Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties
Abstract
:1. Introduction
2. Results
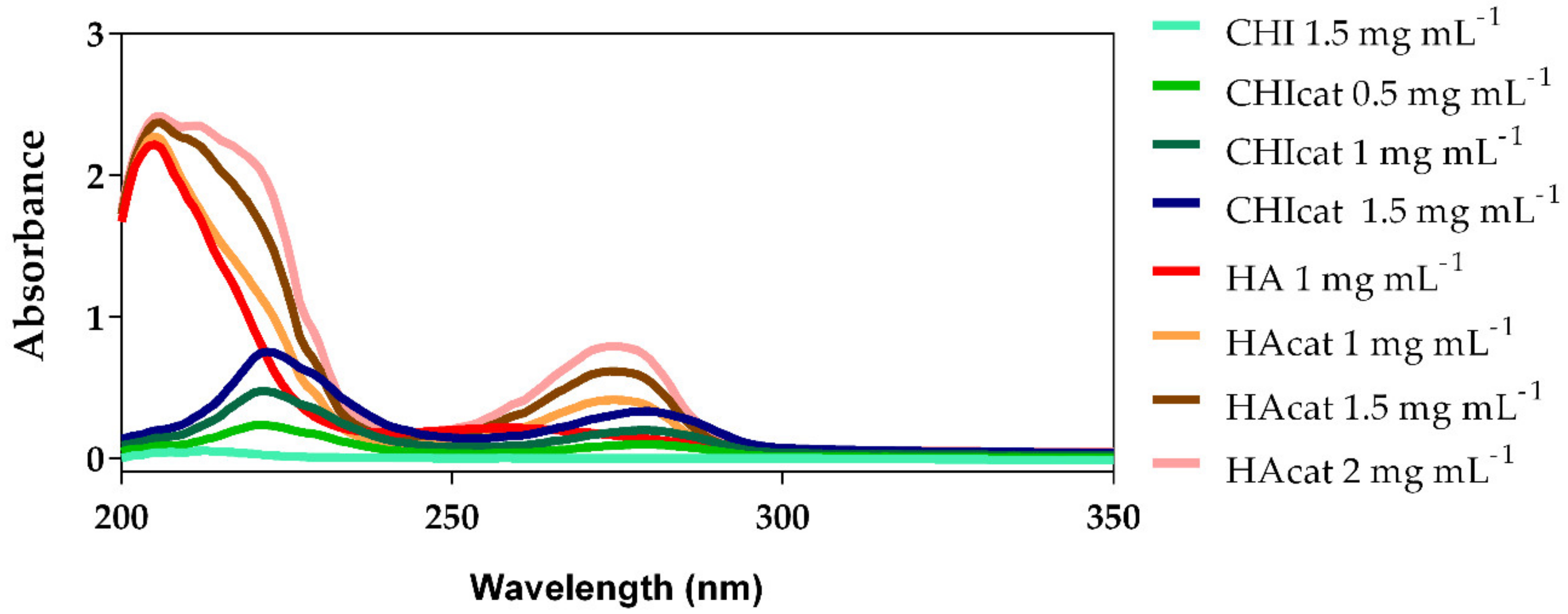
2.1. UV-Vis Analysis of Catechol-Modified Polymers
2.2. Zeta Potential Measurements
2.3. BGNPs’ Morphological and Elemental Characterization
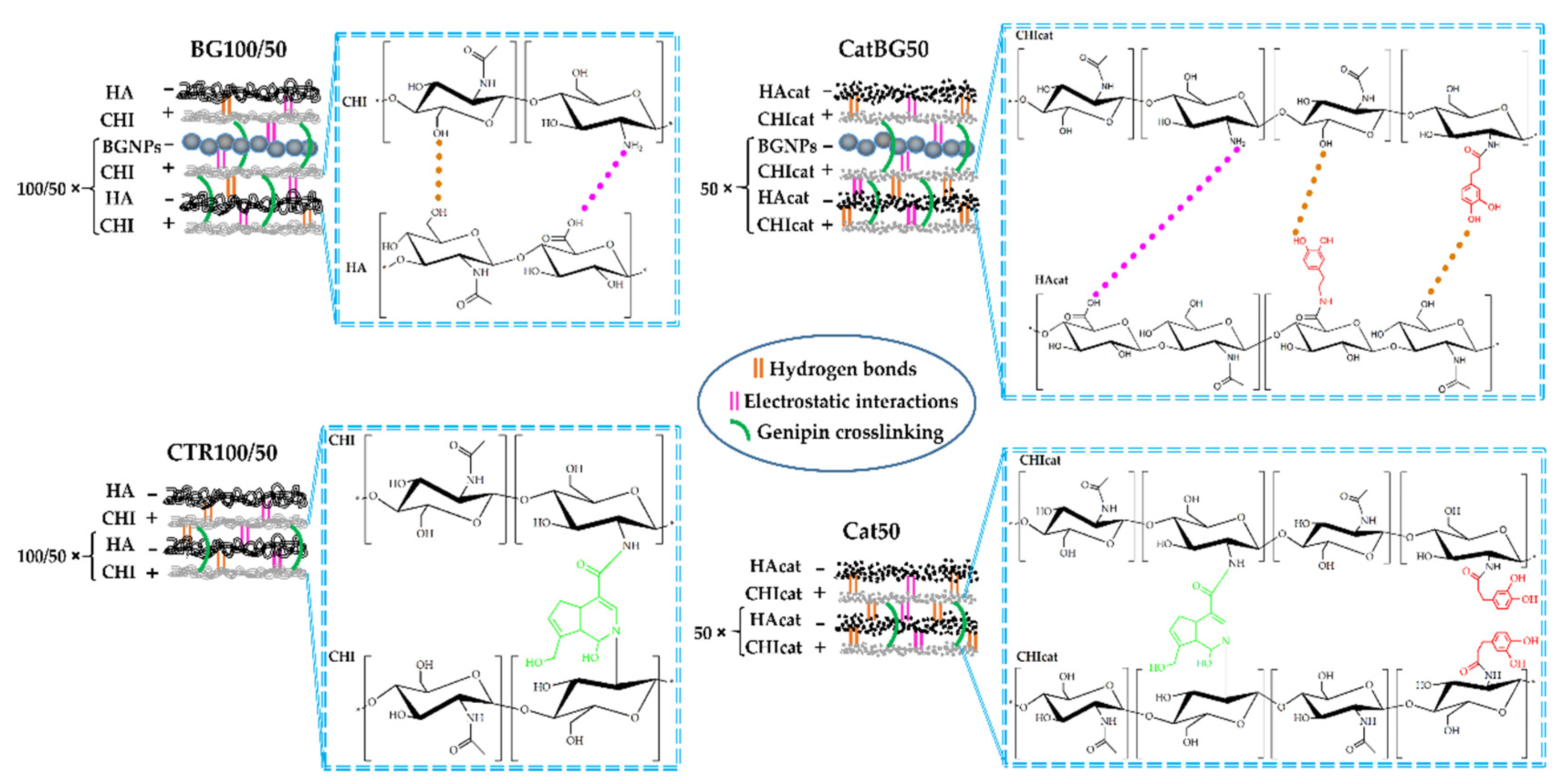
2.4. Production of the LbL Freestanding Films
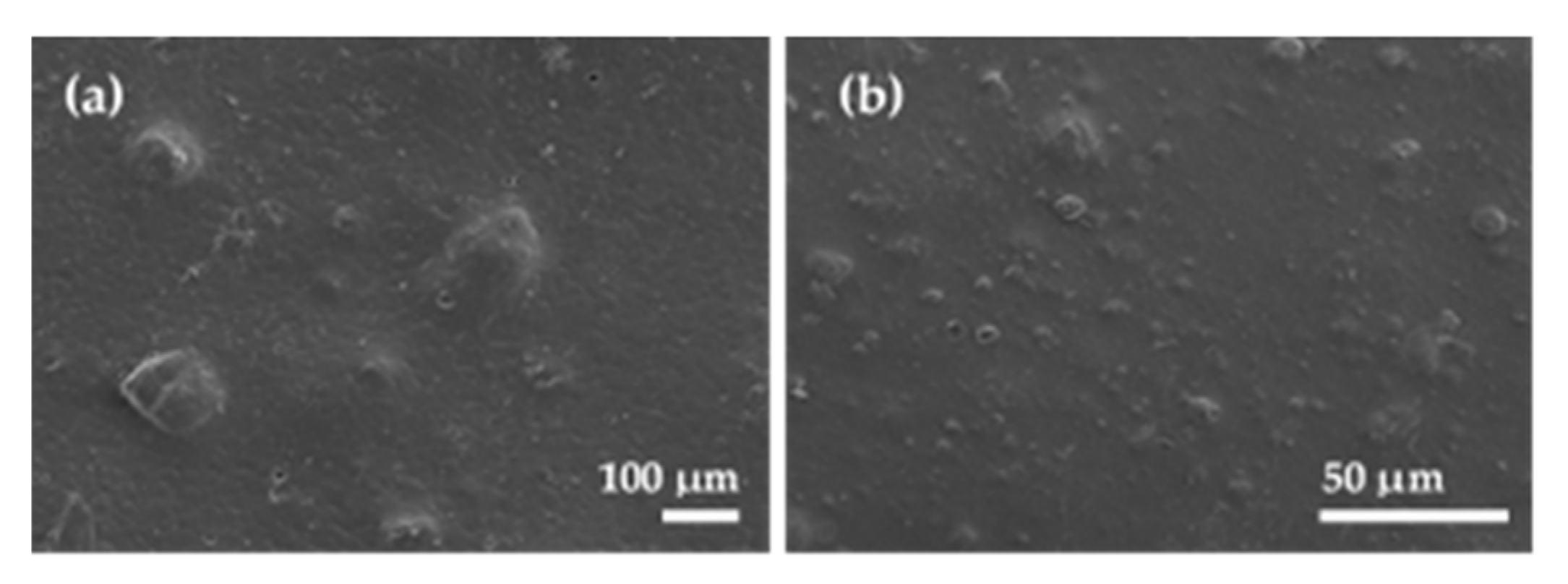
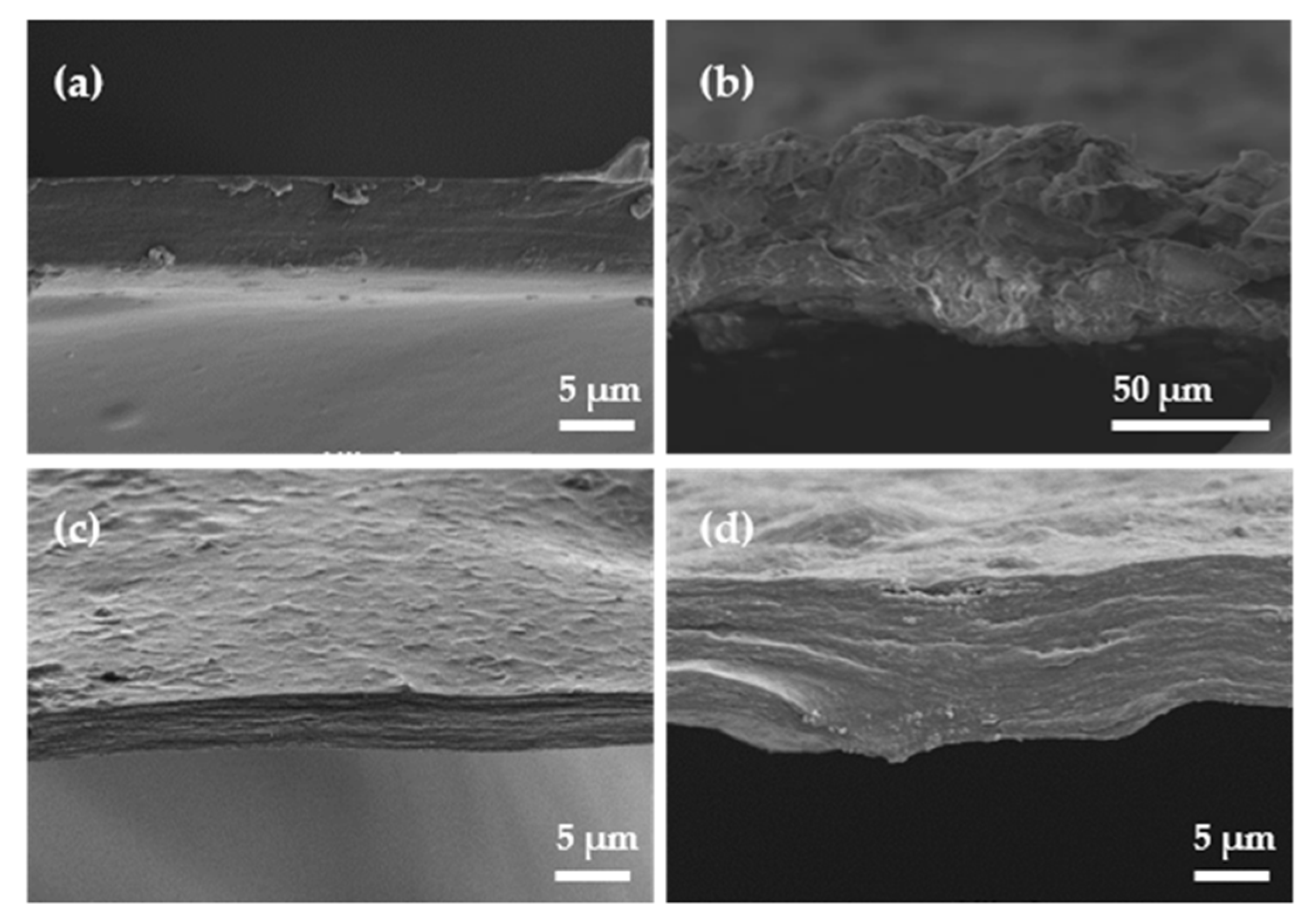
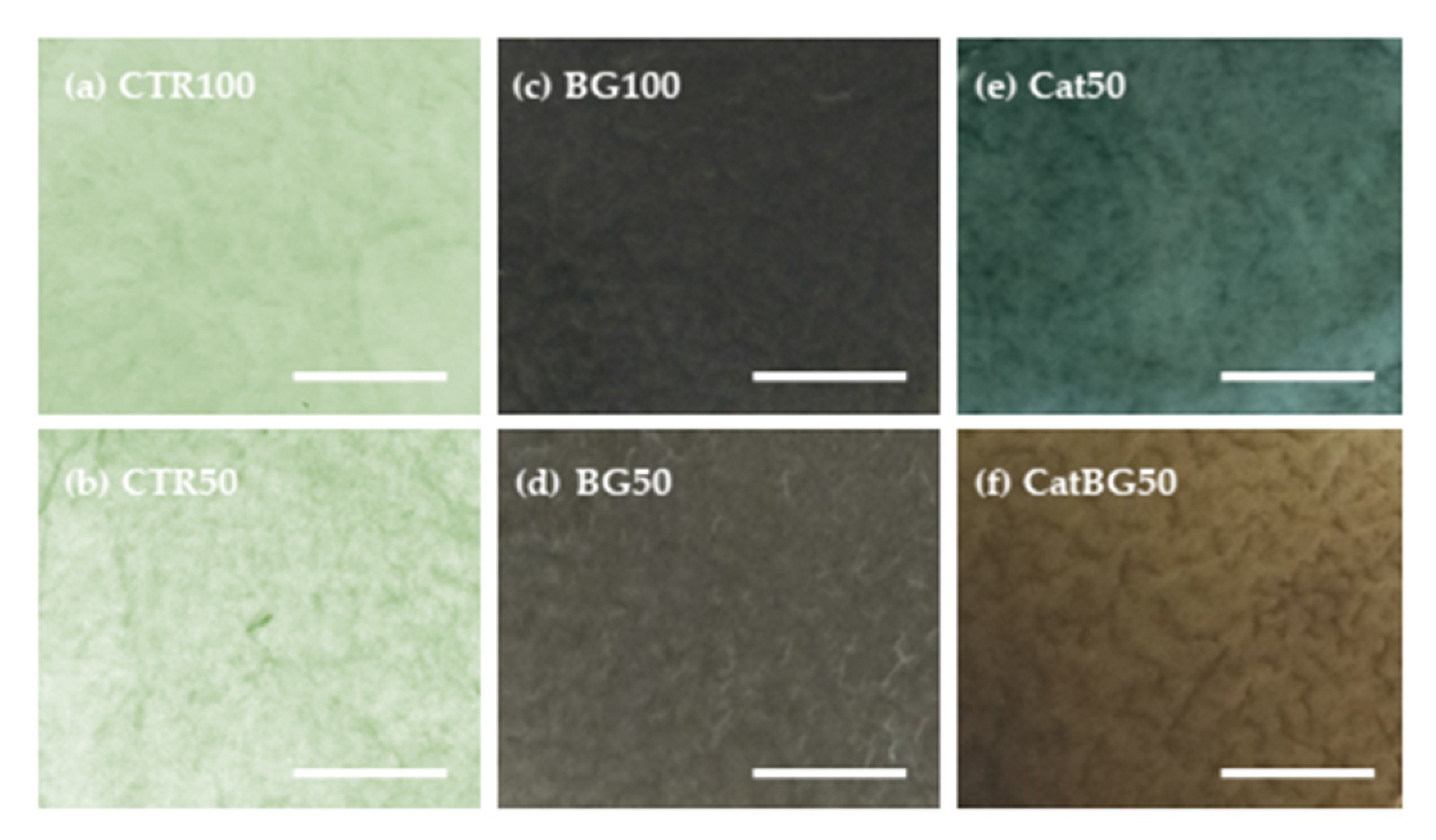
2.5. Films’ Morphological and Topographic Characterization
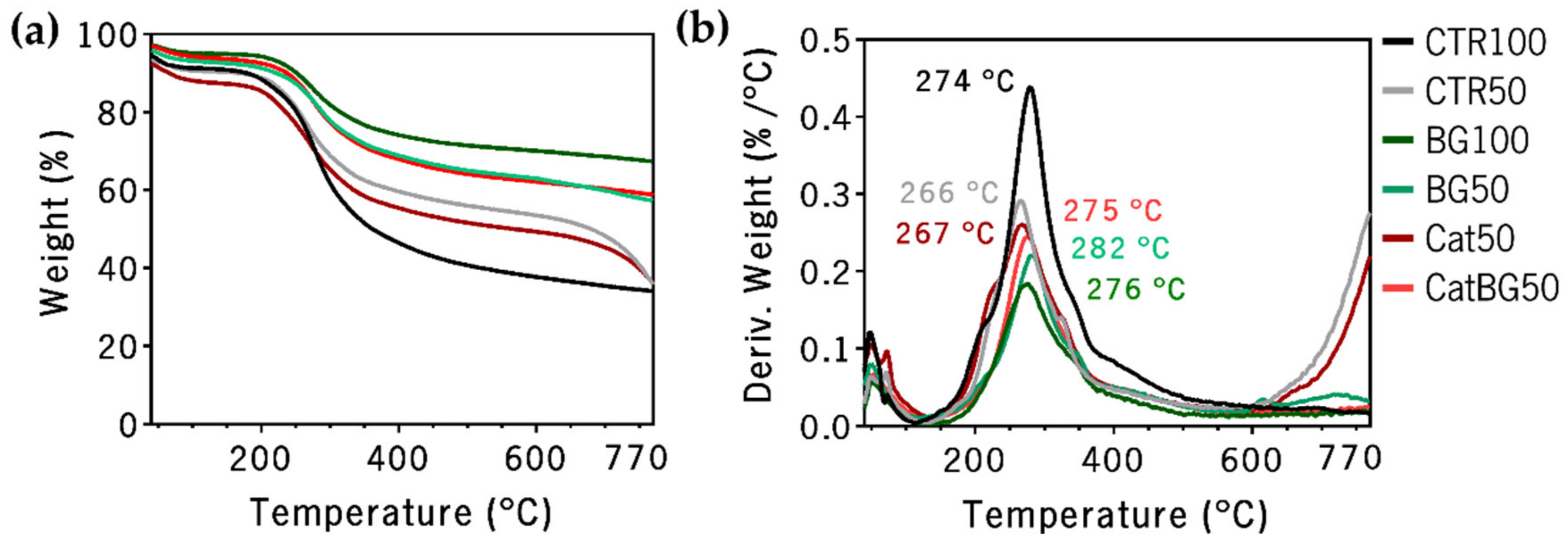
2.6. Thermogravimetric Analysis (TGA)
2.7. Water Contact Angle (WCA) Measurements
2.8. Swelling Behavior and Degradation Studies
2.9. Dynamic Mechanical Analysis (DMA)
2.10. Adhesive Properties
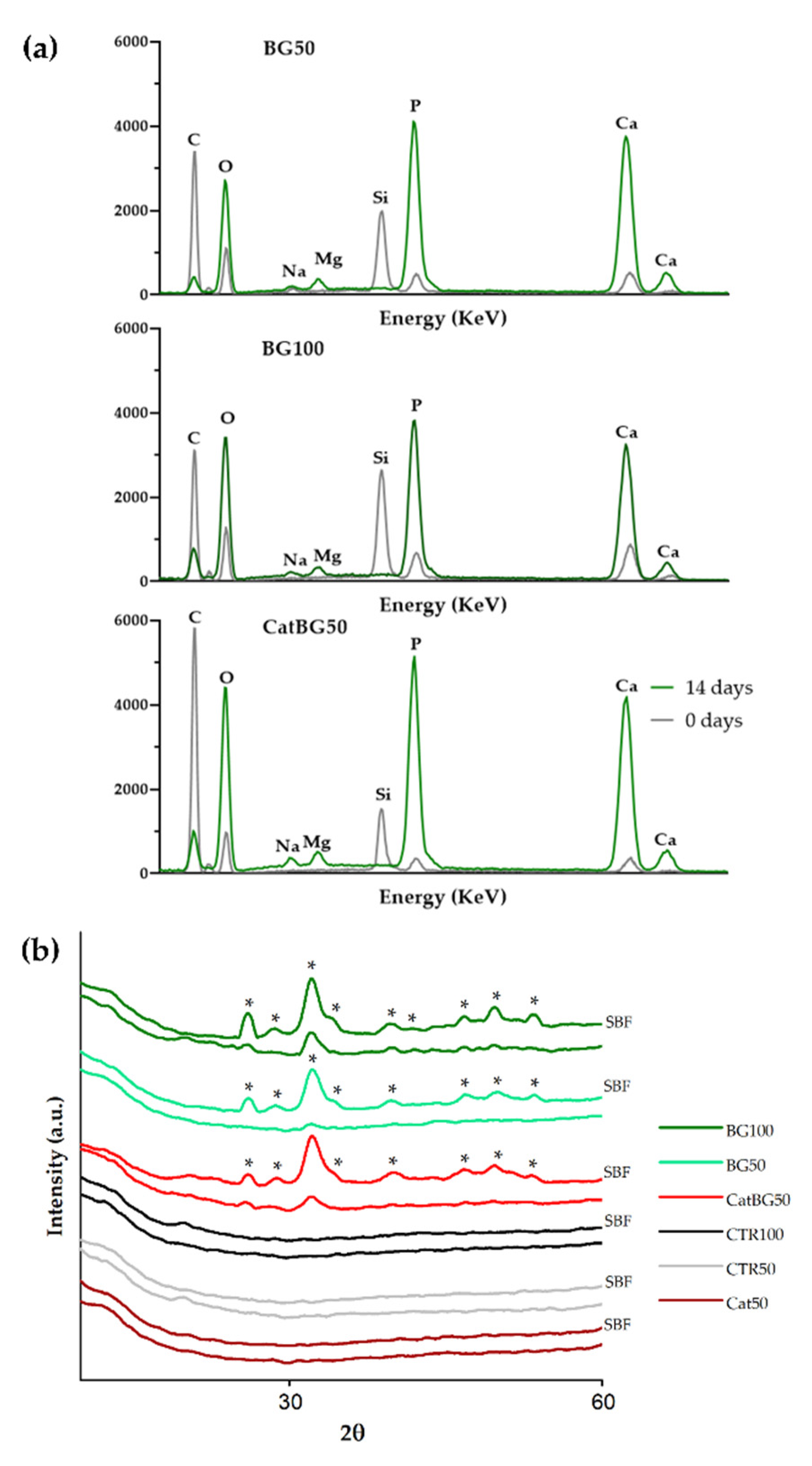
2.11. In Vitro Bioactivity Studies
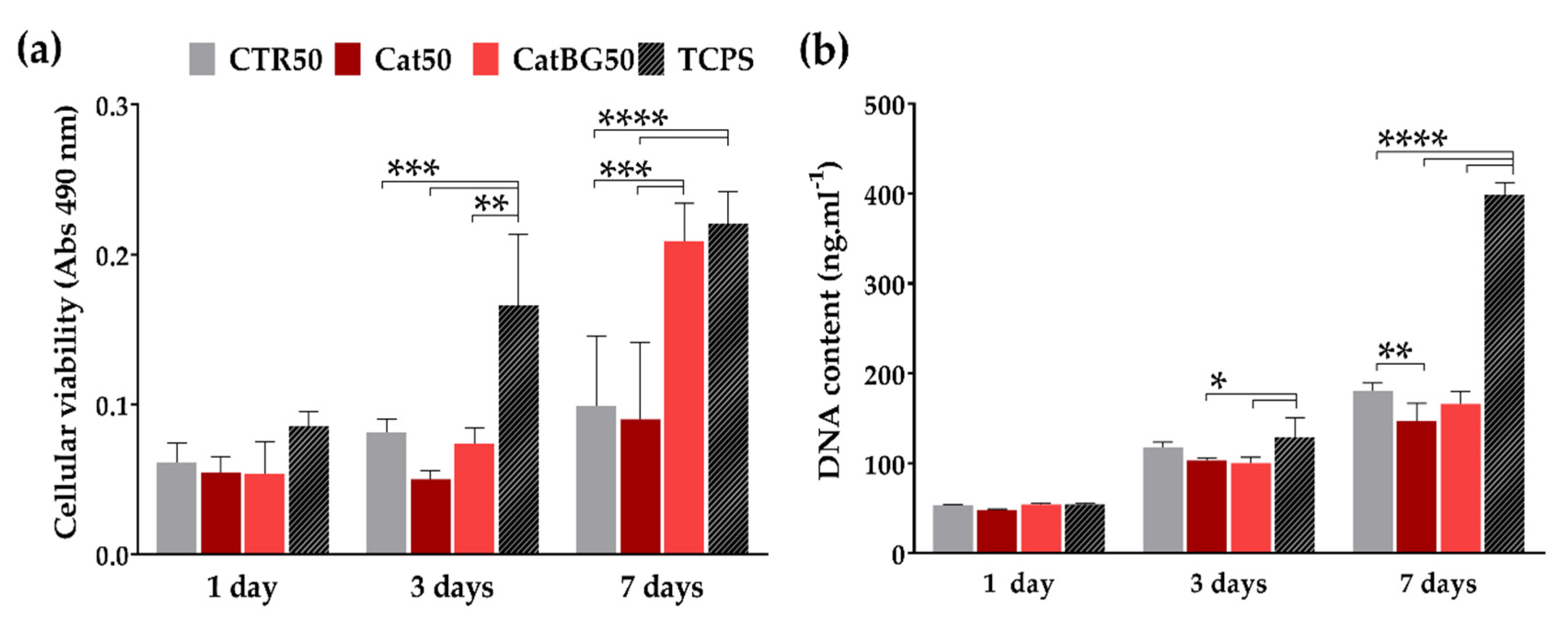
2.12. Cellular Assays
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Catechol-Functionalized Chitosan
4.3. Synthesis of Catechol-Functionalized Hyaluronic Acid
4.4. Production of Ternary Bioglass Nanoparticles
4.5. Freestanding LbL Films through Spin-Coating
4.6. Determination of the Catechol-Degree of Substitution
4.7. Zeta Potential
4.8. Morphological, Topographical, and Elemental Characterization of the Films and of the BGNPs
4.9. Atomic Force Microscopy
4.10. Thermogravimetric Analysis
4.11. Swelling Behavior
4.12. Weight Loss
4.13. WCA Measurements
4.14. Dynamic Mechanical Analysis
4.15. In Vitro Bioactivity
4.16. Cellular Assays
4.16.1. Cell Seeding
4.16.2. MTS Assay
4.16.3. DNA Quantification
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Hydrogens | Habc(C) | H1(D) | H1(A) | H2-6(A) + H3-6(D) | H2(A) | Ac |
|---|---|---|---|---|---|---|
| δ/ppm | 6.8–6.7 | 4.9 | 4.7 | 3.5–4.1 | 3.3 | 2.1 |







References
