2. Results and Discussion
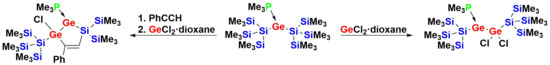
The reaction of
1 with one equivalent of GeCl
2·dioxane resulted in the formation of compound
3 (
Scheme 2,
Figure 1), which can be considered a 1,1-migratory insertion product of GeCl
2 into an Si–Ge bond of
1. The further reaction of
3 with another equivalent of GeCl
2·dioxane caused the selective 1,1-insertion of GeCl
2 into the other Si–Ge bond of
3 to give the digermylgermylene adduct
4 (
Figure 2).
Insertion reactions of GeCl
2 into Ge-element bonds are not very common but have been reported on a few occasions. Most notable is the isomerization of Cl
3GeGeCl
3 to GeCl
4 and GeCl
2, followed by the four-fold insertion of GeCl
2 into all Ge–Cl bonds of GeCl
4 to give neopentagermane Ge(GeCl
3)
4 [
19]. More recently, Rivard and co-workers described the related reaction of an NHC adduct of GeCl
2 with GeCl
2 to give branched oligomers [
20]. The insertion of GeCl
2·dioxane into the Ge–P bond of a germylene phosphane adduct was reported by Wesemann and co-workers [
21], whereas Schnepf and co-workers observed insertion of GeCl
2·dioxane into the S–C bonds of [(Me
3Si)
3CS]
2Ge: [
22].
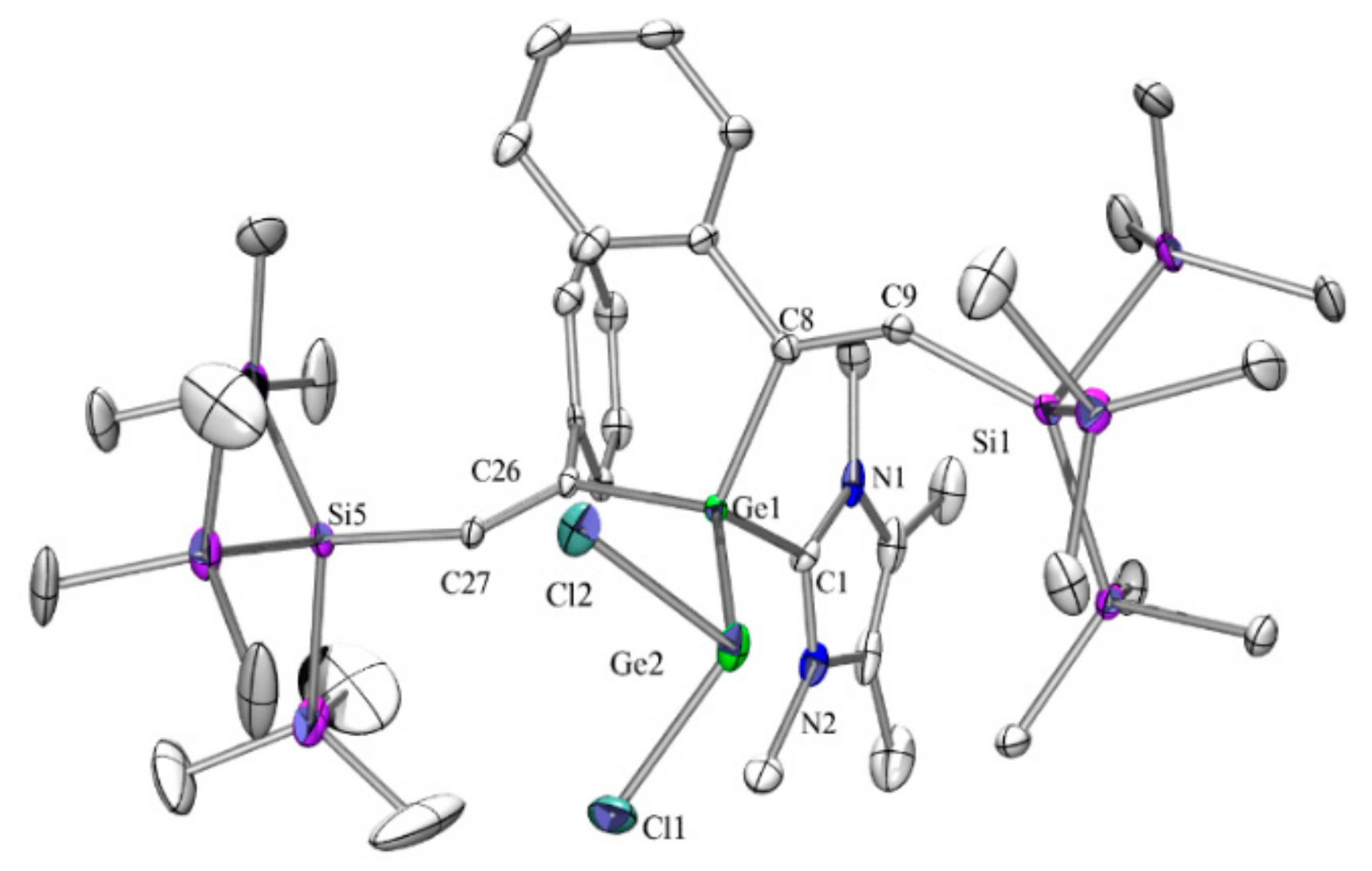
In the solid state structure of
3 (
Figure 1), the elongation of bond distances of divalent germanium to neighboring atoms (Ge(1)–Ge(2) 2.5210(18) Å, Ge(2)–Si(6) 2.428(3) Å) was not as pronounced as was observed before for related cyclic and acyclic compounds [
5,
23,
24]. Consistently, the structure of
4 (
Figure 2) displayed elongated Ge–Ge bonds of 2.4980(9) and 2.5114(9) Å.
In the
29Si NMR spectrum of
3, the Ge(PMe
3)Si(SiMe
3)
3 part (δ = −8.7 (d,
3JSi-P = 11.4 Hz, GeSi(
SiMe
3)
3) and −112.6 (d,
2JSi-P = 16.1 Hz,
Si(SiMe
3)
3) very much resembles the spectrum of
1, while the GeCl
2 unit attached to the second Si(SiMe
3)
