Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017
Abstract
:1. Introduction
2. Sponges and Derived Microbes’ Chemical Diversity
2.1. Class Calcarea
2.1.1. Order Baerida
Family Baeriidae
2.1.2. Order Clathrinida
Family Clathrinidae
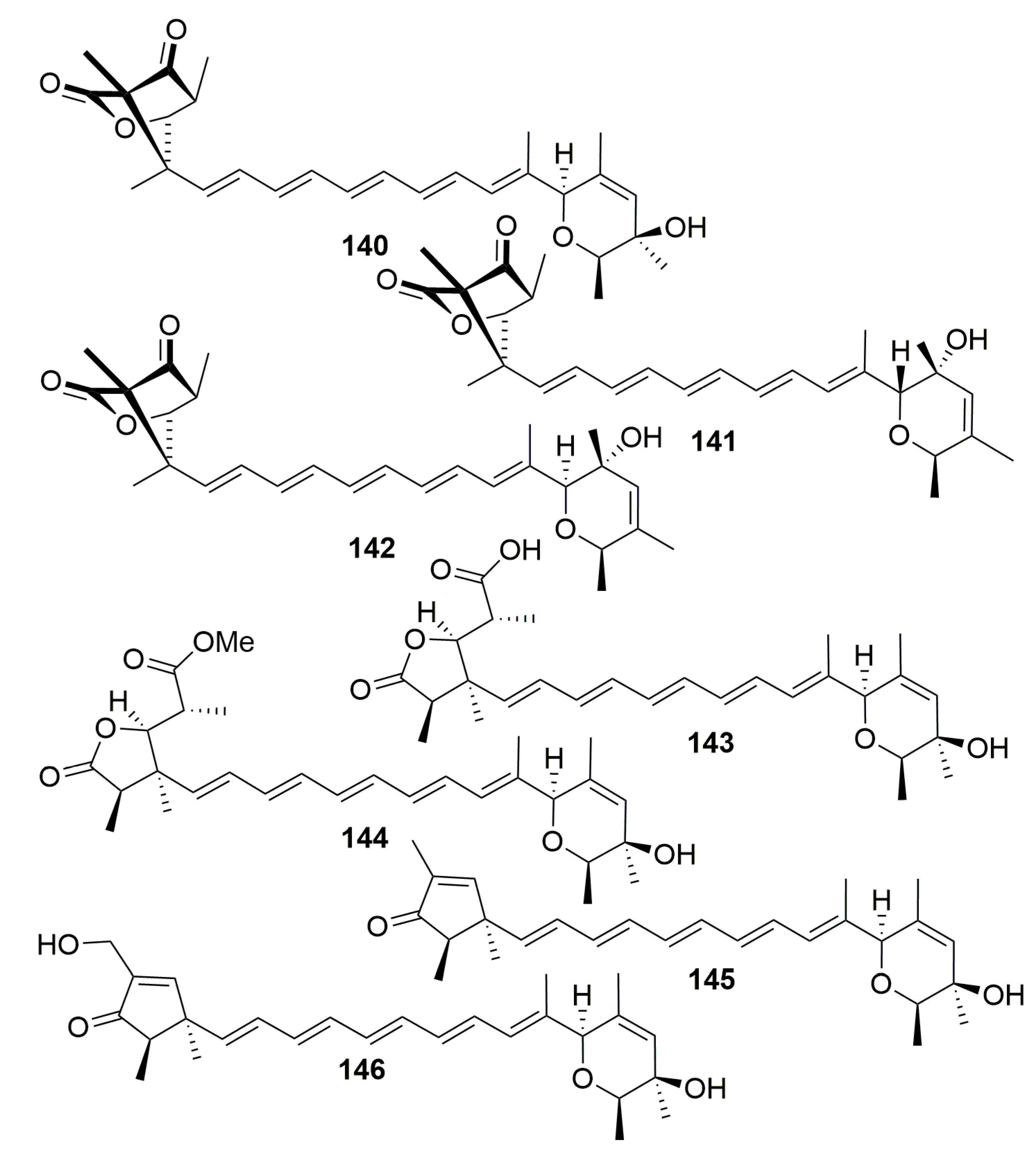
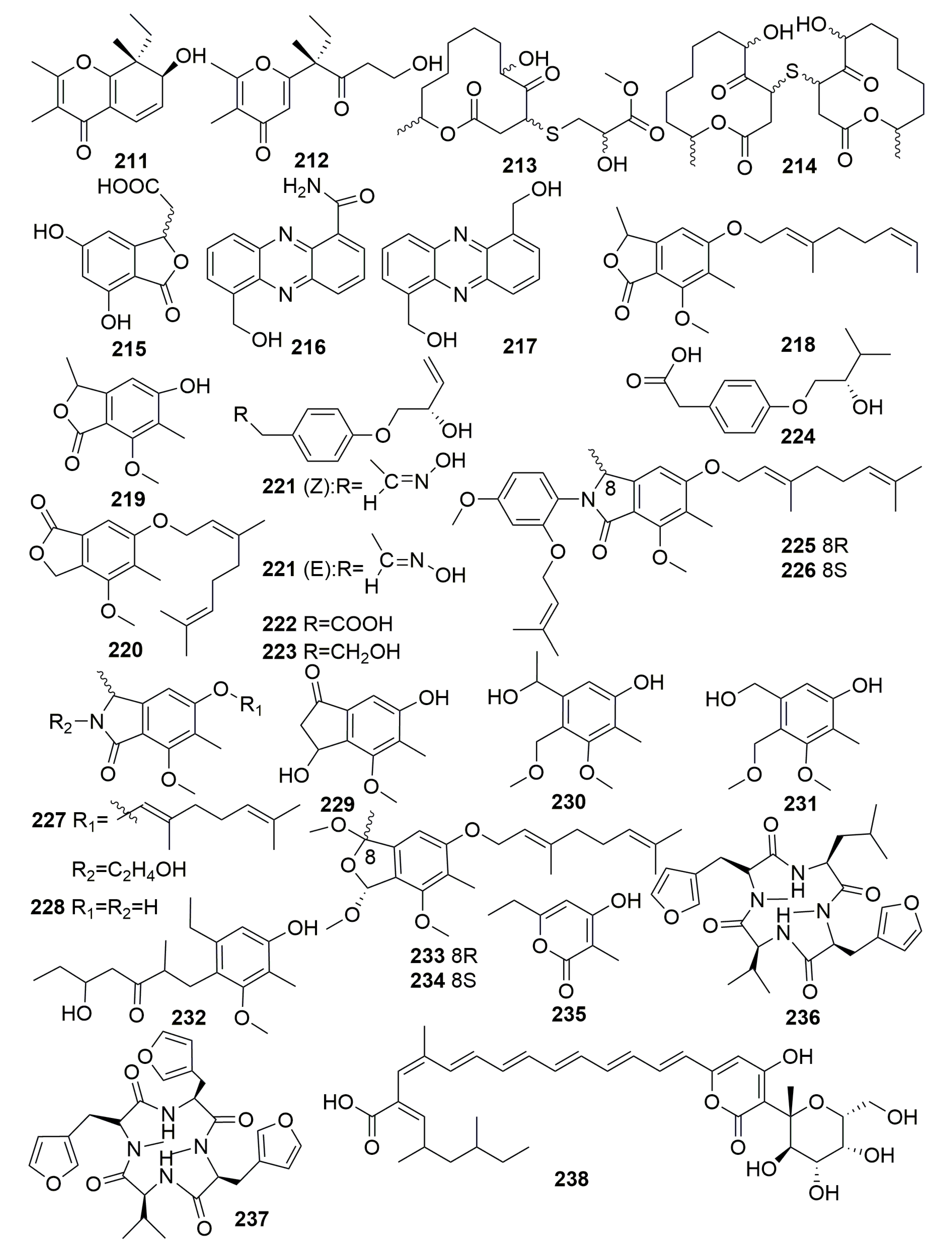
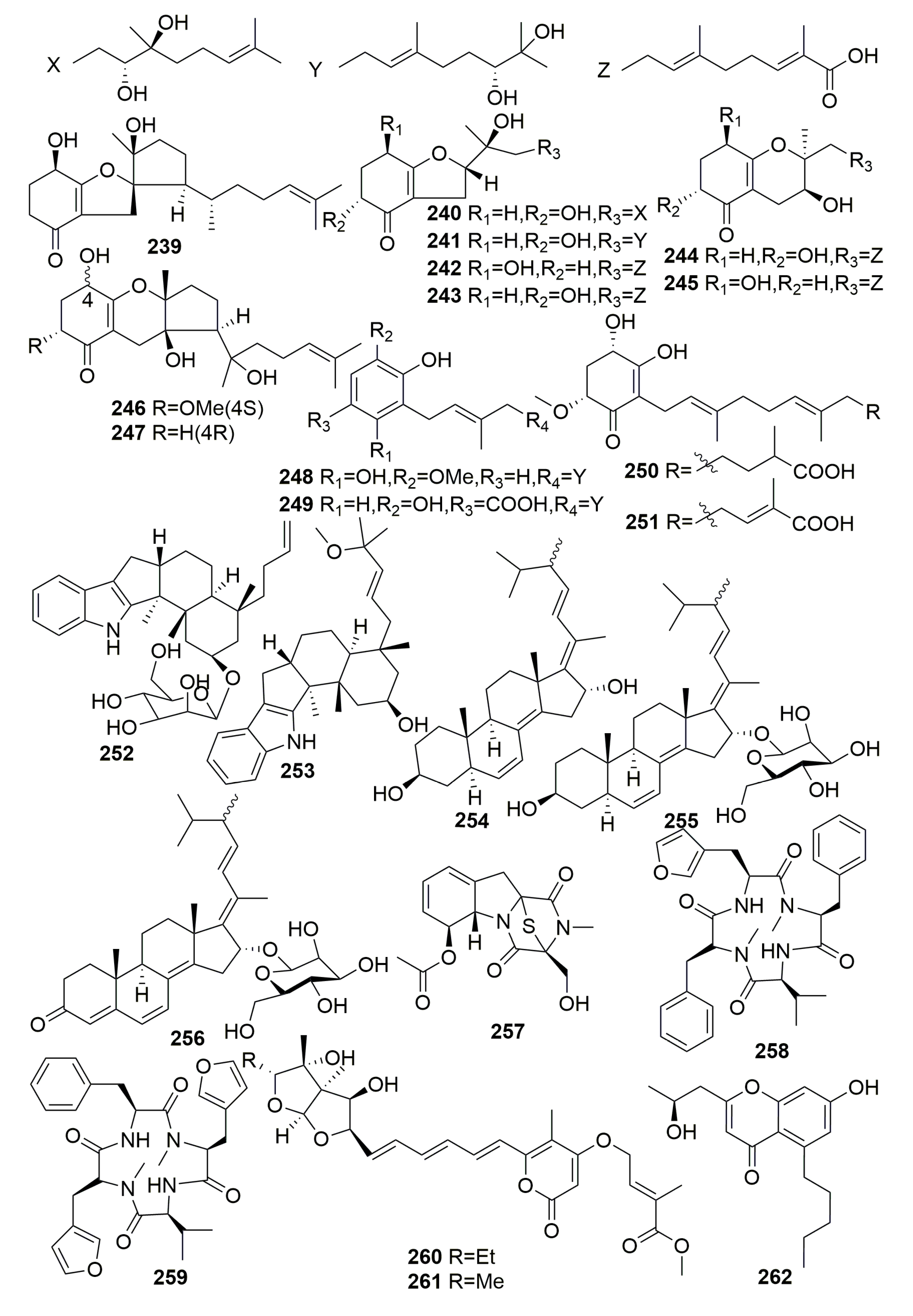
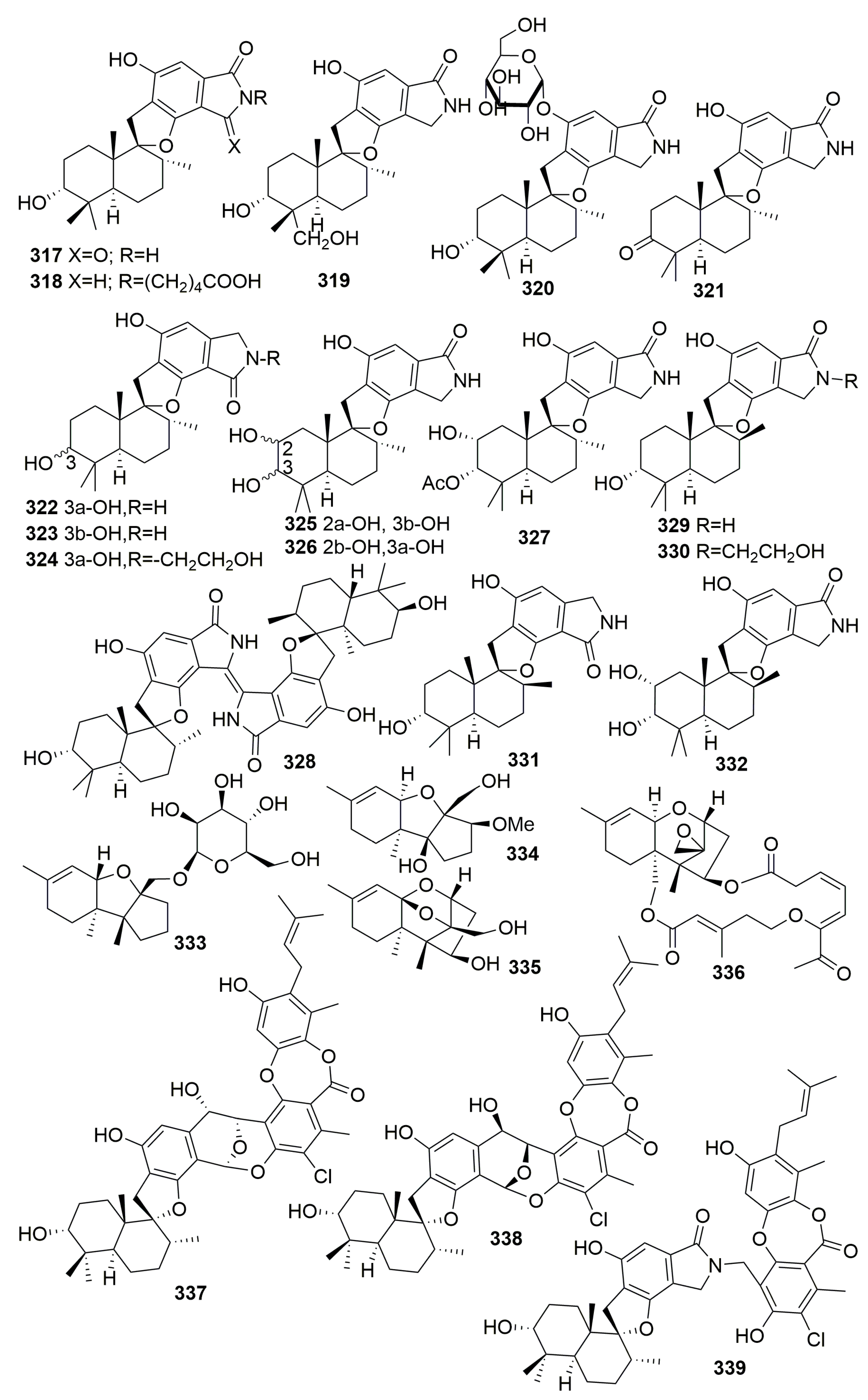
2.2. Class Demospongiae
2.2.1. Order Agelasida
Family Agelasidae
2.2.2. Order Axinellida
Family Axinellidae
Family Raspailiidae
2.2.3. Order Biemnida
Family Rhabderemiidae
2.2.4. Order Chondrillida
Family Chondrillidae
2.2.5. Order Chondrosiida
Family Chondrosiidae
2.2.6. Order Clionaida
Family Clionaidae
2.2.7. Order Dictyoceratida
Family Dysideidae
Family Irciniidae
Family Thorectidae
2.2.8. Order Haplosclerida
Family Callyspongiidae
Family Chalinidae
Family Niphatidae
Family Petrosiidae
2.2.9. Order Poecilosclerida
Family Acarnidae
Family Microcionidae
Family Mycalidae
Family Myxillidae
2.2.10. Order Scopalinida
Family Scopalinidae
2.2.11. Order Sphaerocladina
2.2.12. Order Suberitida
Family Halichondriidae
Family Suberitidae
2.2.13. Order Tethyida
Family Tethyidae
2.2.14. Order Tetractinellida
Family Ancorinidae
Family Geodiidae
Family Neopeltidae
Family Tetillidae
Family Theonellidae
2.2.15. Order Verongiida
Family Aplysinidae
Family Ianthellidae
Family Pseudoceratinidae
2.3. Unidentified
3. Statistical Analysis of Sponge-Derived Microorganisms
3.1. Geographical Distribution of Sponge-Derived Microorganisms
3.2. Biodiversity of Microbial-Associated Sponge Hosts
3.3. Biodiversity of Sponge-Derived Microorganisms
3.4. Chemical Diversity and Bioactive Diversity of Sponge-Derived Microorganisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Douglas, A.E. Symbiotic Interactions; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Li, Z. Advances in marine microbial symbionts in the China Sea and related pharmaceutical metabolites. Mar. Drugs 2009, 7, 113–129. [Google Scholar] [CrossRef]
- Wang, G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J. Ind. Microbiol. Biotechnol. 2006, 33, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Ebel, R.; Edrada, R.A.; Schupp, P.; Lin, W.H.; Wray, V.; Steube, K. Detection of pharmacologically active natural products using ecology. Selected examples from Indopacific marine invertebrates and sponge-derived fungi. Pure Appl. Chem. 2003, 75, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Indraningrat, A.A.; Smidt, H.; Sipkema, D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Mar. Drugs 2016, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Wang, H. Cytotoxic Natural Products from Marine Sponge-Derived Microorganisms. Mar. Drugs 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [Green Version]
- Hardoim, C.C.; Costa, R. Microbial communities and bioactive compounds in marine sponges of the family irciniidae—A review. Mar. Drugs 2014, 12, 5089–5122. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Faheem, M.; Azhar, E.I.; Yasir, M.; Alvi, S.A.; Kamal, M.A.; Ullah, I.; Naseer, M.I. Bacteria from Marine Sponges: A Source of New Drugs. Curr. Drug Metab. 2017, 18, 11–15. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H., II; Singleton, F.L. A marine Micrococcus produces metabolites ascribed to the spongetedania ignis. Experientia 1988, 44, 1021. [Google Scholar] [CrossRef]
- Takagi, M.; Motohashi, K.; Shin-ya, K. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, from the sponge-derived Aspergillus sp. fS14. J. Antibiot. 2010, 63, 393–395. [Google Scholar] [CrossRef] [Green Version]
- Quévrain, E.; Domart-Coulon, I.; Pernice, M.; Bourguet-Kondracki, M.-L. Novel natural parabens produced by a Microbulbifer bacterium in its calcareous sponge host Leuconia nivea. Environ. Microb. 2009, 11, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.C.; De la Fuente Blanco, J.A.; Baz, J.P.; Puentes, J.L.F.; Millan, F.R.; Vazquez, F.E.; Fernandez-Chimeno, R.I.; Gravalos, D.G. 4′-N-methyl-5′-hydroxystaurosporine and 5’-hydroxystaurosporine, new indolocarbazole alkaloids from a marine Micromonospora sp. strain. J. Antibiot. 2000, 53, 895–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumikawa, M.; Khan, S.T.; Komaki, H.; Nagai, A.; Inaba, S.; Takagi, M.; Shin-Ya, K. JBIR-37 and -38, novel glycosyl benzenediols, isolated from the sponge-derived fungus, Acremonium sp. SpF080624G1f01. Biosci. Biotechnol. Biochem. 2009, 73, 2138–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, J.Y.; Hashimoto, J.; Inaba, S.; Takagi, M.; Shin-Ya, K. JBIR-59, a new sorbicillinoid, from a marine-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2010, 63, 203–205. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Takagi, M.; Shin-ya, K. JBIR-124: A novel antioxidative agent from a marine sponge-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2012, 65, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.-Y.; Khan, S.T.; Takagi, M.; Shin-ya, K. JBIR-58, a new salicylamide derivative, isolated from a marine sponge-derived Streptomyces sp. SpD081030ME-02. J. Antibiot. 2010, 63, 267–269. [Google Scholar] [CrossRef]
- Motohashi, K.; Inaba, K.; Fuse, S.; Doi, T.; Izumikawa, M.; Khan, S.T.; Takagi, M.; Takahashi, T.; Shin-ya, K. JBIR-56 and JBIR-57, 2(1H)-Pyrazinones from a Marine Sponge-Derived Streptomyces sp. SpD081030SC-03. J. Nat. Prod. 2011, 74, 1630–1635. [Google Scholar] [CrossRef]
- Neumann, K.; Abdel-Lateff, A.; Wright, A.D.; Kehraus, S.; Krick, A.; Koenig, G.M. Novel sorbicillin derivativs with an unprecedented carbon skeleton from the sponge-derived fungus Trichoderma species. Eur. J. Org. Chem. 2007, 24, 4125. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.; Wright, A.D. Trichopyrone and other constituents from the marine sponge-derived fungus Trichoderma sp. Z. Naturforsch. C J. Biosci. 2009, 64, 186–192. [Google Scholar] [CrossRef]
- Ma, X.; Peng, J.; Wu, G.; Zhu, T.; Li, G.; Gu, Q.; Li, D. Speradines B-D, oxygenated cyclopiazonic acid alkaloids from the sponge-derived fungus Aspergillus flavus MXH-X104. Tetrahedron 2015, 71, 3522–3527. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E.M.; Fekete, A.; Krischke, M.; Stopper, H.; Edrada-Ebel, R.; Mueller, M.J.; Hentschel, U.; Abdelmohsen, U.R. Strepoxazine A, a new cytotoxic phenoxazin from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 4196–4199. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Othman, E.M.; Reimer, A.; Grüne, M.; Kozjak-Pavlovic, V.; Stopper, H.; Hentschel, U.; Abdelmohsen, U.R. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 2786–2789. [Google Scholar] [CrossRef]
- Panizel, I.; Yarden, O.; Ilan, M.; Carmeli, S. Eight New Peptaibols from Sponge-Associated Trichoderma atroviride. Mar. Drugs 2013, 11, 4937–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amagata, T.; Amagata, A.; Tenney, K.; Valeriote, F.A.; Lobkovsky, E.; Clardy, J.; Crews, P. Unusual C25 steroids produced by a sponge-derived Penicillium citrinum. Org. Lett. 2003, 5, 4393–4396. [Google Scholar] [CrossRef]
- Hiort, J.; Maksimenka, K.; Reichert, M.; Perovic-Ottstadt, S.; Lin, W.H.; Wray, V.; Steube, K.; Schaumann, K.; Weber, H.; Proksch, P.; et al. New natural products from the sponge-derived fungus Aspergillus niger. J. Nat. Prod. 2004, 67, 1532–1543. [Google Scholar] [CrossRef]
- Jadulco, R.; Edrada, R.A.; Ebel, R.; Berg, A.; Schaumann, K.; Wray, V.; Steube, K.; Proksch, P. New communesin derivatives from the fungus Penicillium sp. derived from the Mediterranean sponge Axinella verrucosa. J. Nat. Prod. 2004, 67, 78–81. [Google Scholar] [CrossRef]
- Boot, C.M.; Tenney, K.; Valeriote, F.A.; Crews, P. Highly N-methylated linear peptides produced by an atypical sponge-derived Acremonium sp. J. Nat. Prod. 2006, 69, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Boot, C.M.; Amagata, T.; Tenney, K.; Compton, J.E.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Four classes of structurally unusual peptides from two marine-derived fungi: Structures and bioactivities. Tetrahedron 2007, 63, 9903–9914. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Elardo, S.M.; Buback, V.; Gulder, T.A.; Bugni, T.S.; Reppart, J.; Bringmann, G.; Ireland, C.M.; Schirmeister, T.; Hentschel, U. New tetromycin derivatives with anti-trypanosomal and protease inhibitory activities. Mar. Drugs 2011, 9, 1682–1697. [Google Scholar] [CrossRef]