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017, 54, 21–34. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cheng, Q.; Tang, Z. Layered nanocomposites inspired by the structure and mechanical properties of nacre. Chem. Soc. Rev. 2012, 41, 1111–1129. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. Rsc Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Kalpana, S.; Katti, D.R.; Mohanty, B. Biomimetic Lessons Learnt from Nacre. In Biomimetics Learning from Nature; IntechOpen: Rijeka, Croatia, 2010. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Haemers, S.; Koper, G.J.M.; Frens, G. Effect of Oxidation Rate on Cross-Linking of Mussel Adhesive Proteins. Biomacromolecules 2003, 4, 632–640. [Google Scholar] [CrossRef]
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [Green Version]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Dubas, S.T.; Farhat, T.R.; Schlenoff, J.B. Multiple Membranes from “True” Polyelectrolyte Multilayers. J. Am. Chem. Soc. 2001, 123, 5368–5369. [Google Scholar] [CrossRef]
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000, 21, 319–348. [Google Scholar] [CrossRef]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef]
- Lvov, Y.; Onda, M.; Ariga, K.; Kunitake, T. Ultrathin films of charged polysaccharides assembled alternately with linear polyions. J. Biomater. Sci. Polym. Ed. 1998, 9, 345–355. [Google Scholar] [CrossRef]
- Schneider, A.; Vodouhê, C.; Richert, L.; Francius, G.; Le Guen, E.; Schaaf, P.; Voegel, J.-C.; Frisch, B.; Picart, C. Multifunctional Polyelectrolyte Multilayer Films: Combining Mechanical Resistance, Biodegradability, and Bioactivity. Biomacromolecules 2007, 8, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef] [Green Version]
- Mitzi, D.B.; Kosbar, L.L.; Murray, C.E.; Copel, M.; Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 2004, 428, 299–303. [Google Scholar] [CrossRef]
- Jiang, C.; Markutsya, S.; Tsukruk, V.V. Compliant, Robust, and Truly Nanoscale Free-Standing Multilayer Films Fabricated Using Spin-Assisted Layer-by-Layer Assembly. Adv. Mater. 2004, 16, 157–161. [Google Scholar] [CrossRef]