3 group caused a deshielding effect of the chemical shift for the central silicon atom of the Si(SiMe
3)
3 unit to −76.7 ppm. The same was observed for the signal at −73.0 ppm in the
29Si NMR spectrum of
4. With the phenylacetylene insertion product
2 readily available [
5], we were interested in whether the 1,1-migratory insertion of GeCl
2 into the remaining Ge–Si bond of
2 would occur with similar ease. The reaction of vinylgermylene PMe
3 adduct
2 with GeCl
2·dioxane indeed proceeded quantitatively, however, the obtained compound
5 (
Figure 3,
Scheme 3) was not the expected insertion product of the Ge–Si bond, but rather a cyclic germylene PMe
3 adduct.
It is known that germylene phosphane adducts are in equilibrium with the free germylene [
13]. For the formation of
5 we assumed that, in the first step, the respective free germylene of
2 inserted into a Ge–Cl bond of GeCl
2, forming a germylchlorogermylene PMe
3 adduct intermediate (
6). The intramolecular cyclization of
6 can occur via the metathesis of a Ge–Cl with a Si–SiMe
3 bond, releasing Me
3SiCl with simultaneous Si–Ge bond formation (
Scheme 3). In this mechanism, the eventual germylene involves not the original germanium atom but the one introduced by the addition of GeCl
2.
In the solid state structure of 5, the planar ring features of Ge–Ge (2.5052(5) Å) and Ge–P (2.3610(9) Å) bond distances, which are somewhat shorter than that of 3 and 4, were the bond of the germylene atom to the Si(SiMe3)2 unit (2.4409(9) Å), which is longer than in 3 but still short of the 2.4816(8) Å observed for 2. The Si–Ge–Ge angle is very acute (84.97(3)°), with the Ge–PMe3 interaction located almost orthogonal to the ring plane. The 29Si NMR spectrum features the signal for the germylene attached Si(SiMe3)2 unit at −50.1 ppm (d, 2JSi-P = 15 Hz) and that for the CSi(SiMe3)3 at −100.7 (d, 3JSi-P = 2 Hz).
