds from Cuscuta pedicellata ameliorate oxidative stress and upregulate expression of some energy regulatory genes in high fat diet induced obesity in rats. Biomed. Pharmacother. 2018, 108, 1253–1258. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Seymour, M.; Tanone, I.; Lewis, S.; Urcuyo-Llanes, D.; Bolling, S.F.; Bennink, M.R. Blueberry-enriched diets reduce metabolic syndrome and insulin resistance in rats. FASEB J. 2009, 23. [Google Scholar]
- Vuong, T.; Benhaddou-Andaloussi, A.; Brault, A.; Harbilas, D.; Martineau, L.; Vallerand, D.; Ramassamy, C.; Matar, C.; Haddad, P. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKA y mice. Int. J. Obes. 2009, 33, 1166. [Google Scholar] [CrossRef] [Green Version]
- Vendrame, S.; Zhao, A.; Merrow, T.; Klimis-Zacas, D. The effects of wild blueberry consumption on plasma markers and gene expression related to glucose metabolism in the obese Zucker rat. J. Med. Food 2015, 18, 619–624. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Sicari, V.; Pellicanò, T.; Tenuta, M.; Leporini, M.; Menichini, F.; Loizzo, M. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J. Funct. Foods 2016, 25, 477–485. [Google Scholar] [CrossRef]
- Owira, P.; Ojewole, J. Grapefruit juice improves glycemic control but exacerbates metformin-induced lactic acidosis in non-diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 563–570. [Google Scholar]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; de Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; D’Angelo, D.; Lombardi, P.; Mastellone, V. Citrus medica L. cv Diamante (Rutaceae) peel extract improves glycaemic status of Zucker diabetic fatty (ZDF) rats and protects against oxidative stress. J. Enzyme Inhib. Med. Chem. 2016, 31, 1270–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Zengin, G.; Locatelli, M.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multidirectional investigations on different parts of Allium scorodoprasum L. subsp. rotundum (L.) Stearn: Phenolic components, in vitro biological, and in silico propensities. Food Res. Int. 2018, 108, 641–649. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Elaraj, J.; Hamdan, A.; Lebdeh, S.A.; Halawa, T. Evaluation of the hypoglycemic effect of seven wild folkloric edible plants from Palestine. J. Complement. Integr. Med. 2019. [Google Scholar] [CrossRef]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C. A comparative assessment of biological effects and chemical profile of Italian Asphodeline lutea extracts. Molecules 2018, 23, 461. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Ali, M.; Choi, J. Promising inhibitory effects of anthraquinones, naphthopyrone, and naphthalene glycosides, from Cassia obtusifolia on α-glucosidase and human protein tyrosine phosphatases 1B. Molecules 2017, 22, 28. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild edible Swiss chard leaves (Beta vulgaris L. var. cicla): Nutritional, phytochemical composition and biological activities. Food Res. Int. 2019, 119, 612–621. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Tomás, H.; Castilho, P.C. Evaluation of Rubus grandifolius L.(wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. 2019, 123, 443–452. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J. Sci. Food Agric. 2014, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. Inhibition of key enzymes linked to obesity by preparations from Mediterranean dietary plants: Effects on α-amylase and pancreatic lipase activities. Plant Foods Hum. Nutr. 2013, 68, 340–346. [Google Scholar] [CrossRef]
- Conforti, F.; Ioele, G.; Statti, G.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Saoud, D.; Jelassi, A.; Hlila, M.; Goudjil, M.; Ladjel, S.; Jannet, H.B. Biological activities of extracts and metabolites isolated from Anvillea radiata Coss. & Dur.(Asteraceae). S. Afr. J. Bot. 2019, 121, 386–393. [Google Scholar]
- Nicolosi, R.J.; Wilson, T.A.; Lawton, C.; Handelman, G.J. Dietary effects on cardiovascular disease risk factors: Beyond saturated fatty acids and cholesterol. J. Am. Coll. Nutr. 2001, 20, 421S–427S. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Lefebvre, P.J. Troglitazone: Antihyperglycemic activity and potential role in the treatment of type 2 diabetes. Diabetes Care 1999, 22, 1568–1577. [Google Scholar] [CrossRef]
- Klein, S. The national obesity crisis: A call for action. Gastroenterology 2004, 126, 6. [Google Scholar] [CrossRef]
- Conforti, F.; Perri, V.; Menichini, F.; Marrelli, M.; Uzunov, D.; Statti, G.A.; Menichini, F. Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother. Res. 2012, 26, 600–604. [Google Scholar] [CrossRef]
- Inocencio, C.; Rivera, D.; Obón, M.C.; Alcaraz, F.; Barreña, J.-A. A systematic revision of Capparis section Capparis (Capparaceae) 1, 2. Ann. Mo. Bot. Gard. 2006, 93, 122–150. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M. Glucosinolates and fatty acid, sterol, and tocopherol composition of seed oils from Capparis spinosa Var. spinosa and Capparis ovata Desf. Var. canescens (Coss.) Heywood. J. Agric. Food Chem. 2005, 53, 7136–7141. [Google Scholar]