- Tyona, M.D. A theoritical study on spin coating technique. Adv. Mater. Res. 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, P.A.; Johal, M.S.; Holmes, D.J.; Casson, J.L.; Robinson, J.M.; Wang, H.-L. Polyelectrolyte Spin-Assembly. Langmuir Acs J. Surf. Colloids 2002, 18, 168–173. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Xu, M.; Sun, Q.; Su, J.; Wang, J.; Xu, C.; Zhang, T.; Sun, Q. Microbial transformation of geniposide in Gardenia jasminoides Ellis into genipin by Penicillium nigricans. Enzym. Microb. Technol. 2008, 42, 440–444. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, J.; Deming, T.J. Role of l-3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Rodrigues, J.R.; Alves, N.M.; Mano, J.F. Biomimetic polysaccharide/bioactive glass nanoparticles multilayer membranes for guided tissue regeneration. Rsc Adv. 2016, 6, 75988–75999. [Google Scholar] [CrossRef] [Green Version]
- Couto, D.S.; Alves, N.M.; Mano, J.F. Nanostructured multilayer coatings combining chitosan with bioactive glass nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 1741–1748. [Google Scholar] [CrossRef]
- Rego, S.J.; Vale, A.C.; Luz, G.M.; Mano, J.F.; Alves, N.M. Adhesive Bioactive Coatings Inspired by Sea Life. Langmuir Acs J. Surf. Colloids 2016, 32, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.I.; Cibrao, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.L.; Vale, A.C.; Sousa, M.P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial bioadhesive layer-by-layer coatings for orthopedic applications. J. Mater. Chem. B 2016, 4, 5385–5393. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.P.; Mano, J.F. Cell-Adhesive Bioinspired and Catechol-Based Multilayer Freestanding Membranes for Bone Tissue Engineering. Biomimetics 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired multilayer membranes as potential adhesive patches for skin wound healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhang, J.; Zhong, Y.; Chu, M.; Chang, W.; Yao, Z. Mussel-inspired bio-compatible free-standing adhesive films assembled layer-by-layer with water-resistance. Rsc Adv. 2018, 8, 18904–18912. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic Acid Catechol: A Biopolymer Exhibiting a pH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Ghadban, A.; Ahmed, A.S.; Ping, Y.; Ramos, R.; Arfin, N.; Cantaert, B.; Ramanujan, R.V.; Miserez, A. Bioinspired pH and magnetic responsive catechol-functionalized chitosan hydrogels with tunable elastic properties. Chem. Commun. 2016, 52, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Lin, L.; Dalsin, J.L.; Messersmith, P.B. Biomimetic Anchor for Surface-Initiated Polymerization from Metal Substrates. J. Am. Chem. Soc. 2005, 127, 15843–15847. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, Calcium-Free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Joo, H.; Byun, E.; Lee, M.; Hong, Y.; Lee, H.; Kim, P. Biofunctionalization via flow shear stress resistant adhesive polysaccharide, hyaluronic acid-catechol, for enhanced in vitro endothelialization. J. Ind. Eng. Chem. 2016, 34, 14–20. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/Pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release Off. J. Control. Release Soc. 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Min, Y.; Hammond, P.T. Catechol-Modified Polyions in Layer-by-Layer Assembly to Enhance Stability and Sustain Release of Biomolecules: A Bioinspired Approach. Chem. Mater. 2011, 23, 5349–5357. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Golniya, Z.; Daniels, A.U. Bioactive glass nanoparticles with negative zeta potential. Ceram. Int. 2011, 37, 2311–2316. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol-gel for biomedical applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef]