Both NMR spectroscopic results and XRD data showed selective formation of only one isomer of
5. It is reasonable to assume that the other possible isomer where PMe
3 and Cl are on different sides of the ring plane is less stable. Since we have previously shown that the dissociation of PMe
3 from silylated germylenes is a facile process, re-association of PMe
3 would occur in a selective way to form the more thermodynamically stable product [
13].
Compound
3 offered the opportunity to also study the reaction sequence of 1,1-insertion of GeCl
2 followed by 1,2-insertion of an alkyne. In particular, the regioselectivity of the alkyne insertion reaction was of interest. As the germylene atom of adduct
3 has two weak bonds, the question arose whether the alkyne would insert into the Ge–Si bond or into the, presumably, weaker Ge–Ge bond. Much to our surprise, we observed two products. One was the double regioselective insertion of Me
3SiC≡CH (
7), not into the expected Ge–Si and Ge–Ge bonds adjacent to the germylene atom but into the two different Ge–Si bonds presented in
3 (
Scheme 4). While the structure of compound
7 was unequivocally established by single crystal XRD analysis, the structure of the other compound
8a was assigned by NMR spectroscopy as the product of a single alkyne insertion into the germylene–Si bond.
The outcome of the experiment was rationalized by assuming that the first step in the reaction occurred analogously to what is shown in
Scheme 1. The 1,2-insertion of the alkyne into the (Me
3Si)
3Si–Ge bond gave the germylene adduct
8a (
Scheme 5). The initial formation of
8a was consistent with NMR spectroscopic analysis of the reaction course. Dissociation of PMe
3 can lead to the free germylene of
8a, which can rearrange to 1,2-dichlorodigermene
8b and, in a further rearrangement step, lead to isomer
8c, where formally the two germanium atoms of
8a and
8c exchanged valency (
Scheme 5). The insertion of another equivalent of Me
3SiC≡CH into the (Me
3Si)
3Si–Ge bond of isomer
8c led to
7.
The solid state structure of
7 (
Figure 4) again revealed elongated distances to the divalent germylene atom of 2.5178(14) and 2.016(8) Å for the Ge–Ge and Ge–C bonds. It is interesting to note that, within the molecule, seven consecutively connected atoms (P–Ge–Ge–C=C–Si–Si) were almost coplanar, with a second plane defined by Ge–C=C–Si–S perpendicular to the first. The
29Si NMR spectroscopic analysis of the intermediate
8a showed that the signal for the silicon atom attached to the GeCl
2 unit at −78.6 ppm was close to the related signal of
3, whereas the central silicon atom of the vinylated Si(SiMe
3)
3 group appeared at a typical chemical shift of −87.3 ppm. For the final product
7, these signals were observed at −85.3 and −95.0 ppm.
When an N-heterocyclic carbene (NHC) adduct of the double phenylacetylene insertion product
9 [
13] was subjected to reaction with GeCl
2·dioxane, the germylene lone pair served as a Lewis base to replace the dioxane of GeCl
2 (
10) (
Scheme 6 top reaction,
Figure 5). A similar reaction of Roesky’s germylene with GeCl
2·dioxane has been reported [
25].
The solid state structure of
10 (
Figure 5) formally includes two divalent germanium atoms. Ge1 acts as a Lewis acid to accept the NHC as a base (Ge1–C1 = 2.006(4) Å). In addition Ge1 donates via its lone pair to the GeCl
2 unit (Ge(1)–Ge(2) 2.5628(8) Å). Compared to the GeCl
2 units in compounds
3,
4 and
7, the Ge–Cl distances of
10 were elongated to 2.30 Å, consistent with the divalent character of the germylene atom. As expected, the NMR spectra of
10 were fairly similar to that of
9. The most pronounced difference was found for the
13C signal of the NHC carbene carbon atom, while for
9 a value of 179.9 ppm was observed, the respective signal for
10 can be detected at 150.6 ppm. Similar up-field shifts of the NHC carbene signal were observed previously for cases where the lone pair of a tetrylene NHC adduct acted as a base to a Lewis acid [
26].