- Ozkaya, F.C.; Bedir, E.; Hames, E.E. A new siderophore from sponge associated Pseudomonas fluorescens 4.9.3. Rec. Nat. Prod. 2015, 9, 509–517. [Google Scholar]
- Wu, B.; Ohlendorf, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Acetylcholinesterase Inhibitors from a Marine Fungus Talaromyces sp. Strain LF458. Mar. Biotechnol. 2015, 17, 110–119. [Google Scholar] [CrossRef]
- Küppers, L.; Ebrahim, W.; El-Neketi, M.; Özkaya, F.; Mándi, A.; Kurtán, T.; Orfali, R.; Müller, W.; Hartmann, R.; Lin, W.; et al. Lactones from the Sponge-Derived Fungus Talaromyces rugulosus. Mar. Drugs 2017, 15, 359. [Google Scholar] [CrossRef] [Green Version]
- De Castro, M.V.; Ióca, L.P.; Williams, D.E.; Costa, B.Z.; Mizuno, C.M.; Santos, M.F.C.; de Jesus, K.; Ferreira, É.L.F.; Seleghim, M.H.R.; Sette, L.D.; et al. Condensation of Macrocyclic Polyketides Produced by Penicillium sp. DRF2 with Mercaptopyruvate Represents a New Fungal Detoxification Pathway. J. Nat. Prod. 2016, 79, 1668–1678. [Google Scholar] [CrossRef]
- Ding, L.-J.; Gu, B.-B.; Jiao, W.-H.; Yuan, W.; Li, Y.-X.; Tang, W.-Z.; Yu, H.-B.; Liao, X.-J.; Han, B.-N.; Li, Z.-Y.; et al. New Furan and Cyclopentenone Derivatives from the Sponge-Associated Fungus Hypocrea Koningii PF04. Mar. Drugs 2015, 13, 5579–5592. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Z.; Ju, Z.; Wan, J.; Liao, S.; Lin, X.; Zhang, T.; Zhou, X.; Chen, H.; Tu, Z.; et al. Cytotoxic Cytochalasins from Marine-Derived Fungus Arthrinium arundinis. Planta Med. 2015, 81, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wei, X.; Qin, X.; Lin, X.; Zhou, X.; Liao, S.; Yang, B.; Liu, J.; Tu, Z.; Liu, Y. Arthpyrones A-C, pyridone alkaloids from a sponge-derived fungus Arthrinium arundinis ZSDS1-F3. Org. Lett. 2015, 17, 656–659. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; He, W.; Lin, X.; Zhou, X.; Liu, Y. One Strain-Many Compounds Method for Production of Polyketide Metabolites Using the Sponge-Derived Fungus Arthrinium arundinis ZSDS1-F3. Chem. Nat. Compd. 2017, 53, 373–374. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.-P.; Li, L.-C.; Zhong, B.-L.; Liao, X.-J.; Liu, Y.-H.; Xu, S.-H. Gliomasolides A–E, unusual macrolides from a sponge-derived fungus Gliomastix sp. ZSDS1-F7-2. RSC Adv. 2015, 5, 54645–54648. [Google Scholar] [CrossRef]
- Ding, L.-J.; Yuan, W.; Li, Y.-X.; Liao, X.-J.; Sun, H.; Peng, Q.; Han, B.-N.; Lin, H.-W.; Li, Z.-Y.; Yang, F.; et al. Hypocrol A, a new tyrosol derivative from a sponge-derived strain of the fungus Hypocrea koningii. Nat. Prod. Res. 2016, 30, 1633–1638. [Google Scholar] [CrossRef]
- Ding, L.-J.; Yuan, W.; Liao, X.-J.; Han, B.-N.; Wang, S.-P.; Li, Z.-Y.; Xu, S.-H.; Zhang, W.; Lin, H.-W. Oryzamides A–E, Cyclodepsipeptides from the Sponge-Derived Fungus Nigrospora oryzae PF18. J. Nat. Prod. 2016, 79, 2045–2052. [Google Scholar] [CrossRef]
- Gu, B.-B.; Tang, J.; Wang, S.-P.; Sun, F.; Yang, F.; Li, L.; Xu, Y.; Lin, H.-W. Structure, absolute configuration, and variable-temperature 1H-NMR study of (±)-versiorcinols A–C, three racemates of diorcinol monoethers from the sponge-associated fungus Aspergillus versicolor 16F-11. RSC Adv. 2017, 7, 50254–50263. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Dong, K.; Wang, X.; Zhong, J.; Mu, Y.; Liu, Y.; Huang, X. Polyketide derivatives from a marine-sponge-associated fungus Pestalotiopsis heterocornis. Phytochemistry 2017, 142, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Ma, Q.; Zhong, J.; Liu, Y.; Sun, T.; Wang, J.; Huang, X. New Metabolites and Bioactive Chlorinated Benzophenone Derivatives Produced by a Marine-Derived Fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, I.E.; Gross, H.; Pontius, A.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.-H.; Konig, G.M. Epoxyphomalin A and B, prenylated polyketides with potent cytotoxicity from the marine-derived fungus Phoma sp. Org. Lett. 2009, 11, 5014–5017. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Dethoup, T.; Bessa, L.; Pinto, M.; Gales, L.; Costa, P.; Silva, A.; Kijjoa, A. New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar. Drugs 2014, 12, 5160–5173. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Hou, Y.; Braun, D.; Cohen, H.C.; Xiong, M.P.; Bugni, T.S. First Natural Analogs of the Cytotoxic Thiodepsipeptide Thiocoraline A from a Marine Verrucosispora sp. J. Org. Chem. 2011, 76, 6542–6547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.; Kijjoa, A. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef]
- Liu, S.; Dai, H.; Konuklugil, B.; Orfali, R.S.; Lin, W.; Kalscheuer, R.; Liu, Z.; Proksch, P. Phenolic bisabolanes from the sponge-derived fungus Aspergillus sp. Phytochem. Lett. 2016, 18, 187–191. [Google Scholar] [CrossRef]
- Lang, G.; Wiese, J.; Schmaljohann, R.; Imhoff, J.F. New pentaenes from the sponge-derived marine fungus Penicillium rugulosum: Structure determination and biosynthetic studies. Tetrahedron 2007, 63, 11844–11849. [Google Scholar] [CrossRef]
- San-Martin, A.; Rovirosa, J.; Vaca, I.; Vergara, K.; Acevedo, L.; Vina, D.; Orallo, F.; Chamy, C. New butyrolactone from a marine-derived fungus Aspergillus sp. J. Chil. Chem. Soc. 2011, 56, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Eltamany, E.E.; Abdelmohsen, U.R.; Ibrahim, A.K.; Hassanean, H.A.; Hentschel, U.; Ahmed, S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. EG45. Bioorg. Med. Chem. Lett. 2014, 24, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.; Hentschel, U.; Edrada-Ebel, R. Dereplication Strategies for Targeted Isolation of New Antitrypanosomal Actinosporins A and B from a Marine Sponge Associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [Green Version]
- Grkovic, T.; Abdelmohsen, U.R.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Quinn, R.J. Two new antioxidant actinosporin analogues from the calcium alginate beads culture of sponge-associated Actinokineospora sp. strain EG49. Bioorg. Med. Chem. Lett. 2014, 24, 5089–5092. [Google Scholar] [CrossRef]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Actinomycete Metabolome Induction/Suppression with N-Acetylglucosamine. J. Nat. Prod. 2017, 80, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-E.; Julianti, E.; Oh, H.; Park, W.; Oh, D.-C.; Oh, K.-B.; Shin, J. Stereochemistry of hydroxy-bearing benzolactones: Isolation and structural determination of chrysoarticulins A–C from a marine-derived fungus Chrysosporium articulatum. Tetrahedron Lett. 2013, 54, 3111–3115. [Google Scholar] [CrossRef]
- De Rosa, S.; De Giulio, A.; Tommonaro, G.; Popov, S.; Kujumgiev, A. A β-amino acid containing tripeptide from a Pseudomonas-Alteromonas bacterium associated with a Black Sea sponge. J. Nat. Prod. 2000, 63, 1454–1455. [Google Scholar] [CrossRef]
- Yu, L.-L.; Li, Z.-Y.; Peng, C.-S.; Li, Z.-Y.; Guo, Y.-W. Neobacillamide A, a novel thiazole-containing alkaloid from the marine bacterium Bacillus atrophaeus C89, associated with South China Sea sponge Dysidea avara. Helv. Chim. Acta 2009, 92, 607–612. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Zhang, G.; Philippe, A.; Schmitz, W.; Pimentel-Elardo, S.M.; Hertlein-Amslinger, B.; Hentschel, U.; Bringmann, G. Cyclodysidins A-D, cyclic lipopeptides from the marine sponge-derived Streptomyces strain RV15. Tetrahedron Lett. 2012, 53, 23–29. [Google Scholar] [CrossRef]