- Olmo, N.; Martin, A.I.; Salinas, A.J.; Turnay, J.; Vallet-Regi, M.; Lizarbe, M.A. Bioactive sol-gel glasses with and without a hydroxycarbonate apatite layer as substrates for osteoblast cell adhesion and proliferation. Biomaterials 2003, 24, 3383–3393. [Google Scholar] [CrossRef]
- Prabhu, M.; Ruby Priscilla, S.; Kavitha, K.; Manivasakan, P.; Rajendran, V.; Kulandaivelu, P. In Vitro Bioactivity and Antimicrobial Tuning of Bioactive Glass Nanoparticles Added with Neem (Azadirachta indica) Leaf Powder. Biomed Res. Int. 2014, 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Taccola, S.; Desii, A.; Pensabene, V.; Fujie, T.; Saito, A.; Takeoka, S.; Dario, P.; Menciassi, A.; Mattoli, V. Free-Standing Poly(l-lactic acid) Nanofilms Loaded with Superparamagnetic Nanoparticles. Langmuir Acs J. Surf. Colloids 2011, 27, 5589–5595. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridade, S.G.; Oliveira, N.M.; Reis, R.L.; Mano, J.F. Chitosan–alginate multilayered films with gradients of physicochemical cues. J. Mater. Chem. B 2015, 3, 4555–4568. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-Dependent Thickness Behavior of Sequentially Adsorbed Layers of Weak Polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, L.; Zhang, M.; Chen, X.; Liu, M.; Fan, J.; Liu, J.; Zhou, F.; Wang, Z. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017, 8, 2218. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of Surface Polarity on Water Contact Angle and Interfacial Hydration Structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef]
- Zhu, W.; Peck, Y.; Iqbal, J.; Wang, D.A. A novel DOPA-albumin based tissue adhesive for internal medical applications. Biomaterials 2017, 147, 99–115. [Google Scholar] [CrossRef]
- Secrist, K.E.; Nolte, A.J. Humidity Swelling/Deswelling Hysteresis in a Polyelectrolyte Multilayer Film. Macromolecules 2011, 44, 2859–2865. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Swelling and Smoothing of Polyelectrolyte Multilayers by Salt. Langmuir Acs J. Surf. Colloids 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- Miller, M.D.; Bruening, M.L. Correlation of the Swelling and Permeability of Polyelectrolyte Multilayer Films. Chem. Mater. 2005, 17, 5375–5381. [Google Scholar] [CrossRef]