In a related reaction, we reacted germylene adduct
1 with three equivalents phenylacetylene and one equivalent GeCl
2·dioxane. Unfortunately, the reaction was not very selective but it was possible to select a crystal of PMe
3 germylene adduct
11 under the microscope (
Scheme 6,
Figure 6). It seems reasonable that
11 was formed via the initial formation of the previously described divinylgermylene [
5,
13]. In a similar way, as was proposed for the formation of
5, insertion of the divinylgermylene into a Ge–Cl bond of GeCl
2·dioxane was supposed to give a chlorogermylgermylene. Phenylacetylene can now insert into the Ge–Ge bond of this new germylene, which eventually is isolated as its PMe
3 adduct
11. The germanium atom of the initial germylene then attached to three vinyl substituents. The third vinyl group, however, contained the phenyl group in the 2-position as its regioselectivity was then determined by the newly introduced germylene atom.
The crystal structure of
11 (
Figure 6) features a tetravalent germanium atom with the three Ge–C distances covering a range between 1.94 and 1.96 Å and a Ge–Cl distance of 2.1662(9) Å. Again, the Ge–C bond to the divalent germanium atom Ge2 is longer (2.031(3) Å) and the same is true for the Ge–Cl bond (2.296(1) Å).
3. Experimental Section
General Remarks: All reactions involving air-sensitive compounds were carried out under an atmosphere of dry nitrogen or argon using either Schlenk techniques or a glove box. All solvents were dried using a column based solvent purification system [
27]. Chemicals were obtained from different suppliers and used without further purification. Adducts
1 [
5,
13],
2 [
5,
13,
14], and
9 [13] as well as reagents 1,3,4,5-tetramethylimidazol-2-ylidene (ImMe
4) [
28] and GeCl
2·dioxane [
29] were prepared according to previously published procedures.
The
1H (300 MHz),
13C (75.4 MHz),
31P (121.4 MHz), and
29Si (59.3 MHz) NMR spectra were recorded on a Varian INOVA 300 spectrometer and are referenced to tetramethylsilane (TMS). If not otherwise noted, the solvent used was C
6D
6 and the samples were measured at rt. In the case of the reaction samples, a D
2O capillary was used to provide an external lock frequency signal. To compensate for the low isotopic abundance of
29Si, the INEPT pulse sequence was used for the amplification of the signal [
30,
31].
Elementary analysis was carried out using a Heraeus VARIO ELEMENTAR. For a number of compounds, the obtained elemental analysis showed carbon values that were too low, which is a typical problem for these compounds likely caused by silicon carbide formation during the combustion process.
X-ray Structure Determination: For X-ray structure analyses, the crystals were mounted onto the tip of glass fibers. Data collection was performed with a BRUKER-AXS SMART APEX CCD diffractometer using graphite-monochromated Mo Kα radiation (0.71073 Å). The data were reduced to F
2o and corrected for absorption effects with SAINT [
32] and SADABS [
33,
34], respectively. The structures were solved by direct methods and refined by full-matrix least-squares method (SHELXL97) [
35]. If not noted otherwise all non-hydrogen atoms were refined with anisotropic displacement parameters and all hydrogen atoms were located in calculated positions to correspond to standard bond lengths and angles. Crystallographic data (excluding structure factors) for the structures of compounds
3,
4,
5,
7,
10, and
11 reported in this paper were deposited with the Cambridge Crystallographic Data Center as supplementary publication number CCDC-969562 (
3), 969565 (
4), 1918810 (
5), 1918811 (
7), 1918808 (
10), and 1918809 (
11) (see
Supplementary Materials). The data can be obtained free of charge at:
http://www.ccdc.cam.ac.uk/products/csd/request/. The figures of solid state molecular structures were generated using Ortep-3 as implemented in WINGX [
36] and rendered using POV-Ray 3.6 [
37].