- Mitova, M.; Tommonaro, G.; De Rosa, S. A novel cyclopeptide from a bacterium associated with the marine sponge Ircinia muscarum. Z. Naturforsch. C J. Biosci. 2003, 58, 740–745. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Mühlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stöhr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Bruhn, T.; Schäffler, K.; Steffens, S.; Schmaljohann, R.; Wiese, J.; Imhoff, J.F. Sorbifuranones A–C, sorbicillinoid metabolites from Penicillium strains isolated from Mediterranean sponges. Tetrahedron 2010, 66, 9894–9901. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Rempel, V.; Schnakenburg, G.; Kehraus, S.; Müller, C.E.; König, G.M. Identification of a Potent and Selective Cannabinoid CB1 Receptor Antagonist from Auxarthron reticulatum. ACS Med. Chem. Lett. 2011, 2, 866–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.; Harms, H.; Loef, I.; Kehraus, S.; El Maddah, F.; Arslan, I.; Rempel, V.; Müller, C.; König, G. GPR18 Inhibiting Amauromine and the Novel Triterpene Glycoside Auxarthonoside from the Sponge-Derived Fungus Auxarthron reticulatum. Planta Med. 2015, 81, 1141–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, L.; Lodin, A.; Herold, I.; Ilan, M.; Carmeli, S.; Yarden, O. Sensitivity of Neurospora crassa to a Marine-Derived Aspergillus tubingensis Anhydride Exhibiting Antifungal Activity That Is Mediated by the MAS1 Protein. Mar. Drugs 2014, 12, 4713–4731. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Aktas, N.; Konuklugil, B.; Mándi, A.; Daletos, G.; Lin, W.; Dai, H.; Kurtán, T.; Proksch, P. A new fusarielin analogue from Penicillium sp. isolated from the Mediterranean sponge Ircinia oros. Tetrahedron Lett. 2015, 56, 5317–5320. [Google Scholar] [CrossRef]
- Cohen, E.; Koch, L.; Thu, K.M.; Rahamim, Y.; Aluma, Y.; Ilan, M.; Yarden, O.; Carmeli, S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the Mediterranean sponge Psammocinia sp. collected along the coast of Israel. Bioorg. Med. Chem. 2011, 19, 6587–6593. [Google Scholar] [CrossRef]
- Elissawy, A.M.; Ebada, S.S.; Ashour, M.L.; Özkaya, F.C.; Ebrahim, W.; Singab, A.B.; Proksch, P. Spiroarthrinols A and B, two novel meroterpenoids isolated from the sponge-derived fungus Arthrinium sp. Phytochem. Lett. 2017, 20, 246–251. [Google Scholar] [CrossRef]
- Wu, B.; Wiese, J.; Wenzel-Storjohann, A.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Engyodontochones, Antibiotic Polyketides from the Marine Fungus Engyodontium album Strain LF069. Chemistry 2016, 22, 7452–7462. [Google Scholar] [CrossRef]
- Speitling, M.; Smetanina, O.F.; Kuznetsova, T.A.; Laatsch, H. Bromoalterochromides A and A’, unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM 636T. J. Antibiot. 2007, 60, 36–42. [Google Scholar] [CrossRef]
- Varoglu, M.; Crews, P. Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. J. Nat. Prod. 2000, 63, 41–43. [Google Scholar] [CrossRef]
- Pinheiro, Â.; Dethoup, T.; Bessa, J.; Silva, A.M.S.; Kijjoa, A. A new bicyclic sesquiterpene from the marine sponge associated fungus Emericellopsis minima. Phytochem. Lett. 2012, 5, 68–70. [Google Scholar] [CrossRef]
- Asiri, I.A.M.; Badr, J.M.; Youssef, D.T.A. Penicillivinacine, antimigratory diketopiperazine alkaloid from the marine-derived fungus Penicillium vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- Li, B.; Huang, Q.-X.; Gao, D.; Liu, D.; Ji, Y.-B.; Liu, H.-G.; Lin, W.-H. New C13lipids from the marine-derived fungus Trichoderma harzianum. J. Asian Nat. Prod. Res. 2015, 17, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.-C.; Xu, M.-Y.; Li, H.-J.; Yuan, J.; Tang, G.; Xu, J.; Yang, D.-P.; Lan, W.-J. Discovery of aromadendrane anologues from the marine-derived fungus Scedosporium dehoogii F41-4 by NMR-guided isolation. RSC Adv. 2016, 6, 94763–94770. [Google Scholar] [CrossRef]
- Cao, Q.-X.; Wei, J.-H.; Deng, R.; Feng, G.-K.; Zhu, X.-F.; Lan, W.-J.; Li, H.-J. Two New Pyripyropenes from the Marine Fungus Fusarium lateritium 2016F18-1. Chem. Biodivers. 2017, 14, e1600298. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Wray, V.; Berg, A.; Grafe, U.; Sudarsono; Brauers, G.; Proksch, P. Novel spiciferone derivatives from the fungus Drechslera hawaiiensis isolated from the marine sponge Callyspongia aerizusa. Z. Naturforsch. C J. Biosci. 2000, 55, 218–221. [Google Scholar] [CrossRef]
- Jadulco, R.; Proksch, P.; Wray, V.; Sudarsono; Berg, A.; Graefe, U. New Macrolides and Furan Carboxylic Acid Derivative from the Sponge-Derived Fungus Cladosporium herbarum. J. Nat. Prod. 2001, 64, 527–530. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Choi, E.J.; Kwon, H.C.; Ham, J.; Yang, H.O. 6-Hydroxymethyl-1-phenazine-carboxamide and 1,6-phenazinedimethanol from a marine bacterium, Brevibacterium sp. KMD 003, associated with marine purple vase sponge. J. Antibiot. 2009, 62, 621–624. [Google Scholar] [CrossRef]
- Almeida, C.; Kehraus, S.; Prudêncio, M.; König, G.M. Marilones A–C, phthalides from the sponge-derived fungus Stachylidium sp. Beilstein J. Org. Chem. 2011, 7, 1636–1642. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.; Part, N.; Bouhired, S.; Kehraus, S.; Koenig, G.M. Stachylines A-D from the sponge-derived fungus Stachylidium sp. J. Nat. Prod. 2011, 74, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, C.; Hemberger, Y.; Schmitt, S.M.; Bouhired, S.; Natesan, L.; Kehraus, S.; Dimas, K.; Gutschow, M.; Bringmann, G.; Konig, G.M. Marilines A-C: Novel phthalimidines from the sponge-derived fungus Stachylidium sp. Chemistry 2012, 18, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Eguereva, E.; Kehraus, S.; Koenig, G.M. Unprecedented polyketides from a marine sponge-associated Stachylidium sp. J. Nat. Prod. 2013, 76, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Maddah, F.E.; Kehraus, S.; Schnakenburg, G.; König, G.M. Endolides A and B, Vasopressin and Serotonin-Receptor Interacting N-Methylated Peptides from the Sponge-Derived Fungus Stachylidium sp. Org. Lett. 2016, 18, 528–531. [Google Scholar] [CrossRef]
- Peng, J.; Jiao, J.; Li, J.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Pyronepolyene C-glucosides with NF-κB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, G.; Zhu, T.; Kurtán, T.; Mándi, A.; Jiao, J.; Li, J.; Qi, X.; Gu, Q.; Li, D. Meroterpenoids with Diverse Ring Systems from the Sponge-Associated Fungus Alternaria sp. JJY-32. J. Nat. Prod. 2013, 76, 1946–1957. [Google Scholar] [CrossRef]
- Harms, H.; Rempel, V.; Kehraus, S.; Kaiser, M.; Hufendiek, P.; Muller, C.E.; Konig, G.M. Indoloditerpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GPR18 and cannabinoid receptors. J. Nat. Prod. 2014, 77, 673–677. [Google Scholar] [CrossRef]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; Koenig, G.M. Aβ-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef]
- Harms, H.; Orlikova, B.; Ji, S.; Nesaei-Mosaferan, D.; Konig, G.M.; Diederich, M. Epipolythiodiketopiperazines from the Marine Derived Fungus Dichotomomyces cejpii with NF-kappaB Inhibitory Potential. Mar. Drugs 2015, 13, 4949–4966. [Google Scholar] [CrossRef] [Green Version]
- El Maddah, F.; Kehraus, S.; Nazir, M.; Almeida, C.; König, G.M. Insights into the Biosynthetic Origin of 3-(3-Furyl) alanine in Stachylidium sp. 293 K04 Tetrapeptides. J. Nat. Prod. 2016, 79, 2838–2845. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Lin, X.-P.; Wang, Z.; Zhou, X.-F.; Qin, X.-C.; Kaliyaperumal, K.; Zhang, T.-Y.; Tu, Z.-C.; Liu, Y. Asteltoxins with antiviral activities from the marine sponge-derived fungus Aspergillus sp. SCSIO XWS02F40. Molecules 2016, 21, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperry, S.; Samuels, G.J.; Crews, P. Vertinoid Polyketides from the Saltwater Culture of the Fungus Trichoderma longibrachiatum Separated from a Haliclona Marine Sponge. J. Org. Chem. 1998, 63, 10011–10014. [Google Scholar] [CrossRef]
- Wang, G.-Y.-S.; Abrell, L.M.; Avelar, A.; Borgeson, B.M.; Crews, P. New hirsutane-based sesquiterpenes from salt water cultures of a marine sponge-derived fungus and the terrestrial fungus Coriolus consors. Tetrahedron 1998, 54, 7335–7342. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Steffens, S.; Günther, E.; Schaumann, K. Evariquinone, isoemericellin, and stromemycin from a sponge derived strain of the fungus Emericella variecolor. Phytochemistry 2003, 63, 437–443. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Gulder, T.A.M.; Hentschel, U.; Bringmann, G. Cebulactams A1 and A2, new macrolactams isolated from Saccharopolyspora cebuensis, the first obligate marine strain of the genus Saccharopolyspora. Tetrahedron Lett. 2008, 49, 6889–6892. [Google Scholar] [CrossRef]