- Almodóvar, J.; Place, L.W.; Gogolski, J.; Erickson, K.; Kipper, M.J. Layer-by-Layer Assembly of Polysaccharide-Based Polyelectrolyte Multilayers: A Spectroscopic Study of Hydrophilicity, Composition, and Ion Pairing. Biomacromolecules 2011, 12, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Hankiewicz, J.; Swierczek, E. Lysozyme in human body fluids. Clin. Chim. Acta 1974, 57, 205–209. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene Oxide as a Matrix for Enzyme Immobilization. Langmuir Acs J. Surf. Colloids 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Vale, A.C.; Pereira, P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial free-standing polysaccharide composite films inspired by the sea. Int. J. Biol. Macromol. 2019, 133, 933–944. [Google Scholar] [CrossRef]
- Ankerfors, C.; Pettersson, T.; Wågberg, L. AFM adhesion imaging for the comparison of polyelectrolyte complexes and polyelectrolyte multilayers. Soft Matter 2012, 8, 8298–8301. [Google Scholar] [CrossRef]
- Schaefer, D.M.; Gomez, J. Atomic Force Microscope Techniques for Adhesion Measurements. J. Adhes. 2000, 74, 341–359. [Google Scholar] [CrossRef]
- Notley, S.M.; Eriksson, M.; Wågberg, L. Visco-elastic and adhesive properties of adsorbed polyelectrolyte multilayers determined in situ with QCM-D and AFM measurements. J. Colloid Interface Sci. 2005, 292, 29–37. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition Mechanisms in Layer-by-Layer or Step-by-Step Deposition Methods: From Elastic and Impermeable Films to Soft Membranes with Ion Exchange Properties. Isrn Mater. Sci. 2012, 2012, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, J.; Dong, X.; Zhang, L.; Zeng, H. A mesoporous bioactive glass/polycaprolactone composite scaffold and