Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of SPME Procedure
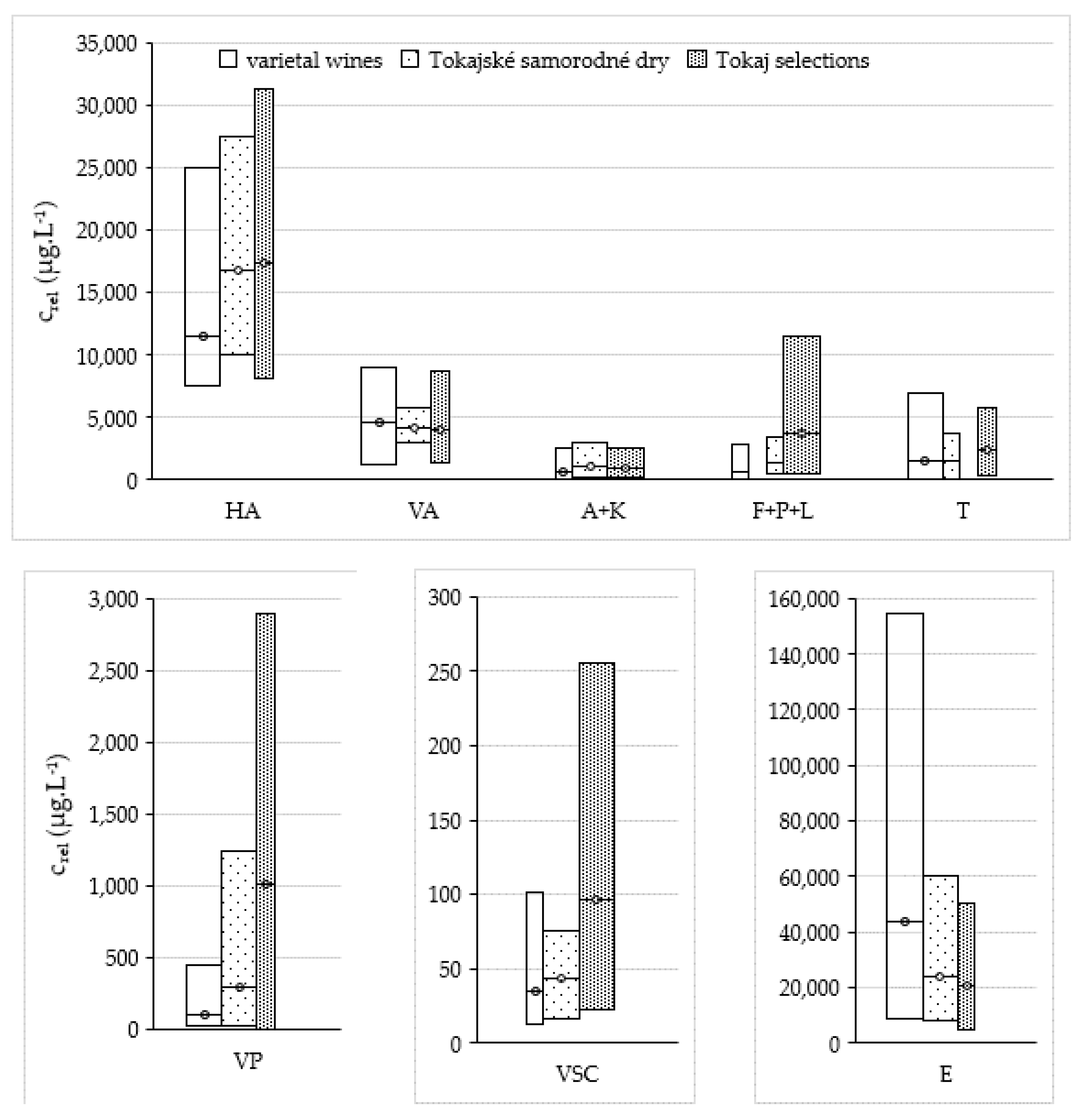
2.2. VOC Profile
2.3. Multivariate Analysis of Tokaj Wines’ Volatile Compounds
3. Materials and Methods
3.1. Wine Samples
3.2. Analysis of VOC Profiles by GC×GC
3.3. Chemicals
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007; Official Journal of the European Union; European Union L9; European Union: Brussels, Belgium, 2013; pp. 671–854.