- Izumikawa, M.; Khan, S.T.; Komaki, H.; Takagi, M.; Shin-ya, K. JBIR-31, a new teleocidin analog, produced by salt-requiring Streptomyces sp. NBRC 105896 isolated from a marine sponge. J. Antibiot. 2010, 63, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, K.; Takagi, M.; Shin-ya, K. Tetrapeptides possessing a unique skeleton, JBIR-34 and JBIR-35, isolated from a sponge-derived actinomycete, Streptomyces sp. Sp080513GE-23. J. Nat. Prod. 2010, 73, 226–228. [Google Scholar] [CrossRef]
- Motohashi, K.; Takagi, M.; Shin-ya, K. Tetracenoquinocin and 5-iminoaranciamycin from a sponge-derived Streptomyces sp. Sp080513GE-26. J. Nat. Prod. 2010, 73, 755–758. [Google Scholar] [CrossRef]
- Izumikawa, M.; Kawahara, T.; Hwang, J.-H.; Takagi, M.; Shin-ya, K. JBIR-107, a new metabolite from the marine-sponge-derived actinomycete, Streptomyces tateyamensis NBRC 105047. Biosci. Biotechnol. Biochem. 2013, 77, 663–665. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, L.; Liu, D.; Proksch, P.; Zhou, D.; Lin, W. Truncateols A–N, new isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with anti-H1N1 virus activities. Tetrahedron 2015, 71, 2708–2718. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Angupyrones A-E, α-Pyrone Analogues with ARE-Activation from a Sponge-Associated Fungus Truncatella angustata. Chem. Biodivers. 2017, 14, e1700236. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Isocoumarin Derivatives from the Sponge-Associated Fungus Peyronellaea glomerate with Antioxidant Activities. Chem. Biodivers. 2016, 13, 1186–1193. [Google Scholar] [CrossRef]
- Zhu, H.; Hua, X.-x.; Gong, T.; Pang, J.; Hou, Q.; Zhu, P. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem. Lett. 2013, 6, 392–396. [Google Scholar] [CrossRef]
- Gong, T.; Zhen, X.; Li, B.-J.; Yang, J.-L.; Zhu, P. Two new monoterpenoid α-pyrones from a fungus Nectria sp. HLS206 associated with the marine sponge Gelliodes carnosa. J. Asian Nat. Prod. Res. 2015, 17, 633–637. [Google Scholar] [CrossRef]
- Zhen, X.; Gong, T.; Liu, F.; Zhang, P.-C.; Zhou, W.-Q.; Li, Y.; Zhu, P. A New Analogue of Echinomycin and a New Cyclic Dipeptide from a Marine-Derived Streptomyces sp. LS298. Mar. Drugs 2015, 13, 6947–6961. [Google Scholar] [CrossRef] [Green Version]
- Gesner, S.; Cohen, N.; Ilan, M.; Yarden, O.; Carmeli, S. Pandangolide 1a, a metabolite of the sponge-associated fungus Cladosporium sp., and the absolute stereochemistry of pandangolide 1 and iso-cladospolide B. J. Nat. Prod. 2005, 68, 1350–1353. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cen, S.; Proksch, P.; Lin, W. Isoindolinone-type alkaloids from the sponge-derived fungus Stachybotrys chartarum. Tetrahedron 2014, 70, 7010–7015. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A-P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, Y.; Li, X.; Cheng, Z.; Huang, J.; Proksch, P.; Lin, W. Chartarolides A-C, novel meroterpenoids with antitumor activities. Tetrahedron Lett. 2017, 58, 1826–1829. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Punctaporonins H–M: Caryophyllene-Type Sesquiterpenoids from the Sponge-Associated Fungus Hansfordia sinuosae. Mar. Drugs 2014, 12, 3904–3916. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Li, Y.; Liu, D.; Ma, M.; Chen, J.; Lin, W. New Resorcinol Derivatives from a Sponge-Derived Fungus Hansfordia sinuosae. Chem. Biodivers. 2017, 14, e1700059. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Li, Y.; Li, Y.; Ma, M.; Chen, J.-L. Salicylic acid derivatives and phenylspirodrimanes from the sponge-associated fungus Hansfordia sinuosae. J. Asian Nat. Prod. Res. 2018, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Steffens, S.; Schaumann, K. Petrosifungins A and B, novel cyclodepsipeptides from a sponge-derived strain of Penicillium brevicompactum. J. Nat. Prod. 2004, 67, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Elbandy, M.; Shinde, P.B.; Dang, H.T.; Hong, J.; Bae, K.S.; Jung, J.H. Furan metabolites from the sponge-derived yeast Pichia membranifaciens. J. Nat. Prod. 2008, 71, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Elbandy, M.; Shinde, P.B.; Hong, J.; Bae, K.S.; Kim, M.A.; Lee, S.M.; Jung, J.H. α-pyrones and yellow pigments from the sponge-derived fungus Paecilomyces lilacinus. Bull. Korean Chem. Soc. 2009, 30, 188–192. [Google Scholar]
- Lopez-Gresa, M.P.; Cabedo, N.; Gonzalez-Mas, M.C.; Ciavatta, M.L.; Avila, C.; Primo, J. Terretonins E and F, Inhibitors of the Mitochondrial Respiratory Chain from the Marine-Derived Fungus Aspergillus insuetus. J. Nat. Prod. 2009, 72, 1348–1351. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Dang, H.T.; Hong, J.-K.; Lee, C.-O.; Bae, K.-S.; Kim, D.-K.; Jung, J.-H. A Cytotoxic Lipopeptide from the Sponge-Derived Fungus Aspergillus versicolor. Bull. Korean Chem. Soc. 2010, 31, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-M.; Dang, H.T.; Li, J.; Zhang, P.; Hong, J.-K.; Lee, C.-O.; Jung, J.-H. A Cytotoxic Fellutamide Analogue from the Sponge-Derived Fungus Aspergillus versicolor. Bull. Korean Chem. Soc. 2011, 32, 3817–3820. [Google Scholar] [CrossRef] [Green Version]
- Li, J.L.; Huang, L.; Liu, J.; Song, Y.; Gao, J.; Jung, J.H.; Liu, Y.; Chen, G. Acetylcholinesterase inhibitory dimeric indole derivatives from the marine actinomycetes Rubrobacter radiotolerans. Fitoterapia 2015, 102, 203–207. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Huang, L.; Ni, M.; Zhao, Y.; Fan, H.; Bao, X. Antichlamydial Dimeric Indole Derivatives from Marine Actinomycete Rubrobacter radiotolerans. Planta Med. 2017, 83, 805–811. [Google Scholar] [CrossRef]
- Buttachon, S.; May Zin, W.W.; Dethoup, T.; Gales, L.; Pereira, J.A.; Silva, A.M.; Kijjoa, A. Secondary Metabolites from the Culture of the Marine Sponge-Associated Fungi Talaromyces tratensis and Sporidesmium circinophorum. Planta Med. 2016, 82, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.H.; Li, J.; Fu, H.Z.; Proksch, P. Four novel hydropyranoindeno-derivatives from marine fungus Aspergillus versicolor. Chin. Chem. Lett. 2001, 12, 435–438. [Google Scholar]
- Edrada, R.A.; Heubes, M.; Brauers, G.; Wray, V.; Berg, A.; Graefe, U.; Wohlfarth, M.; Muehlbacher, J.; Schaumann, K.; Bringmann, G.; et al. Online analysis of xestodecalactones A-C, novel bioactive metabolites from the fungus Penicillium cf. montanense and their subsequent isolation from the sponge Xestospongia exigua. J. Nat. Prod. 2002, 65, 1598–1604. [Google Scholar]
- Lin, W.; Brauers, G.; Ebel, R.; Wray, V.; Berg, A.; Sudarsono; Proksch, P. Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. J. Nat. Prod. 2003, 66, 57–61. [Google Scholar] [CrossRef]
- Ingavat, N.; Dobereiner, J.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Aspergillusol A, an α-Glucosidase Inhibitor from the Marine-Derived Fungus Aspergillus aculeatus. J. Nat. Prod. 2009, 72, 2049–2052. [Google Scholar] [CrossRef]
- Ingavat, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Asperaculin A, a sesquiterpenoid from a marine-derived fungus, Aspergillus aculeatus. J. Nat. Prod. 2011, 74, 1650–1652. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.-L.; Yang, R.-Y.; Zheng, C.-J.; Chen, Y.-Y.; Fu, X.-M.; Qian, P.-Y.; She, Z.-G.; Voogd, N.J.; et al. Antibacterial Bisabolane-Type Sesquiterpenoids from the Sponge-Derived Fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.L.; Shao, C.L.; Chen, J.F.; Guo, Z.Y.; Fu, X.M.; Chen, M.; Chen, Y.Y.; Li, R.; de Voogd, N.J.; She, Z.G.; et al. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria. Bioorg. Med. Chem. Lett. 2012, 22, 1326–1329. [Google Scholar] [CrossRef]
- Supong, K.; Thawai, C.; Suwanborirux, K.; Choowong, W.; Supothina, S.; Pittayakhajonwut, P. Antimalarial and antitubercular C-glycosylated benz[α]anthraquinones from the marine-derived Streptomyces sp. BCC45596. Phytochem. Lett. 2012, 5, 651–656. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with Anti-HIV Activity from the Sponge-Derived Fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, H.; Li, F.; Zhu, T.; Gu, Q.; Li, D. Stachybotrin G, a sulfate meroterpenoid from a sponge derived fungus Stachybotrys chartarum MXH-X73. Tetrahedron Lett. 2015, 56, 7053–7055. [Google Scholar] [CrossRef]