its bioactivity behavior. J. Biomed. Mater. Res. Part A 2008, 84A, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ouyang, W.; Lawuyi, B.; Martoni, C.; Prakash, S. Reaction of chitosan with genipin and its fluorogenic attributes for potential microcapsule membrane characterization. J. Biomed. Mater. Res. Part A 2005, 75, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hillberg, A.L.; Holmes, C.A.; Tabrizian, M. Effect of genipin cross-linking on the cellular adhesion properties of layer-by-layer assembled polyelectrolyte films. Biomaterials 2009, 30, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.-H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol-gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Jones, D.S. Dynamic mechanical analysis of polymeric systems of pharmaceutical and biomedical significance. Int. J. Pharm. 1999, 179, 167–178. [Google Scholar] [CrossRef]
- Fujie, T.; Kinoshita, M.; Shono, S.; Saito, A.; Okamura, Y.; Saitoh, D.; Takeoka, S. Sealing effect of a polysaccharide nanosheet for murine cecal puncture. Surgery 2010, 148, 48–58. [Google Scholar] [CrossRef]
- Fujie, T.; Matsutani, N.; Kinoshita, M.; Okamura, Y.; Saito, A.; Takeoka, S. Adhesive, Flexible, and Robust Polysaccharide Nanosheets Integrated for Tissue-Defect Repair. Adv. Funct. Mater. 2009, 19, 2560–2568. [Google Scholar] [CrossRef]
- Fujie, T.; Okamura, Y.; Takeoka, S. Ubiquitous Transference of a Free-Standing Polysaccharide Nanosheet with the Development of a Nano-Adhesive Plaster. Adv. Mater. 2007, 19, 3549–3553. [Google Scholar] [CrossRef]
- Guo, Y.-B.; Wang, D.-G.; Zhang, S.-W. Adhesion and friction of nanoparticles/polyelectrolyte multilayer films by AFM and micro-tribometer. Tribol. Int. 2011, 44, 906–915. [Google Scholar] [CrossRef]
- Morita, M.; Ohmi, T.; Hasegawa, E.; Kawakami, M.; Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 1990, 68, 1272–1281. [Google Scholar] [CrossRef]