- COMMISSION DELEGATED REGULATION (EU) 2019/33 of 17 October 2018 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Applications for Protection of Designations of Origin, Geographical Indications and Traditional Terms in the Wine Sector, the Objection Procedure, Restrictions of Use, Amendments to Product Specifications, Cancellation of Protection, and Labelling and Presentation; Official Journal of the European Union L9; European Union: Brussels, Belgium, 2019; pp. 2–45.
- Definition of the vitivinicultural products by code; Document of International Organization of Vine and Wine. Paris, France, Edition 2018 ed. Available online: http://www.oiv.int/en/technical-standards-and-documents/products-definition-and-labelling/definition-of-the-vitivinicultural-products-by-code-sheet (accessed on 3 February 2020).
- Furdíková, K.; Machyňáková, A.; Drtilová, T.; Klempová, T.; Ďurčanská, K.; Špánik, I. Comparison of volatiles in noble-rotten and healthy grape berries of Tokaj. LWT 2019, 105, 37–47. [Google Scholar] [CrossRef]
- Miklósy, É.; Kerényi, Z. Comparison of the volatile aroma components in noble rotted grape berries from two different locations of the Tokaj wine district in Hungary. Anal. Chim. Acta 2004, 513, 177–181. [Google Scholar] [CrossRef]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae—Their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Magyar, I.; Bene, Z.S. Morphological and taxonomic study on mycobiota of noble rotted grapes in the Tokaj wine district. Acta Aliment. 2006, 35, 237–246. [Google Scholar] [CrossRef]
- Act No. 313/2009 on Viticulture and Winegrowing; Collection of Laws of the Slovak Republic 2009; Ministry of Justice of Slovak Republic: Bratislava, Slovakia, 2009; pp. 1–28.
- Miklósy, É.; Kalmár, Z.; Kerényi, Z. Identification of some characteristic aroma compounds in noble rotted grape berries and Aszú wines from Tokaj by GC-MS. Acta Aliment. 2004, 33, 215–226. [Google Scholar] [CrossRef]
- Magyar, I. Botrytized wines. In Advances in Food and Nutrition Research; Academic Press: Burlington, VT, USA, 2011; Volume 63, pp. 147–206. [Google Scholar]
- Magyar, I.; Soos, J. Botrytized wines—Current perspectives. Int. J. Wine Res. 2016, 8, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, E.; Dubourdieu, D.; Darriet, P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chem. 2007, 103, 536–545. [Google Scholar] [CrossRef]
- Sarrazin, E.; Shinkaruk, S.; Tominaga, T.; Bennetau, B.; Freärot, E.; Dubourdieu, D. Odorous impact of volatile thiols on the aroma of young botrytized sweet wines: Identification and quantification of new sulfanyl alcohols. J. Agric. Food Chem. 2007, 55, 1437–1444. [Google Scholar] [CrossRef]
- Thibon, C.; Shinkaruk, S.; Jourdes, M.; Bennetau, B. Aromatic potential of botrytized white wine grapes: Identification and quantification of new cysteine-Sconjugate flavour precursors. Anal. Chim. Acta 2010, 660, 190–196. [Google Scholar] [CrossRef]
- Machyňáková, A.; Khvalbota, L.; Furdíková, K.; Drtilová, T.; Špánik, I. Characterization of volatile organic compounds in Slovak Tokaj wines. J. Food Nutr. Res. 2019, 58, 307–318. [Google Scholar]
- Compendium of International Methods of Wine and Must Analysis; Document of International Organization of Vine and Wine. Paris, France, Edition 2018 ed. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 3 February 2020).