3.1. Dichloro[tris(trimethylsilyl)silyl]germyl[tris(trimethylsilyl)silyl]germylene·PMe3 Adduct (3)
Method A: A solution of tris(trimethylsilyl)silyl potassium (0.31 mmol) in THF (2 mL) was slowly added dropwise to a vigorously stirred solution of GeCl2·dioxane (72 mg, 0.31 mmol) and trimethylphosphane (11 mg, 0.16 mmol) in THF (40 mL) at −30 °C. Stirring of the reaction mixture was continued for 1 h at rt. After the solvent was removed, the residue was extracted with pentane (3 × 5 mL). The orange extract was concentrated to 3 mL and stored at −35 °C. Yellow crystalline 3 (54 mg, 43%) was obtained.
Method B: GeCl2·dioxane (194 mg, 0.84 mmol) in THF (2 mL) was added to a stirred solution of 1 (1.40 mmol) in THF (15 mL) at −30 °C. After stirring of the reaction mixture for 1 h at rt, further work-up proceeded analogous to Method A. Yellow crystalline 3 (376 mg, 56%) was obtained.
Mp = 116–119 °C. NMR: δ in ppm: 1H: 1.20 (d, 9H, 2JH-P = 10.7 Hz, PMe3), 0.46 (s, 27H, SiMe3), 0.43 (s, 27H, SiMe3). 13C{1H}: 17.1 (d, 1JC-P = 25.3 Hz, PMe3), 3.1 (SiMe3), 4.2 (SiMe3). 29Si{1H}: −8.2 (GeCl2Si(SiMe3)3), −8.7 (d, 3JSi-P = 11.4 Hz, GeSi(SiMe3)3), −76.7 (GeSi(SiMe3)3), −112.6 (d, 2JSi-P = 16.1 Hz, Si(SiMe3)3). 31P{1H}: −15.2. Anal. calcd for C21H63Cl2Ge2PSi8 (787.55): C 32.03, H 8.06. Found: C 31.93, H 8.09.
3.2. Bis{dichloro[tris(trimethylsilyl)silyl]germyl}germylene·PMe3 Adduct (4)
GeCl2·dioxane (173 mg, 0.75 mmol) in THF (2 mL) was added to a stirred solution of 1 (289 mg, 0.40 mmol) in THF (10 mL) at −30 °C. After 10 min the solvent was removed and the product was extracted with pentane (3 × 5 mL). The solution was concentrated to 5 mL and stored at −35 °C. Pale yellow crystalline 4 (118 mg, 34%) was obtained. Mp = 90–92 °C. NMR: δ in ppm: 1H: 1.27 (d, 9H, 2JH-P = 11.7 Hz, PMe3), 0.45 (s, 54H, SiMe3). 13C{1H}: 29.9 (PMe3), 2.9 (SiMe3). 29Si{1H}: −8.0 (SiMe3), −73.0 (Si(SiMe3)3). 31P{1H}: −8.7. Anal. calcd for C21H63Cl4Ge3PSi8 (931.08): C 27.09, H 6.82. Found: C 26.80, H 6.69.