- Conforti, F.; Modesto, S.; Menichini, F.; Statti, G.A.; Uzunov, D.; Solimene, U.; Duez, P.; Menichini, F. Correlation between environmental factors, chemical composition, and antioxidative properties of caper species growing wild in Calabria (South Italy). Chem. Biodiv. 2011, 8, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Argentieri, M.P.; Avato, P.; Menichini, F.; Conforti, F. Inhibitory effect on lipid absorption and variability of chemical constituents from Capparis sicula subsp. sicula and Capparis orientalis. Chem. Biodiv. 2016, 13, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; La Grotteria, S.; Araniti, F.; Conforti, F. Investigation of the potential health benefits as lipase inhibitor and antioxidant of Leopoldia comosa (L.) Parl.: Variability of chemical composition of wild and cultivated bulbs. Plant Food Hum. Nutr. 2017, 72, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Casacchia, T.; Sofo, A.; Casaburi, I.; Marrelli, M.; Conforti, F.; Statti, G.A. Antioxidant, enzyme-inhibitory and antitumor activity of the wild dietary plant Muscari comosum (L.) Mill. Int. J. Plant Biol. 2017, 8, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Araniti, F.; Statti, G.; Conforti, F. Metabolite profiling and biological properties of aerial parts from Leopoldia comosa (L.) Parl.: Antioxidant and anti-obesity potential. S. Afr. J. Bot. 2019, 120, 104–111. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wang, M.; He, J.; Wang, Y.; Zhang, D.; Zhou, F.; Ji, B. In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented oats (Avena sativa L.) and synergistic effect of three phenolic acids. J. Agric. Food Chem. 2012, 60, 7245–7251. [Google Scholar] [CrossRef]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [Green Version]
- Kalt, W.; Foote, K.; Fillmore, S.; Lyon, M.; Van Lunen, T.; McRae, K. Effect of blueberry feeding on plasma lipids in pigs. Br. J. Nutr. 2008, 100, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Biophys. Acta 2005, 1733, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Hou, M.; Xia, X.; Zhu, H.; Tang, Z.; Wang, Q.; Li, Y.; Chi, D.; Yu, X.; Zhao, T. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: The role of the PPARgamma-LXRalpha-ABCA1 pathway. J. Biol. Chem. 2005, 280, 36792–36801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bo, C.; Cao, Y.; Roursgaard, M.; Riso, P.; Porrini, M.; Loft, S.; Møller, P. Anthocyanins and phenolic acids from a wild blueberry (Vaccinium angustifolium) powder counteract lipid accumulation in THP-1-derived macrophages. Eur. J. Nutr. 2016, 55, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Kahn, C.R. Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 143. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS ONE 2015, 10, e0123795. [Google Scholar] [CrossRef]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gospin, R.; Sandu, O.; Gambina, K.; Tiwari, A.; Bonkowski, M.; Hawkins, M. Resveratrol improves insulin resistance with anti-inflammatory and ‘browning’ effects in adipose tissue of overweight humans. J. Invest. Med. 2016, 64, 814–815. [Google Scholar] [CrossRef] [Green Version]
- UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 3 January 2020).
- Giacosa, A.; Barale, R.; Bavaresco, L.; Gatenby, P.; Gerbi, V.; Janssens, J.; Johnston, B.; Kas, K.; La Vecchia, C.; Mainguet, P. Cancer prevention in Europe: The Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 2013, 22, 90–95. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Buckland, G.; Bach, A.; Serra-Majem, L. Obesity and the Mediterranean diet: A systematic review of observational and intervention studies. Obes. Rev. 2008, 9, 582–593. [Google Scholar] [CrossRef]
- Schröder, H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef]
- Schröder, H.; Marrugat, J.; Vila, J.; Covas, M.I.; Elosua, R. Adherence to the traditional Mediterranean diet is inversely associated with body mass index and obesity in a Spanish population. J. Nutr. 2004, 134, 3355–3361. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Balaji, M.; Ganjayi, M.S.; Kumar, G.E.H.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrelli, M.; Statti, G.; Conforti, F. A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders. Molecules 2020, 25, 649. https://doi.org/10.3390/molecules25030649
Marrelli M, Statti G, Conforti F. A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders. Molecules. 2020; 25(3):649. https://doi.org/10.3390/molecules25030649
Chicago/Turabian StyleMarrelli, Mariangela, Giancarlo Statti, and Filomena Conforti. 2020. "A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders" Molecules 25, no. 3: 649. https://doi.org/10.3390/molecules25030649
APA StyleMarrelli, M., Statti, G., & Conforti, F. (2020). A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders. Molecules, 25(3), 649. https://doi.org/10.3390/molecules25030649