- Marquez, A.; Serratosa, M.P.; Merida, K.; Zea, L.; Moyano, L. Optimization and validation of anautomated DHS–TD–GC–MS method for the determination of aromatic esters in sweet wines. Talanta 2014, 123, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Karine, P.; Nicolli, K.P.; Welke, J.E.; Closs, M.; Caramão, E.B.; Costa, G.; Manfroi, V.; Zini, C.A. Characterization of the Volatile Profile of Brazilian Moscatel Sparkling Wines Through Solid Phase Microextraction and Gas Chromatography. J. Braz. Chem. Soc. 2015, 26, 1411–1430. [Google Scholar]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Asfaw, A.A.; Aspromonte, J.; Wolfs, K.; Van Schepdael, A.; Adams, E. Overview of sample introduction techniques prior to GC for the analysis of volatiles in solid materials. J. Sep. Sci. 2019, 42, 214–225. [Google Scholar]
- Vyviurska, O.; Špánik, I. Assessment of Tokaj varietal wines with comprehensive two-dimensional gas chromatography coupled to high resolution mass spectrometry. Microchem. J. 2020, 152, 104385. [Google Scholar] [CrossRef]
- Lopez Pinar, A.; Rauhut, D.; Ruehl, E.; Buettner, A. Effects of bunch rot (Botrytis cinerea) and powdery mildew (Erysiphe necator) fungal diseases on wine aroma. Front. Chem. 2017, 5, 20. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Elsevier, Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Furdíková, K.; Makyšová, K.; Špánik, I. Effect of indigenous S. cerevisiae strains on higher alcohols, volatile acids, and esters in wine. Czech J. Food Sci. 2017, 35, 131–142. [Google Scholar]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory Threshold of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and Concentrations in Young Riesling and Non-Riesling Wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of oak-derived compounds on the olfactory perception of barrel-aged wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar]
- NIST WebBook Chemie Database. Available online: https://webbook.nist.gov/chemistry/ (accessed on 3 February 2020).
- Lima, A.; Pereira, J.A.; Baraldi, I.; Malheiro, R. Cooking impact in colour, pigments and volatile composition of grapevine leaves (Vitis vinifera L. var. Malvasia Fina and Touriga Franca). Food Chem. 2017, 221, 1197–1205. [Google Scholar] [PubMed] [Green Version]
Sample Availability: Samples of the compound are not available from the authors. |


| RI | Compound | FR | Varietal Wines | Tokajské Samorodné Dry | Tokaj Selections | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg | Min | Max | Avg | Min | Max | Avg | Min | Max | |||
| 1657 | methyl (2Z)-3,7-dimethylocta-2,6-dienoate (methyl nerate) | 54.1 | - | nd | nd | 110 | nd | 237 | - | nd | nd |
| 1520 | (E)-6-methylhept-2-en-4-one | 46.6 | - | nd | nd | 295 | 132 | 678 | - | nd | nd |
| 2450 | 2-[(1E)-1,3-butadien-1-yl]-1,3,4-trimethylbenzene (TPB) | 46.1 | - | nd | nd | - | nd | nd | 246 | 47 | 491 |
| 1666 | diethyl butanedioate (diethyl succinate) | 43.0 | 1006 | 459 | 3863 | 2492 | 1071 | 3721 | 6293 | 1818 | 10590 |
| 1911 | 5-butyloxolan-2-one (γ-octalactone) | 39.7 | 8 | nd | 30 | 199 | 52 | 495 | 13 | nd | 66 |
| 1567 | 5-methylfuran-2-carbaldehyde (5-methylfurfural) | 35.9 | 7 | 2 | 34 | 30 | 9 | 74 | 271 | 31 | 623 |
| 2395 | 4-ethoxy-4-oxobutanoic acid (monoethyl succinate) | 33.3 | 242 | 82 | 764 | 619 | 268 | 1272 | 1182 | 461 | 2164 |