- Harinantenaina Rakotondraibe, L.; Rasolomampianina, R.; Park, H.-Y.; Li, J.; Slebodnik, C.; Brodie, P.J.; Blasiak, L.C.; Hill, R.; TenDyke, K.; Shen, Y.; et al. Antiproliferative and antiplasmodial compounds from selected Streptomyces species. Bioorg. Med. Chem. Lett. 2015, 25, 5646–5649. [Google Scholar] [CrossRef] [Green Version]
- Bugni, T.S.; Bernan, V.S.; Greenstein, M.; Janso, J.E.; Maiese, W.M.; Mayne, C.L.; Ireland, C.M. Brocaenols A-C: Novel Polyketides from a Marine-Derived Penicillium brocae. J. Org. Chem. 2003, 68, 6846. [Google Scholar] [CrossRef] [Green Version]
- Prompanya, C.; Dethoup, T.; Gales, L.; Lee, M.; Pereira, J.; Silva, A.; Pinto, M.; Kijjoa, A. New Polyketides and New Benzoic Acid Derivatives from the Marine Sponge-Associated Fungus Neosartorya quadricincta KUFA 0081. Mar. Drugs 2016, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Kumla, D.; Shine Aung, T.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.; Inácio, Â.; Costa, P.; Lee, M.; Sekeroglu, N.; et al. A New Dihydrochromone Dimer and Other Secondary Metabolites from Cultures of the Marine Sponge-Associated Fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213. Mar. Drugs 2017, 15, 375. [Google Scholar] [CrossRef] [Green Version]
- Christian, O.E.; Compton, J.; Christian, K.R.; Mooberry, S.L.; Valeriote, F.A.; Crews, P. Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomospis asparagi. J. Nat. Prod. 2005, 68, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.H.; Zhu, W.M.; Gu, Q.Q.; Fang, Y.C.; Duan, L.; Cui, C.B. A new cytotoxic compound from Penicillium auratiogriseum, symbiotic or epiphytic fungus of sponge Mycale plumose. Chin. Chem. Lett. 2005, 16, 1227–1229. [Google Scholar]
- Xin, Z.H.; Fang, Y.; Du, L.; Zhu, T.; Duan, L.; Chen, J.; Gu, Q.-Q.; Zhu, W.-M. Aurantiomides A-C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J. Nat. Prod. 2007, 70, 853–855. [Google Scholar] [CrossRef]
- Feher, D.; Barlow, R.S.; Lorenzo, P.S.; Hemscheidt, T.K. A 2-substituted prodiginine, 2- (p-hydroxybenzyl) prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, K.; Inaba, S.; Takagi, M.; Shin-ya, K. JBIR-15, a new aspochracin derivative, isolated from a sponge-derived fungus, Aspergillus sclerotiorum Huber Sp080903f04. Biosci. Biotechnol. Biochem. 2009, 73, 1898–1900. [Google Scholar] [CrossRef] [Green Version]
- Gomes, N.M.; Dethoup, T.; Singburaudom, N.; Gales, L.; Silva, A.M.S.; Kijjoa, A. Eurocristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 2012, 5, 717–720. [Google Scholar] [CrossRef]
- May Zin, W.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinto, M.; Gales, L.; Pereira, J.; Silva, A.; Sekeroglu, N.; et al. New Cyclotetrapeptides and a New Diketopiperzine Derivative from the Marine Sponge-Associated Fungus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, K.; Kehraus, S.; Guetschow, M.; Koenig, G.M. Cytotoxic and HLE-inhibitory tetramic acid derivatives from marine-derived fungi. Nat. Prod. Commun. 2009, 4, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, J.; Stewart, A.; van Wagoner, R.; Elliott, E.; Bourdelais, A.; Wright, J. Monacyclinones, New Angucyclinone Metabolites Isolated from Streptomyces sp. M7_15 Associated with the Puerto Rican Sponge Scopalinaruetzleri. Mar. Drugs 2015, 13, 4682–4700. [Google Scholar] [CrossRef] [Green Version]
- Antia, B.S.; Aree, T.; Kasettrathat, C.; Wiyakrutta, S.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry 2011, 72, 816–820. [Google Scholar] [CrossRef]
- Noinart, J.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.; Urbatzka, R.; Freitas, S.; Lee, M.; Silva, A.; Pinto, M.; et al. A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207. Mar. Drugs 2017, 15, 139. [Google Scholar] [CrossRef] [Green Version]
- Leman-Loubière, C.; Le Goff, G.; Retailleau, P.; Debitus, C.; Ouazzani, J. Sporothriolide-Related Compounds from the Fungus Hypoxylon monticulosum CLL-205 Isolated from a Sphaerocladina Sponge from the Tahiti Coast. J. Nat. Prod. 2017, 80, 2850–2854. [Google Scholar] [CrossRef]
- Amagata, T.; Doi, M.; Ohta, T.; Minoura, K.; Numata, A. Absolute stereostructures of novel cytotoxic metabolites, gymnastatins A-E, from a Gymnascella species separated from a Halichondria sponge. J. Chem. Soc. Perkin Trans. 1 1998, 21, 3585–3600. [Google Scholar] [CrossRef]
- Amagata, T.; Minoura, K.; Numata, A. Gymnasterones, novel cytotoxic metabolite produced by a fungal strain from sponge. Tetrahedron Lett. 1998, 39, 3773–3774. [Google Scholar] [CrossRef]
- Amagata, T.; Minoura, K.; Numata, A. Gymnastatins F-H, cytostatic metabolites from the sponge-derived fungus Gymnascella dankaliensis. J. Nat. Prod. 2006, 69, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Amagata, T.; Takigawa, K.; Minoura, K. Gymnastatins I-K, Cancer Cell Growth Inhibitors from a Sponge-Derived Gymnascella dankaliensis. Heterocycles 2010, 81, 897. [Google Scholar]
- Amagata, T.; Doi, M.; Tohgo, M.; Minoura, K.; Numata, A. Dankasterone, a new class of cytotoxic steroid produced by a Gymnascella species from a marine sponge. Chem. Commun. 1999, 14, 1321–1322. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Minoura, K.; Numata, A. Gymnastatins and dankastatins, growth inhibitory metabolites of a Gymnascella species from a Halichondria sponge. J. Nat. Prod. 2008, 71, 340–345. [Google Scholar] [CrossRef]
- Amagata, T.; Usami, Y.; Minoura, K.; Ito, T.; Numata, A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998, 51, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Wicke, C.; Hueners, M.; Wray, V.; Nimtz, M.; Bilitewski, U.; Lang, S. Production and structure elucidation of glycoglycerolipids from a marine sponge-associated Microbacterium species. J. Nat. Prod. 2000, 63, 621–626. [Google Scholar] [CrossRef]
- Nagai, K.; Kamigiri, K.; Arao, N.; Suzumura, K.-I.; Kawano, Y.; Yamaoka, M.; Zhang, H.; Watanabe, M.; Suzuki, K. YM-266183 and YM-266184, novel thiopeptide antibiotics produced by Bacillus cereus isolated from a marine sponge. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological properties. J. Antibiot. 2003, 56, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Kanoh, K.; Wisespongp, P.; Nishijima, M.; Shizuri, Y. Clonostachysins A and B, new anti-dinoflagellate cyclic peptides from a marine-derived fungus. J. Antibiot. 2005, 58, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Mitova, M.I.; Lang, G.; Wiese, J.; Imhoff, J.F. Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. J. Nat. Prod. 2008, 71, 824–827. [Google Scholar] [CrossRef]
- Schneemann, I.; Kajahn, I.; Ohlendorf, B.; Zinecker, H.; Erhard, A.; Nagel, K.; Wiese, J.; Imhoff, J.F. Mayamycin, a Cytotoxic Polyketide from a Streptomyces Strain Isolated from the Marine Sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Labes, A.; Wiese, J.; Bruhn, T.; Bringmann, G.; Imhoff, J. Nature’s Lab for Derivatization: New and Revised Structures of a Variety of Streptophenazines Produced by a Sponge-Derived Streptomyces Strain. Mar. Drugs 2014, 12, 1699–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Yang, X.; Kang, J.S.; Choi, H.D.; Son, B.W. Chlorohydroaspyrones A and B, antibacterial aspyrone derivatives from the marine-derived fungus Exophiala sp. J. Nat. Prod. 2008, 71, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Ito, Y.; Suzuki, M.; Hirota, A. Indole derivatives from a marine sponge-derived yeast as DPPH radical scavengers. J. Nat. Prod. 2009, 72, 2069–2071. [Google Scholar] [CrossRef]
- Schneemann, I.; Ohlendorf, B.; Zinecker, H.; Nagel, K.; Wiese, J.; Imhoff, J.F. Nocapyrones A-D, γ-Pyrones from a Nocardiopsis Strain Isolated from the Marine Sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1444–1447. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Mizutani, Y.; Umebayashi, Y.; Inno, N.; Kawashima, M.; Kikuchi, T.; Tanaka, R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014, 55, 662–664. [Google Scholar] [CrossRef]