3.3. 1,2-Digerma-3-sila-1-chloro-1-tris(trimethylsilyl)silyl-3,3-bis(trimethylsilyl)-5-phenylcyclopent-4-ene-2-ylidene−PMe3 Adduct (5)
GeCl2·dioxane (23 mg, 0.1 mmol) was added to a stirring solution of 2 (75 mg, 0.1 mmol) in THF (2 mL). After 2 h the solvent was removed and the residue was extracted with pentane (3 × 5 mL). The solution was concentrated to 0.5 mL and stored at −35 °C. Pale yellow crystalline 5 (57 mg, 74%) was obtained. Mp = 122–124 °C. NMR: δ in ppm: 1H: 7.90 (d, J = 7.7 Hz, 2H, o-H), 7.15 (d, J = 7.4 Hz, 2H, m-H), 7.08 (s, 1H, CH), 6.99 (t, J = 7.4 Hz, 1H, p-H), 1.12 (d, 2JH-P = 10.7 Hz, PMe3), 0.34 (s, 9 H, SiMe3), 0.31 (s, 27 H, SiMe3), 0.15 (s, 9 H, SiMe3). 13C{1H}: 171.3, 147.4, 146.6, 128.2, 126.7, 17.0 (d, 1JC-P = 26 Hz, PMe3), 3.0 (SiMe3), 1.9 (SiMe3), 1.2 (SiMe3). 29Si{1H}: −8.7 (SiMe3), −11.1 (d, 3JSi-P = 13 Hz, SiMe3), −11.7 (d, 3JSi-P = 17 Hz, SiMe3), −50.1 (d, 2JSi-P = 15 Hz, GeSi(SiMe3)3, −100.7 (d, 3JSi-P = 2 Hz, Si(SiMe3)2). 31P{1H}: −13.9. Anal. calcd for C26H60Ge2Si7PCl (781.04): C 39.98, H 7.74. Found: C 39.74, H 7.70.
3.4. Adducts 7 and 8a
A solution of 3 (50 mg, 0.064 mmol) and trimethylsilylacetylene (10 mg, 0.11 mmol) in in THF (2 mL) was stirred at ambient temperature. After 2 h, the 29Si NMR suggested formation of the new product 8a but still some 3 was present. After a further 12 h, all starting material was consumed and another new product was formed. When no further progress was detected, the solvent was removed with a vacuum and the residue dissolved in pentane (2mL). Light yellow crystals (30 mg) containing a mixture of 7 and 8a formed after 12 h at −35 °C. Under the microscope, a suitable crystal of 7 was selected for single crystal XRD analysis. NMR: δ in ppm: Compound 8a (THF/D2O-capillary): 1H: 7.67 (d, J = 2.5 Hz, 1H, C=CH), 1.58 (d, 2JH-P = 9.7 Hz, PMe3), 0.39 (s, 9 H, SiMe3), 0.36 (s, 27 H, SiMe3), 0.27 (s, 9 H, SiMe3). 29Si{1H}: −8.2 (CSiMe3), −10.9 Si(SiMe3), −11.5 Si(SiMe3), −78.6 (GeCl2Si(SiMe3)3, −87.3 (CSi(SiMe3)2). 31P{1H}: −29.8.
Compound 7 (C6D6): 1H: 8.47 (s, 1H, C=CH), 8.02 (s, 1H, C=CH), 0.79 (d, 2JH-P = 7.5 Hz, PMe3), 0.34 (s, 27 H, Si(SiMe3)3), 0.32 (s, 27 H, Si(SiMe3)3), 0.26 (s, 9 H, SiMe3). 0.18 (s, 9 H, SiMe3). 29Si{1H}: −7.2 (CSiMe3), −8.2 (CSiMe3), −11.0 (Si(SiMe3)), −11.9 (Si(SiMe3)), −85.3 (Si(SiMe3)3, −95.0 (Si(SiMe3)2). 31P{1H}: −15.7.
3.5. Adduct 10
First, 9 (40 mg, 0.045 mmol) and GeCl2·dioxane (10 mg, 0.045 mmol) were stirred in THF (1 mL) at rt for 48 h. Next, pentane (1 mL) was added. After storage at −35 °C yellow crystalline 10 (22 mg, 47%) was obtained. Mp = 121–122 °C. NMR: δ in ppm: 1H: 7.12 (m, 2H), 6.99 (m, 4H), 6.61 (br, 4H), 6.28 (s, 1H), 3.33 (s, 6H, N–Me), 2.10 (s, 6H, C–Me), 0.20 (s, 54H, SiMe3). 13C{1H}: 150.6, 150.4, 147.0, 145.2, 130.8, 128.7, 127.5, 126.8, 34.0 (N–Me), 9.5 (C–Me), 1.5 (SiMe3). 29Si{1H}: −12.4 (SiMe3), −84.3 (Siq). Anal. calcd for C41H78Cl2Ge2N2Si8 (1039.94): C 47.35, H 7.56, N 2.69. Found: C 47.23, H 7.54, N 2.67.