| 2191 | 2-methoxy-4-prop-2-enylphenol (eugenol) | 30.6 | - | nd | nd | 26 | nd | 40 | 24 | nd | 56 |
| 1860 | 2-methoxyphenol (guaiacol) | 29.6 | - | nd | nd | 2 | nd | 5 | 11 | nd | 24 |
| 1964 | (4R,5R)-5-butyl-4-methyloxolan-2-one (cis-whiskey lactone) | 27.2 | - | nd | nd | 189 | nd | 495 | 519 | 148 | 1212 |
| 1515 | ethyl 2-hydroxy-4-methylpentanoate | 25.8 | 41 | nd | 151 | 95 | 59 | 180 | 222 | 87 | 429 |
| 1820 | butyl ethyl butanedioate | 25.0 | 31 | 12 | 152 | 161 | 31 | 346 | 384 | 134 | 864 |
| 1869 | phenylmethanol | 23.8 | 14 | nd | 35 | 25 | 16 | 32 | 61 | 14 | 124 |
| 2031 | diethyl 2-hydroxybutanedioate (diethyl malate) | 22.5 | 62 | 24 | 281 | 147 | 34 | 300 | 998 | nd | 2428 |
| 2166 | methyl 14-methylpentadecanoate | 21.7 | 120 | nd | 282 | 49 | nd | 91 | nd | nd | nd |
| 1630 | 2-phenylacetaldehyde | 21.4 | 89 | 8 | 269 | 208 | nd | 355 | 277 | 162 | 469 |
| 1686 | 1,1,6-trimethyl-2H-naphthalene (TDN) | 21.2 | 141 | nd | 812 | 576 | nd | 1120 | 1375 | 217 | 3081 |
| 1420 | ethyl octanoate | 20.3 | 13,788 | 4410 | 26,523 | 6219 | 2342 | 14,581 | 3261 | 743 | 7355 |
| 1567 | ethyl 4-oxopentanoate | 20.2 | 1 | nd | 6 | 1 | nd | 5 | 45 | nd | 153 |
| 1770 | ethyl 2-phenylacetate | 19.4 | 266 | 87 | 676 | 683 | 286 | 1282 | 1224 | 523 | 2901 |
| 1457 | furan-2-carbaldehyde (furfural) | 18.6 | 42 | nd | 291 | 123 | 13 | 199 | 1208 | 98 | 3520 |
| 1364 | ethyl 2-hydroxypropanoate | 18.2 | 180 | nd | 1037 | 1560 | 397 | 4151 | 2043 | 39 | 4715 |
| 1520 | benzaldehyde | 17.2 | 191 | 101 | 427 | - | nd | nd | 291 | 34 | 551 |
| 1467 | 2-ethylhexan-1-ol | 16.7 | 68 | 22 | 182 | 180 | 83 | 339 | 261 | 10 | 671 |
| 2235 | ethyl hexadecanoate | 16.4 | 1345 | nd | 2410 | 612 | nd | 1554 | 187 | nd | 1532 |
| 1631 | 4-O-ethyl 1-O-methyl butanedioate (ethyl methyl succinate) | 16.2 | 1 | nd | 17 | 5 | nd | 17 | 81 | nd | 203 |
| 1935 | 2-ethylhexanoic acid | 16.2 | nd | nd | nd | nd | nd | nd | 40 | nd | 110 |
| 1896 | 2-phenylethanol | 16.0 | 2767 | 1778 | 5285 | 4976 | 3042 | 7180 | 6602 | 2885 | 14,758 |
| Sample | Count | Vintage | Residual Sugars (g·L−1) | Alcohol (% v/v) | |
|---|---|---|---|---|---|
| Varietal wines | Furmint | 8 | 2013–2016 | ≤4 | 11–15 |
| Lipovina | 6 | 2015–2016 | |||
| Muscat Lunel | 7 | 2013–2016 | |||
| Tokajské samorodné dry | 8 | 2008–2016 | ≤ 10 | 11–13 | |
| Tokaj selections | Tokajský výber 3-putňový | 4 | 2000–2009 | 60 – 90 | 10–12 |
| Tokajský výber 4-putňový | 4 | 2000–2009 | 90–120 | ||
| Tokajský výber 5-putňový | 4 | 2000–2004 | 120–150 | ||
| Tokajský výber 6-putňový | 5 | 2002–2011 | ≥150 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdíková, K.; Machyňáková, A.; Drtilová, T.; Špánik, I. Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds. Molecules 2020, 25, 669. https://doi.org/10.3390/molecules25030669
Furdíková K, Machyňáková A, Drtilová T, Špánik I. Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds. Molecules. 2020; 25(3):669. https://doi.org/10.3390/molecules25030669
Chicago/Turabian StyleFurdíková, Katarína, Andrea Machyňáková, Tereza Drtilová, and Ivan Špánik. 2020. "Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds" Molecules 25, no. 3: 669. https://doi.org/10.3390/molecules25030669
APA StyleFurdíková, K., Machyňáková, A., Drtilová, T., & Špánik, I. (2020). Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds. Molecules, 25(3), 669. https://doi.org/10.3390/molecules25030669