- Yamada, T.; Umebayashi, Y.; Kawashima, M.; Sugiura, Y.; Kikuchi, T.; Tanaka, R. Determination of the Chemical Structures of Tandyukisins B–D, Isolated from a Marine Sponge-Derived Fungus. Mar. Drugs 2015, 13, 3231–3240. [Google Scholar] [CrossRef] [Green Version]
- Suzue, M.; Kikuchi, T.; Tanaka, R.; Yamada, T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett. 2016, 57, 5070–5073. [Google Scholar] [CrossRef]
- Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C-E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus. Mar. Drugs 2017, 15, 169. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J. Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, Q.-L.; Ji, N.-Y.; Liu, B.; Zhang, W.; Cao, X.-P. Deoxyuridines from the Marine Sponge Associated Actinomycete Streptomyces microflavus. Mar. Drugs 2011, 9, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-B.; Xi, T.; Li, J.; Wang, P.; Li, F.-C.; Lin, Y.-C.; Qin, S. Lobophorin C and D, New Kijanimicin Derivatives from a Marine Sponge-Associated Actinomycetal Strain AZS17. Mar. Drugs 2011, 9, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ren, B.; Chen, C.; Yu, K.; Liu, X.; Zhang, Y.; Yang, N.; He, H.; Liu, X.; Dai, H.; et al. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl. Microbiol. Biot. 2014, 98, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mou, Y.; Hu, J.; Wang, N.; Zhao, L.; Liu, L.; Wang, S.; Meng, D. Cytotoxic polyphenols from a sponge-associated fungus Aspergillus versicolor Hmp-48. Chem. Biodivers. 2014, 11, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, L.; Jiménez, C.; Rodríguez, J.; Areche, C.; Chávez, R.; Henríquez, M.; de la Cruz, M.; Díaz, C.; Segade, Y.; Vaca, I. 3-Nitroasterric Acid Derivatives from an Antarctic Sponge-Derived Pseudogymnoascus sp. Fungus. J. Nat. Prod. 2015, 78, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.F.; Fang, S.T.; Li, W.Z.; Liu, S.J.; Wang, J.H.; Xia, C.H. A new minor diketopiperazine from the sponge-derived fungus Simplicillium sp. YZ-11. Nat. Prod. Res. 2015, 29, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhao, J.; Ding, L.; Huang, C.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.; Gerwick, W.H.; et al. 5-Hydroxycyclopenicillone, a New β-Amyloid Fibrillization Inhibitor from a Sponge-Derived Fungus Trichoderma sp. HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef] [Green Version]
- Mitova, M.; Popov, S.; De Rosa, S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J. Nat. Prod. 2004, 67, 1178–1181. [Google Scholar] [CrossRef]
- Liu, H.; Edrada-Ebel, R.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S.; Muller, W.E.G.; Wray, V.; Lin, W.; Proksch, P. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. J. Nat. Prod. 2009, 72, 1585–1588. [Google Scholar] [CrossRef]
- Liu, H.-B.; Edrada-Ebel, R.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S.; Mueller, W.E.G.; Wray, V.; Lin, W.-H.; Proksch, P. Ophiobolin Sesterterpenoids and Pyrrolidine Alkaloids from the Sponge-Derived Fungus Aspergillus ustus. Helv. Chim. Acta 2011, 94, 623–631. [Google Scholar] [CrossRef]
- Simmons, L.; Kaufmann, K.; Garcia, R.; Schwär, G.; Huch, V.; Müller, R. Bendigoles D–F, bioactive sterols from the marine sponge-derived Actinomadura sp. SBMs009. Bioorg. Med. Chem. 2011, 19, 6570–6575. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, I.; Ryu, S.-Y.; Won, D.H.; Giri, A.G.; Wang, W.; Choi, H.; Chin, J.; Hahn, D.; Kim, E.; et al. Acredinones A and B, Voltage-Dependent Potassium Channel Inhibitors from the Sponge-Derived Fungus Acremonium sp. F9A015. J. Nat. Prod. 2015, 78, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; Ohlendorf, B.; Blümel, M.; Schmaljohann, R.; Imhoff, J.F. Phylogenetic Identification of Fungi Isolated from the Marine Sponge Tethya aurantium and Identification of Their Secondary Metabolites. Mar. Drugs 2011, 9, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Bruhn, T.; Bringmann, G.; Imhoff, J. Helicusin E, Isochromophilone X and Isochromophilone XI: New Chloroazaphilones Produced by the Fungus Bartalinia robillardoides Strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Debbab, A.; Wray, V.; Lin, W.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef] [Green Version]
- Julianti, E.; Oh, H.; Jang, K.H.; Lee, J.K.; Lee, S.K.; Oh, D.-C.; Oh, K.-B.; Shin, J. Acremostrictin, a Highly Oxygenated Metabolite from the Marine Fungus Acremonium strictum. J. Nat. Prod. 2011, 74, 2592–2594. [Google Scholar] [CrossRef]
- Julianti, E.; Oh, H.; Lee, H.-S.; Oh, D.-C.; Oh, K.-B.; Shin, J. Acremolin, a new 1H-azirine metabolite from the marine-derived fungus Acremonium strictum. Tetrahedron Lett. 2012, 53, 2885–2886. [Google Scholar] [CrossRef]
- Rahbk, L.; Sperry, S.; Piper, J.E.; Crews, P. Deoxynortrichoharzin, a new polyketide from the saltwater culture of a sponge-derived Paecilomyces fungus. J. Nat. Prod. 1998, 61, 1571–1573. [Google Scholar] [CrossRef]
- Li, H.; Shinde, P.B.; Lee, H.J.; Yoo, E.S.; Lee, C.-O.; Hong, J.; Choi, S.H.; Jung, J.H. Bile acid derivatives from a sponge-associated bacterium Psychrobacter sp. Arch. Pharm. Res. 2009, 32, 857–862. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, B.; Dang, H.T.; Hong, J.; Lee, H.J.; Yoo, E.S.; Bae, K.S.; Jung, J.H. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J. Nat. Prod. 2009, 72, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Lee, C.-O.; Bae, K.S.; Hong, J.; Jung, J.H. Indole oligomers from a marine sponge-associated bacterium Psychrobacter sp. Biochem. Syst. Ecol. 2010, 38, 839–841. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, P.; Lee, Y.M.; Hong, J.; Yoo, E.S.; Bae, K.S.; Jung, J.H. Oxygenated hexylitaconates from a marine sponge-derived fungus Penicillium sp. Chem. Pharm. Bull. 2011, 59, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jung, J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Schulz, B.; Wray, V.; Totzke, F.; Kubbutat, M.H.G.; Müller, W.E.G.; Hamacher, A.; Kassack, M.U.; Lin, W.; Proksch, P. Arthrinins A–D: Novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg. Med. Chem. 2011, 19, 4644–4651. [Google Scholar] [CrossRef] [PubMed]
- Bultel-Ponce, V.; Berge, J.-P.; Debitus, C.; Nicolas, J.-L.; Guyot, M. Metabolites from the sponge-associated bacterium Pseudomonas species. Mar. Biotechnol. 1999, 1, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, K.; Kamino, K.; Leleo, G.; Adachi, K.; Shizuri, Y. Pseudoalterobactin A and B, new siderophores excreted by marine bacterium Pseudoalteromonas sp. KP20-4. J. Antibiot. 2003, 56, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Izumikawa, M.; Khan, S.T.; Takagi, M.; Shin-ya, K. Sponge-derived Streptomyces producing isoprenoids via the mevalonate pathway. J. Nat. Prod. 2010, 73, 208–212. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, C.; Long, H.; Chen, R.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Varioxiranols A–G and 19-O-Methyl-22-methoxypre-shamixanthone, PKS and Hybrid PKS-Derived Metabolites from a Sponge-Associated Emericella variecolor Fungus. J. Nat. Prod. 2015, 78, 2461–2470. [Google Scholar] [CrossRef]
- Wu, Q.; Long, H.-L.; Liu, D.; Proksch, P.; Lin, W.-H. Varioxiranols I-L, new lactones from a sponge-associated Emericella variecolor fungus. J. Asian Nat. Prod. Res. 2015, 17, 1137–1145. [Google Scholar] [CrossRef]
- Long, H.; Cheng, Z.; Huang, W.; Wu, Q.; Li, X.; Cui, J.; Proksch, P.; Lin, W. Diasteltoxins A–C, Asteltoxin-Based Dimers from a Mutant of the Sponge-Associated Emericella variecolor Fungus. Org. Lett. 2016, 18, 4678–4681. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-L.; Gao, Y.; Xue, D.-Q.; Liu, H.-L.; Peng, C.-S.; Zhang, F.-L.; Li, Z.-Y.; Guo, Y.-W. Streptomycindole, an indole alkaloid from a marine Streptomyces sp. DA22 associated with South China Sea sponge Craniella australiensis. Helv. Chim. Acta 2011, 94, 1838–1842. [Google Scholar] [CrossRef]
- Okada, M.; Sugita, T.; Wong, C.P.; Wakimoto, T.; Abe, I. Identification of Pyridinium with Three Indole Moieties as an Antimicrobial Agent. J. Nat. Prod. 2017, 80, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lu, M.-C.; Chung, H.-M.; Weng, C.-F.; Su, J.-H.; Yang, Y.-T.; Su, Y.-D.; Chang, Y.-C.; Kuo, J.; Wu, Y.-C.; et al. Bafilomycin M, a new cytotoxic bafilomycin produced by a Streptomyces sp. isolated from a marine sponge Theonella sp. Tetrahedron Lett. 2016, 57, 4863–4865. [Google Scholar] [CrossRef]