- Picart, C.; Elkaim, R.; Richert, L.; Audoin, F.; Arntz, Y.; Da Silva Cardoso, M.; Schaaf, P.; Voegel, J.-C.; Frisch, B. Primary Cell Adhesion on RGD-Functionalized and Covalently Crosslinked Thin Polyelectrolyte Multilayer Films. Adv. Funct. Mater. 2005, 15, 83–94. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |









| Polyelectrolyte | CHI | CHIcat | HA | HAcat | BGNPs |
|---|---|---|---|---|---|
| ζ (mV) | 23.37 ± 2.22 | 19.47 ± 1.80 | −20.43 ± 1.65 | −18.40 ± 1.80 | −20.50 ± 0.76 |
| Formulations | Spin Coating Parameters | ||
|---|---|---|---|
| CTR100 | (CHI-HA-CHI-HA)*100-(CHI-HA) | Deposit amount | 1.5 mL * |
| CTR50 | (CHI-HA-CHI-HA)*50-(CHI-HA) | Spin speed | 2000 rpm |
| BG100 | (CHI-HA-CHI-BGNPs)*100-(CHI-HA) | Spin acceleration | 1300 m s−1 |
| BG50 | (CHI-HA-CHI-BGNPs)*50-(CHI-HA) | Spinning time | 10 s |
| Cat50 | (CHIcat-HAcat-CHIcat-HAcat)*50-(CHIcat-HAcat) | Washing spin time | 5 s |
| CatBG50 | (CHIcat-HAcat-CHIcat-BGNPs)*50-(CHIcat-HAcat) | Contact time | 5–7 s |
| Formulations | Thickness (µm) | |
|---|---|---|
| Crosslinked | Uncrosslinked | |
| CTR100 | 5.98 ± 0.06 | 6.15 ± 0.10 |
| CTR50 | 5.12 ± 0.05 | - |
| BG100 | 53.26 ± 5.53 | 48.56 ± 2.01 |
| BG50 | 40.68 ± 4.68 | - |
| Cat50 | 7.58 ± 0.12 | 3.35 ± 0.21 |
| CatBG50 | 13.06 ± 0.57 | 10.5 ± 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.; Vale, A.C.; A. Pires, R.; Botelho, G.; Reis, R.L.; Alves, N.M. Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties. Molecules 2020, 25, 840. https://doi.org/10.3390/molecules25040840
Moreira J, Vale AC, A. Pires R, Botelho G, Reis RL, Alves NM. Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties. Molecules. 2020; 25(4):840. https://doi.org/10.3390/molecules25040840
Chicago/Turabian StyleMoreira, Joana, Ana C. Vale, Ricardo A. Pires, Gabriela Botelho, Rui L. Reis, and Natália M. Alves. 2020. "Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties" Molecules 25, no. 4: 840. https://doi.org/10.3390/molecules25040840