3.6. Adduct 11
Phenylacetylene (87 mg, 0.85 mmol) was added to a stirred solution of 1 (0.27 mmol) in THF (5 mL) and after 1 h GeCl2·dioxane (86 mg, 0.37 mmol) was added. The completeness of conversion was determined by 29Si NMR after 24 h. The solvent was removed and the residue was extracted with toluene (5 × 5 mL). The solvent was removed to yield a brown solid (229 mg). Crystallization from toluene gave pale yellow crystals. 29Si NMR analysis indicated formation of at least four different compounds of which 11 is likely the major component. NMR: δ in ppm: 29Si{1H}: −11.5 (SiMe3), −84.9 (Siq). The selection of a well-shaped crystal from the batch under the microscope allowed the single crystal XRD analysis of 11.
4. Conclusions
Here, we reported a few examples of a main group of analogs of what would be called 1,2-migratory insertions in transition metal chemistry. Mono-substituted alkynes were found to insert into the Si–Ge bonds of disilylated germylene PMe
3 adducts [
5,
13,
14]. The current account extends this chemistry to main group analogs of 1,1-migratory insertions. Reactions of one or two equivalents of GeCl
2·dioxane with the PMe
3 adduct of [(Me
3Si)
3Si]
2Ge: gave either the mono- or diinsertion product of GeCl
2 insertion into the Ge–Si bonds. Only a few examples of insertion reactions of GeCl
2 into weak E–E bonds of group 14 oligomers are known [
19,
20,
21,
22], and the current chemistry seems to provide an interesting pathway to precursors for unsaturated group 14 cluster molecules.
How subtle changes in the starting material can change the reaction course profoundly became evident when we subjected another silylated germylene to reaction with GeCl2·dioxane. This time the alkyne mono-insertion product (Me3Si)3SiGe:(Ph)C=CHSi(SiMe3)3 served as the precursor and, instead of the expected GeCl2 insertion into the Si–Ge bond, we observed the substrate acting as germylene inserting into a Ge–Cl bond. The resulting chlorogermylchlorogermylene reacted further by trimethylsilyl chloride elimination to a cyclic germylene adduct.
In an attempt to reverse the order of 1,1 and 1,2-insertion, we next reacted the GeCl2 mono-insertion product (Me3Si)3SiGe:GeCl2Si(SiMe3)3 with Me3SiC≡CH. An unexpected mixture of alkyne mono- and diinsertion products was obtained, where the first alkyne insertion proceeded into the Si–Ge bond of the germylene but the second insertion did not occur into the Ge–Ge bond as expected but into the Ge–Si bond of the second Si(SiMe3)3 unit. For this to happen, the involved germanium atoms needed to swap oxidation states, meaning that the two Cl atoms of the GeCl2 would migrate to the divalent Ge-atom. We assumed that this rearrangement proceeds via known 1,2-shifts of chlorides involving germylene-digermene-germylene interconversion.
As expected, in the eventual reaction of a divinylgermylene with GeCl2·dioxane no germylene insertion chemistry was observed. In this case only the exchange of the dioxane ligand of GeCl2 against the divinylgermylene base could be detected. A final one-pot reaction of [(Me3Si)3Si]2Ge:·PMe3 with one equivalent GeCl2·dioxane and three equivalents of phenylacetylene gave a number of products with an interesting trivinylated chlorogermane, where a chlorogermylene is attached to one of the vinyl units. We suppose that this compound forms by initial divinylgermylene formation, followed by insertion of this germylene into a Ge–Cl bond of GeCl2. The thus formed germylene with a germyl and a chloride substituent can insert a third alkyne into the Ge–Ge bond. Since the newly introduced Ge atom now acts as the germylene the regiochemistry of the last inserted vinyl unit is reversed with respect to the central germanium atom.
We think that the demonstrated chemistry bears the potential become a useful method in the chemistry of heavy group 14 compounds. The facile introduction of GeCl2 into molecules that already contain a certain degree of unsaturation opens an entry into precursor chemistry of interesting oligogermanium cluster chemistry.