- Brauers, G.; Edrada, R.A.; Ebel, R.; Proksch, P.; Wray, V.; Berg, A.; Graefe, U.; Schaechtele, C.; Totzke, F.; Finkenzeller, G.; et al. Anthraquinones and betaenone derivatives from the sponge-associated fungus Microsphaeropsis species: Novel inhibitors of protein kinases. J. Nat. Prod. 2000, 63, 739–745. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, B.-G.; Brauers, G.; Guan, H.-S.; Proksch, P.; Ebel, R. Microsphaerones A and B, two novel γ-pyrone derivatives from the sponge-derived fungus Microsphaeropsis sp. J. Nat. Prod. 2002, 65, 772–775. [Google Scholar] [CrossRef]
- Brauers, G.; Ebel, R.; Edrada, R.; Wray, V.; Berg, A.; Graefe, U.; Proksch, P. Hortein, a new natural product from the fungus Hortaea werneckii associated with the sponge Aplysina aerophoba. J. Nat. Prod. 2001, 64, 651–652. [Google Scholar] [CrossRef]
- Sun, D.; Sun, W.; Yu, Y.; Li, Z.; Deng, Z.; Lin, S. A new glutarimide derivative from marine sponge-derived Streptomyces anulatus S71. Nat. Prod. Res. 2014, 28, 1602–1606. [Google Scholar] [CrossRef]
- Suciati; Fraser, J.A.; Lambert, L.K.; Pierens, G.K.; Bernhardt, P.V.; Garson, M.J. Secondary Metabolites of the Sponge-Derived Fungus Acremonium persicinum. J. Nat. Prod. 2013, 76, 1432–1440. [Google Scholar] [CrossRef]
- Amagata, T.; Morinaka, B.I.; Amagata, A.; Tenney, K.; Valeriote, F.A.; Lobkovsky, E.; Clardy, J.; Crews, P. A chemical study of cyclic depsipeptides produced by a sponge-derived fungus. J. Nat. Prod. 2006, 69, 1560–1565. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.-y.; Takagi, M.; Shin-ya, K. New xanthoquinodin-like compounds, JBIR-97, -98 and -99, obtained from marine sponge-derived fungus Tritirachium sp. SpB081112MEf2. J. Antibiot. 2010, 63, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemori, H.; Tenma, M.; Shimazaki, K.; Kobayashi, J.i. Three New Metabolites from the Marine Yeast Aureobasidium pullulans. J. Nat. Prod. 1998, 61, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Abbanat, D.; Bernan, V.S.; Maiese, W.M.; Greenstein, M.; Jompa, J.; Tahir, A.; Ireland, C.M. Novel polyketide metabolites from a species of marine fungi. J. Nat. Prod. 2000, 63, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ui, H.; Shiomi, K.; Yamaguchi, Y.; Masuma, R.; Nagamitsu, T.; Takano, D.; Sunazuka, T.; Namikoshi, M.; Omura, S. Nafuredin, a novel inhibitor of NADH-fumarate reductase, produced by Aspergillus niger FT-0554. J. Antibiot. 2001, 54, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Malmstrom, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Namikoshi, M.; Negishi, R.; Nagai, H.; Dmitrenok, A.; Kobayashi, H. Three new chlorine containing antibiotics from a marine-derived fungus Aspergillus ostianus collected in Pohnpei. J. Antibiot. 2003, 56, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Ookura, R.; Kito, K.; Ooi, T.; Namikoshi, M.; Kusumi, T. Structure Revision of Circumdatins A and B, Benzodiazepine Alkaloids Produced by Marine Fungus Aspergillus ostianus, by X-ray Crystallography. J. Org. Chem. 2008, 73, 4245–4247. [Google Scholar] [CrossRef]
- Kito, K.; Ookura, R.; Yoshida, S.; Namikoshi, M.; Ooi, T.; Kusumi, T. New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Org. Lett. 2008, 10, 225–228. [Google Scholar] [CrossRef]
- Kito, K.; Ookura, R.; Kusumi, T.; Namikoshi, M.; Ooi, T. X-ray structures of two stephacidins, heptacyclic alkaloids from the marine-derived fungus Aspergillus ostianus. Heterocycles 2009, 78, 2101–2106. [Google Scholar] [CrossRef]
- Kong, F.; Singh, M.P.; Carter, G.T. Pseudopyronines A and B, α-pyrones produced by a marine Pseudomonas sp. F92S91, and evidence for the conversion of 4-hydroxy-α-pyrone to 3-furanone. J. Nat. Prod. 2005, 68, 920–923. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.J.; Jang, K.H.; Kim, T.S.; Oh, K.-B.; Shin, J. Cyclic Peptides of the Nocardamine Class from a Marine-Derived Bacterium of the Genus Streptomyces. J. Nat. Prod. 2005, 68, 623–625. [Google Scholar] [CrossRef]
- Cruz, L.J.; Martinez Insua, M.; Perez Baz, J.; Trujillo, M.; Rodriguez-Mias, R.A.; Oliveira, E.; Giralt, E.; Albericio, F.; Canedo, L.M. IB-01212, a new cytotoxic cyclodepsipeptide isolated from the marine fungus Clonostachys sp. ESNA-A009. J. Org. Chem. 2006, 71, 3335–3338. [Google Scholar] [CrossRef]
- Cueto, M.; MacMillan, J.B.; Jensen, P.R.; Fenical, W. Tropolactones A-D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry 2006, 67, 1826–1831. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yoshida, T.; Hosono, H.; Ohta, T.; Yokosawa, H. Hexylitaconic acid: A new inhibitor of p53–HDM2 interaction isolated from a marine-derived fungus, Arthrinium sp. Bioorg. Med. Chem. Lett. 2006, 16, 69–71. [Google Scholar] [CrossRef]
- Xu, J.; Takasaki, A.; Kobayashi, H.; Oda, T.; Yamada, J.; Mangindaan, R.E.P.; Ukai, K.; Nagai, H.; Namikoshi, M. Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J. Antibiot. 2006, 59, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.; Qing-Hua, Z.; Miao-Miao, J.; Jin-Shan, T.; Cheng-Du, M.; Kui, H.; Michio, N.; Nai-Li, W.; Xin-Sheng, Y. Polyketides from a marine sponge-derived fungus Mycelia sterilia and proton-proton long-range coupling. Magn. Reson. Chem. 2008, 46, 1148–1152. [Google Scholar] [CrossRef]
- You, M.-X.; Zhang, H.-P.; Hu, C.-Q. Isolation and characterization of three siderophores from marine bacteria. Chin. J. Chem. 2008, 26, 1332–1334. [Google Scholar] [CrossRef]
- Zhao, L.L.; Gai, Y.; Kobayashi, H.; Hu, C.Q.; Zhang, H.P. 5′-Hydroxyzearalenol, a new β-resorcylic macrolide from Fusarium sp. 05ABR26. Chin. Chem. Lett. 2008, 19, 1089–1092. [Google Scholar] [CrossRef]
- Pruksakorn, P.; Arai, M.; Kotoku, N.; Vilchèze, C.; Baughn, A.D.; Moodley, P.; Jacobs, W.R.; Kobayashi, M. Trichoderins, novel aminolipopeptides from a marine sponge-derived Trichoderma sp., are active against dormant mycobacteria. Bioorg. Med. Chem. Lett. 2010, 20, 3658–3663. [Google Scholar] [CrossRef]
- Takagi, M.; Motohashi, K.; Khan, S.T.; Hashimoto, J.; Shin-ya, K. JBIR-65, a new diterpene, isolated from a sponge-derived Actinomadura sp. SpB081030SC-15. J. Antibiot. 2010, 63, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, T.; Hirokawa, T.; Takagi, M.; Shin-ya, K. Trichostatin Analogues JBIR-109, JBIR-110, and JBIR-111 from the Marine Sponge-Derived Streptomyces sp. RM72. J. Nat. Prod. 2012, 75, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Takagi, M.; Shin-ya, K. Three new depsipeptides, JBIR-113, JBIR-114 and JBIR-115, isolated from a marine sponge-derived Penicillium sp. fS36. J. Antibiot. 2012, 65, 147–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureram, S.; Wiyakrutta, S.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med. 2012, 78, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Rotinsulu, H.; Kaneko, T.; Murakami, K.; Fujiwara, H.; Ukai, K.; Namikoshi, M. A New Dibenz[b,e]oxepine Derivative, 1-Hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a Marine-Derived Fungus, Beauveria bassiana TPU942. Mar. Drugs 2012, 10, 2691–2697. [Google Scholar] [CrossRef] [Green Version]
- Amagata, T.; Tanaka, M.; Yamada, T.; Chen, Y.-P.; Minoura, K.; Numata, A. Additional cytotoxic substances isolated from the sponge-derived Gymnascella dankaliensis. Tetrahedron Lett. 2013, 54, 5960–5962. [Google Scholar] [CrossRef]
- Kong, X.; Ma, X.; Xie, Y.; Cai, S.; Zhu, T.; Gu, Q.; Li, D. Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their antitubercular activities. Arch. Pharm. Res. 2013, 36, 739–744. [Google Scholar] [CrossRef]
- Martin, J.; Crespo, G.; Palomo, S.; Gonzalez, I.; Tormo, J.R.; de la Cruz, M.; Anderson, M.; Hill, R.T.; Vicente, F.; Genilloud, O.; et al. Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar. Drugs 2013, 11, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Mosadeghzad, Z.; Zuriati, Z.; Asmat, A.; Gires, U.; Wickneswari, R.; Pittayakhajonwut, P.; Farahani, G.H.N. Chemical components and bioactivity of the marine-derived fungus Paecilomyces sp. collected from Tinggi Island, Malaysia. Chem. Nat. Compd. 2013, 49, 621–625. [Google Scholar] [CrossRef]
- Qi, J.; Shao, C.-L.; Li, Z.-Y.; Gan, L.-S.; Fu, X.-M.; Bian, W.-T.; Zhao, H.-Y.; Wang, C.-Y. Isocoumarin Derivatives and Benzofurans from a Sponge-Derived Penicillium sp. Fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef]
- Kim, M.C.; Hwang, E.; Kim, T.; Ham, J.; Kim, S.Y.; Kwon, H.C. Nocatriones A and B, Photoprotective Tetracenediones from a Marine-Derived Nocardiopsis sp. J. Nat. Prod. 2014, 77, 2326–2330. [Google Scholar] [CrossRef]
- Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Fukuda, A.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorg. Med. Chem. Lett. 2014, 24, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, S.-H.; Shin, Y.; Bae, M.; Kim, B.-Y.; Lee, S.; Oh, K.-B.; Shin, J.; Oh, D.-C. A New Benzofuran Glycoside and Indole Alkaloids from a Sponge-Associated Rare Actinomycete, Amycolatopsis sp. Mar. Drugs 2014, 12, 2326–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Wang, B.-G. N-Formyllapatin A, a new N-formylspiroquinazoline derivative from the marine-derived fungus Penicillium adametzioides AS-53. Phytochem. Lett. 2014, 10, 145–148. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Jiang, W.-L.; Xu, G.-M.; Huang, C.-G.; Wang, B.-G. Bisthiodiketopiperazines and Acorane Sesquiterpenes Produced by the Marine-Derived Fungus Penicillium adametzioides AS-53 on Different Culture Media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef]
- Liu, Y.; Mandi, A.; Li, X.M.; Meng, L.H.; Kurtan, T.; Wang, B.G. Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7’-pyrazino[1,2-b] [1,2] oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640–3652. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhu, T.; Gu, Q.; Xi, R.; Wang, W.; Li, D. Structures and antiviral activities of butyrolactone derivatives isolated from Aspergillus terreus MXH-23. J. Ocean U. China 2014, 13, 1067–1070. [Google Scholar] [CrossRef]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2014, 68, 121–125. [Google Scholar] [CrossRef]
- Sato, S.; Iwata, F.; Fukae, T.; Katayama, M. Neomacquarimicin: A new macquarimicin analog from marine-derived actinomycete. J. Antibiot. 2014, 67, 479–482. [Google Scholar] [CrossRef]
- Wang, J.-F.; Lin, X.-P.; Qin, C.; Liao, S.-R.; Wan, J.-T.; Zhang, T.-Y.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B.; et al. Antimicrobial and antiviral sesquiterpenoids from sponge-associated fungus, Aspergillus sydowii ZSDS1-F6. J. Antibiot. 2014, 67, 581–583. [Google Scholar] [CrossRef]
- Wang, J.-F.; Xu, F.-Q.; Wang, Z.; Lu, X.; Wan, J.-T.; Yang, B.; Zhou, X.-F.; Zhang, T.-Y.; Tu, Z.-C.; Liu, Y. A new naphthalene glycoside from the sponge-derived fungus Arthrinium sp. ZSDS1-F3. Nat. Prod. Res. 2014, 28, 1070–1074. [Google Scholar] [CrossRef]
- Yi-Lei, N.; Yun-Dan, W.; Chuan-Xi, W.; Ru, L.; Yang, X.; Dong-Sheng, F.; Hong, J.; Yun-Yang, L. Compounds from marine-derived Verrucosispora sp. FIM06054 and their potential antitumour activities. Nat. Prod. Res. 2014, 28, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhao, C.; Hao, J.; Wang, C.; Wang, W.; Huang, X.; Zhu, W. New α-glucosidase inhibitors from a marine sponge-derived fungus, Aspergillus sp. OUCMDZ-1583. RSC Adv. 2015, 5, 68852–68863. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Li, Z.; Yu, Z.; Sun, T. Stereochemical investigation of a novel biological active substance from the secondary metabolites of marine fungus Penicillium chrysogenum SYP-F-2720. J. Mex. Chem. Soc. 2015, 59, 53–58. [Google Scholar]
- Ngokpol, S.; Suwakulsiri, W.; Sureram, S.; Lirdprapamongkol, K.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Drimane Sesquiterpene-Conjugated Amino Acids from a Marine Isolate of the Fungus Talaromyces minioluteus (Penicillium minioluteum). Mar. Drugs 2015, 13, 3567–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.; Dethoup, T.; Silva, A.; Kijjoa, A. A New Cyclic Hexapeptide and a New Isocoumarin Derivative from the Marine Sponge-Associated Fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-L.; Shao, C.-L.; Gan, L.-S.; Wang, M.; Wang, C.-Y. Chromone Derivatives from a Sponge-Derived Strain of the Fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Shao, C.-L.; Wang, C.-Y.; Wang, M.; Yang, L.-J.; Wang, C.-Y. Naphthalenones and Depsidones from a Sponge-Derived Strain of the Fungus Corynespora cassiicola. Molecules 2016, 21, 160. [Google Scholar] [CrossRef] [Green Version]
- Che, Q.; Tan, H.; Han, X.; Zhang, X.; Gu, Q.; Zhu, T.; Li, D. Naquihexcin A, a S-Bridged Pyranonaphthoquinone Dimer Bearing an Unsaturated Hexuronic Acid Moiety from a Sponge-Derived Streptomyces sp. HDN-10-293. Org. Lett. 2016, 18, 3358–3361. [Google Scholar] [CrossRef]
- Igarashi, Y.; Asano, D.; Sawamura, M.; In, Y.; Ishida, T.; Imoto, M. Ulbactins F and G, Polycyclic Thiazoline Derivatives with Tumor Cell Migration Inhibitory Activity from Brevibacillus sp. Org. Lett. 2016, 18, 1658–1661. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Austalides S-U, New Meroterpenoids from the Sponge-Derived Fungus Aspergillus aureolatus HDN14-107. Mar. Drugs 2016, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Sekurova, O.; Pérez-Victoria, I.; Martín, J.; Degnes, K.; Sletta, H.; Reyes, F.; Zotchev, S. New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07. Molecules 2016, 21, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lin, X.-P.; Ju, Z.-R.; Liao, X.-J.; Huang, X.-J.; Zhang, C.; Zhao, B.-X.; Xu, S.-H. Aspergchromones A and B, two new polyketides from the marine sponge-associated fungus Aspergillus sp. SCSIO XWS03F03. J. Asian Nat. Prod. Res. 2017, 19, 684–690. [Google Scholar] [CrossRef] [PubMed]

















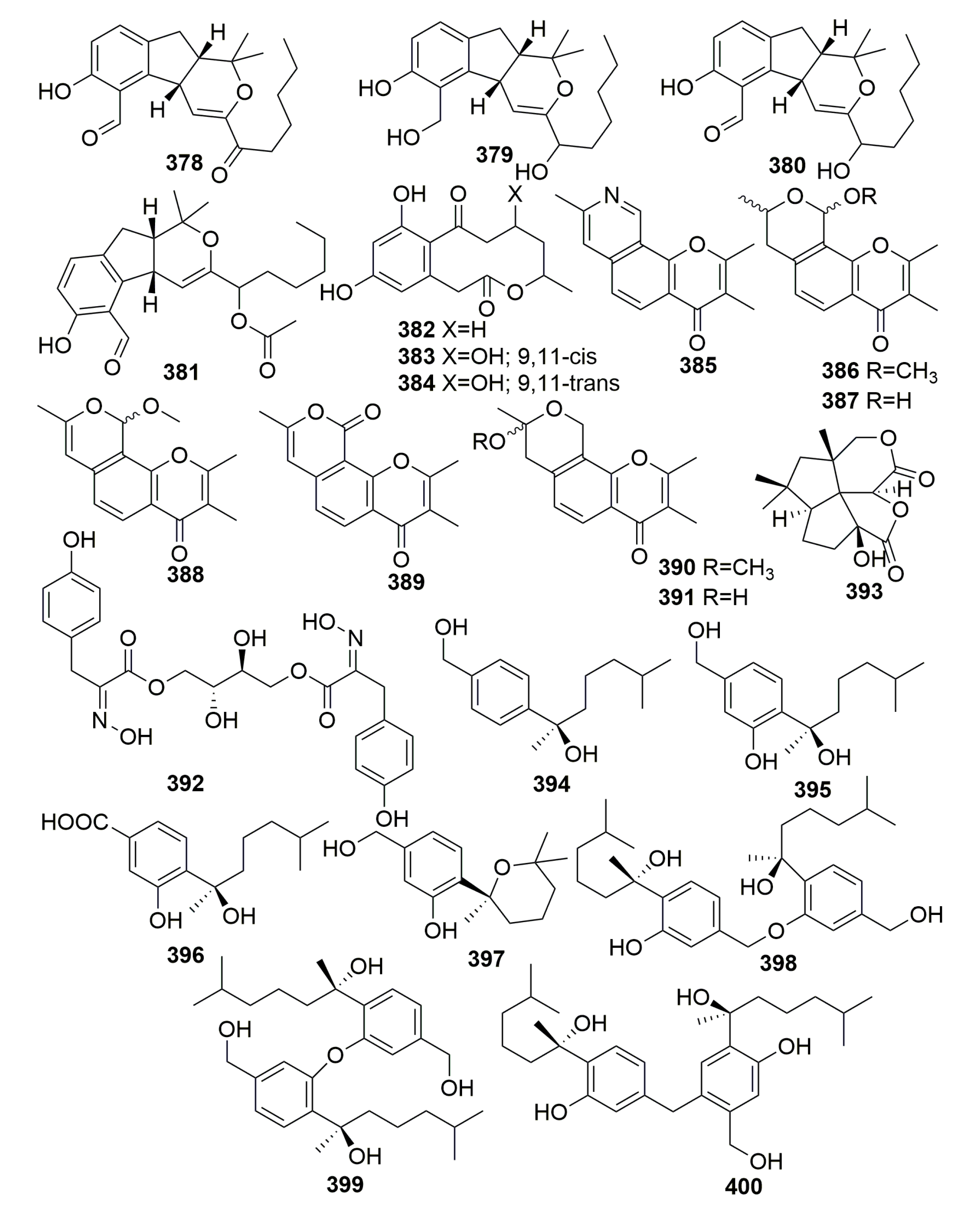






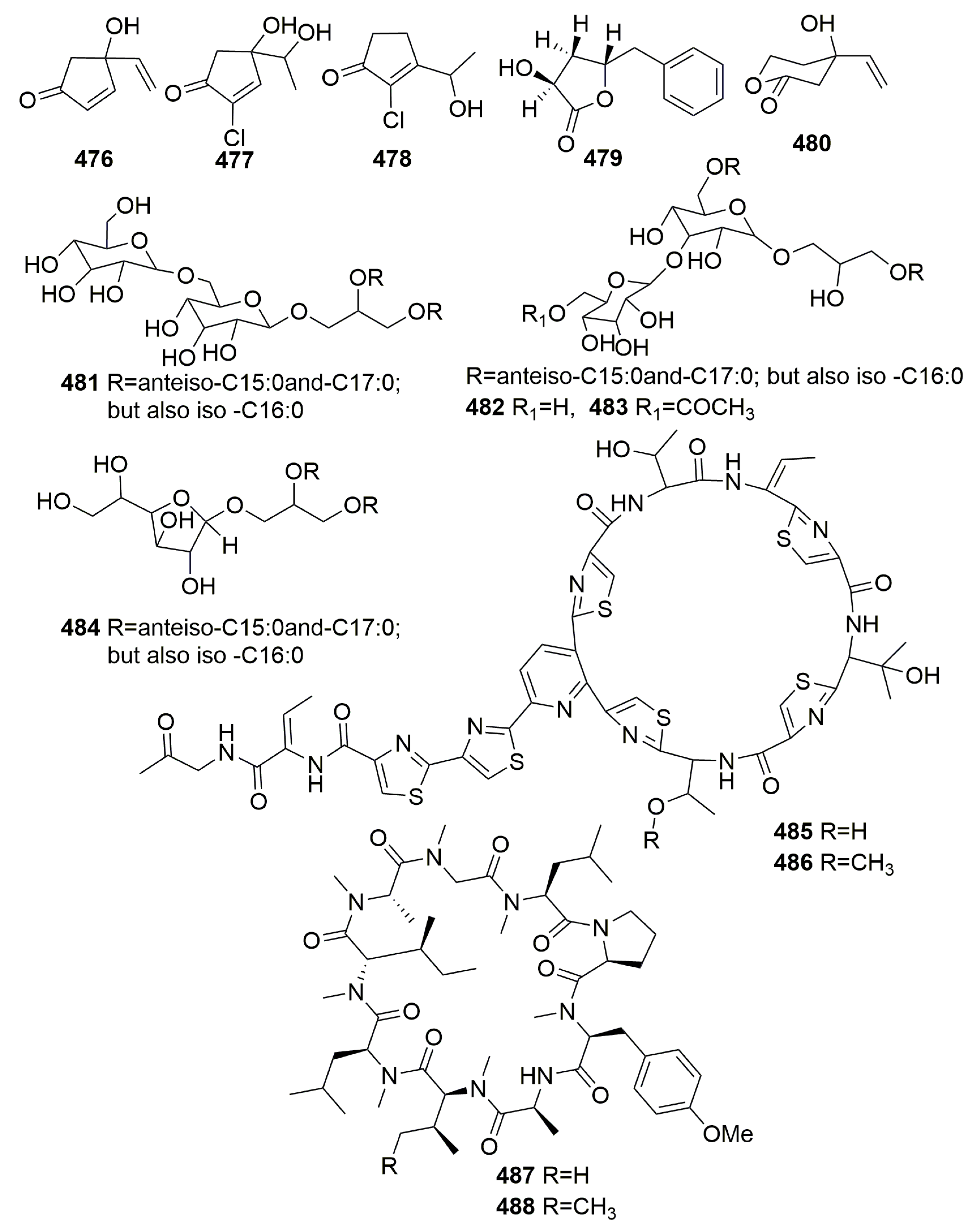



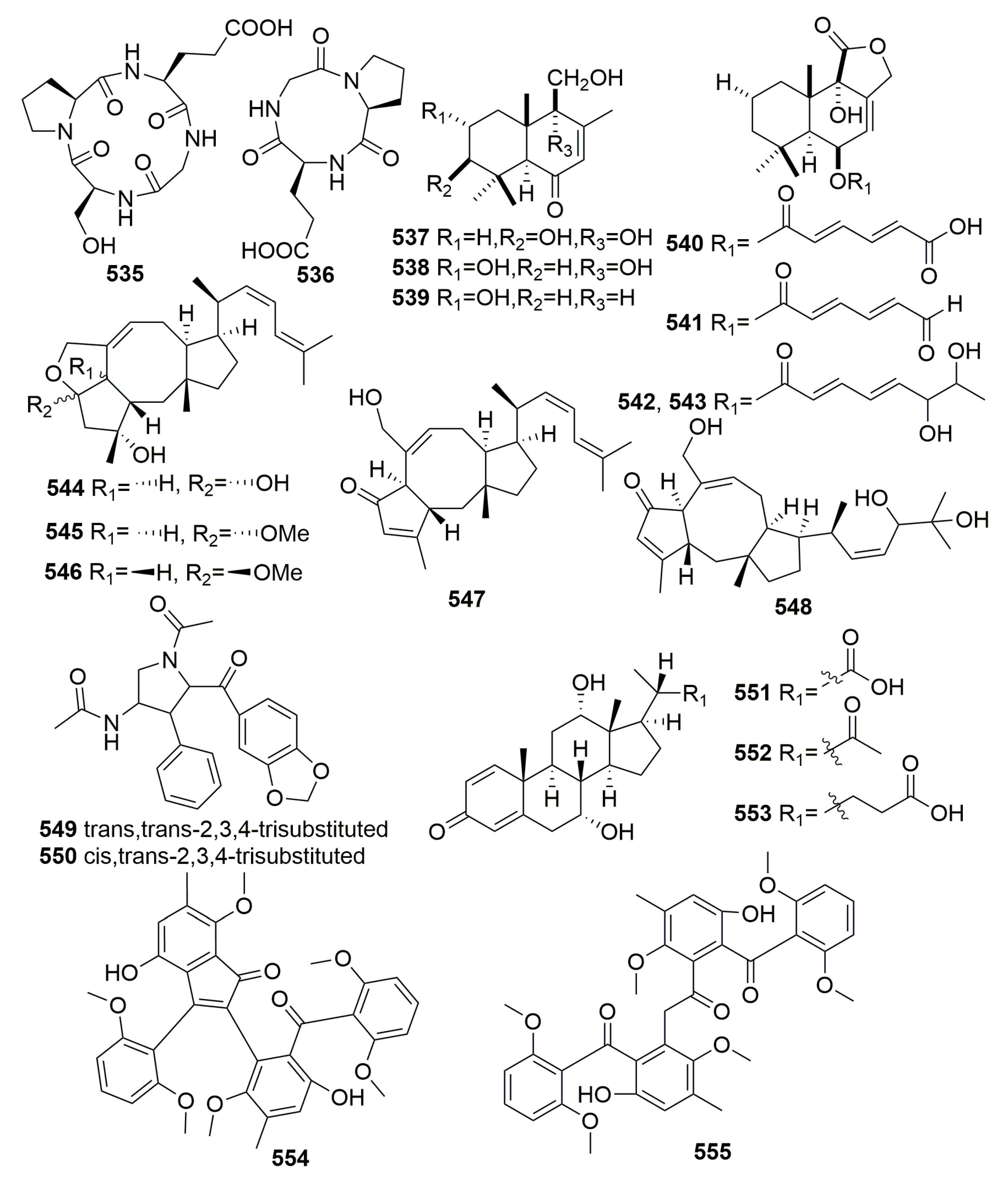






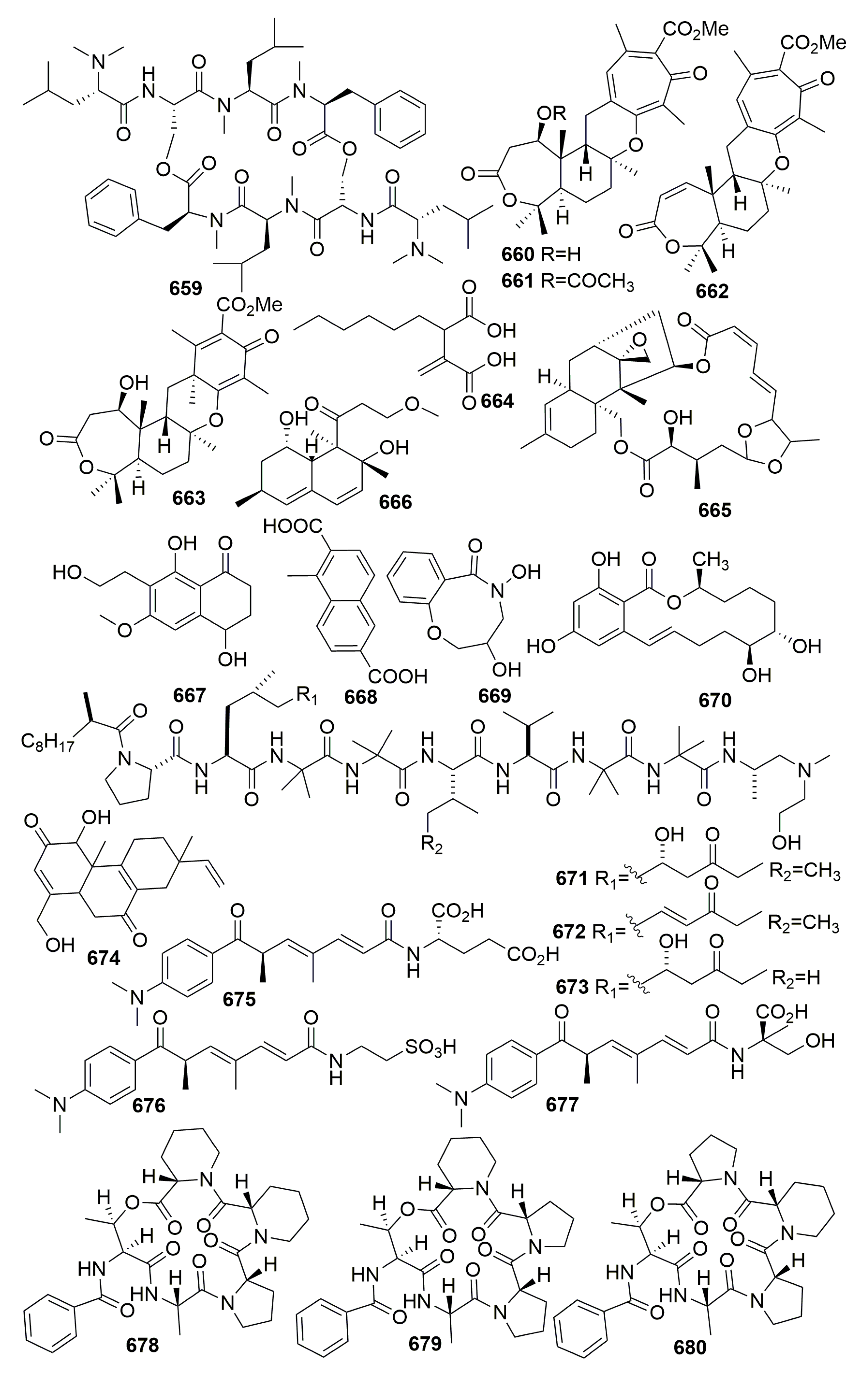









| Title | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 |
|---|---|---|---|---|---|---|---|---|
| 1 | AcAib | AcAib | AcAib | AcAib | AcAib | AcAib | AcAib | AcAib |
| 2 | Ala | Ala | Ala | Ala | Ala | Ala | Ala | Ala |
| 3 | Ala | Ala | Ala | Ala | Ala | Ala | Ala | Ala |
| 4 | Aib | Aib | Aib | Aib | Aib | Aib | Aib | Aib |
| 5 | Iva | Aib | Aib | Aib | Iva | Iva | Aib | Iva |
| 6 | Gln | Gln | Gln | Gln | Gln | Gln | Gln | Gln |
| 7 | Aib | Aib | Aib | Aib | Aib | Aib | Aib | Aib |
| 8 | Aib | Aib | Aib | Aib | Aib | Aib | Aib | Aib |
| 9 | Aib | Ala | Ala | Ala | Aib | Ala | Aib | Ala |
| 10 | Ser | Ser | Ser | Ser | Ser | Ser | Ser | Ser |
| 11 | Leu | Leu | Leu | Leu | Leu | Leu | Leu | Leu |
| 12 | Aib | Aib | Aib | Aib | Aib | Aib | Aib | Aib |
| 13 | Pro | Pro | Pro | Pro | Pro | Pro | Pro | Pro |
| 14 | Leu | Leu | Val | Val | Val | Val | Leu | Val |
| 15 | Aib | Aib | Aib | Aib | Aib | Aib | Aib | Aib |
| 16 | Ile | Ile | Ile | Ile | Ile | Ile | Ile | Ile |
| 17 | Glu | Gln | Gln | Glu-OMe | Glu-OMe | Glu-OMe | Glu-OMe | Gln |
| 18 | Gln | Gln | Gln | Gln | Gln | Gln | Gln | Gln |
| 19 | Pheol | Pheol | Pheol | Pheol | Pheol | Pheol | Pheol | Pheol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-M.; Tang, X.-L.; Sun, Y.-T.; Song, D.-Y.; Cheng, Y.-J.; Liu, H.; Li, P.-L.; Li, G.-Q. Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017. Molecules 2020, 25, 853. https://doi.org/10.3390/molecules25040853
Cheng M-M, Tang X-L, Sun Y-T, Song D-Y, Cheng Y-J, Liu H, Li P-L, Li G-Q. Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017. Molecules. 2020; 25(4):853. https://doi.org/10.3390/molecules25040853
Chicago/Turabian StyleCheng, Mei-Mei, Xu-Li Tang, Yan-Ting Sun, Dong-Yang Song, Yu-Jing Cheng, Hui Liu, Ping-Lin Li, and Guo-Qiang Li. 2020. "Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017" Molecules 25, no. 4: 853. https://doi.org/10.3390/molecules25040853
APA StyleCheng, M.-M., Tang, X.-L., Sun, Y.-T., Song, D.-Y., Cheng, Y.-J., Liu, H., Li, P.-L., & Li, G.-Q. (2020). Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017. Molecules, 25(4), 853. https://doi.org/10.3390/molecules25040853







