Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites
Abstract
1. Introduction
2. Results
2.1. X-ray Fluorescence
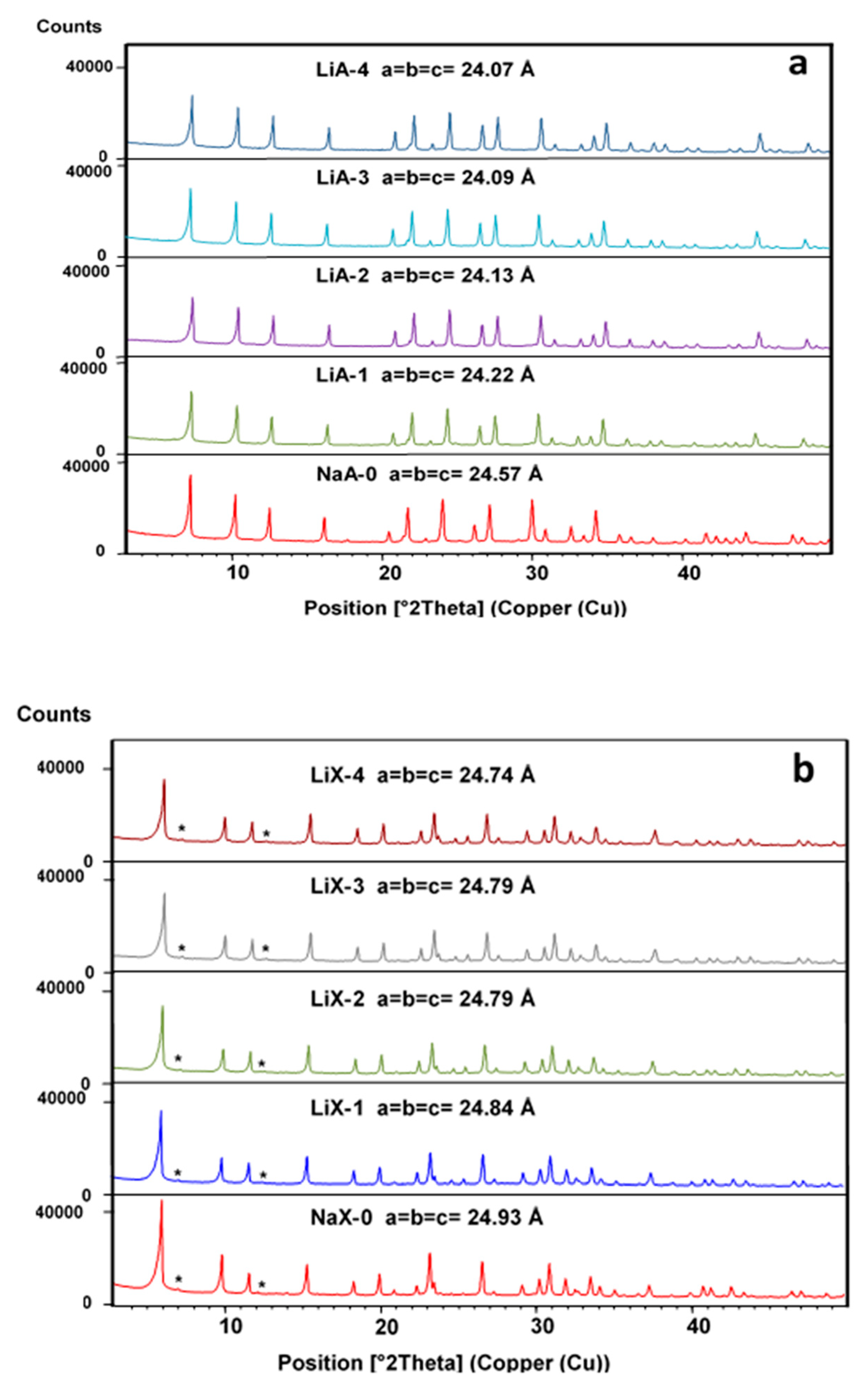
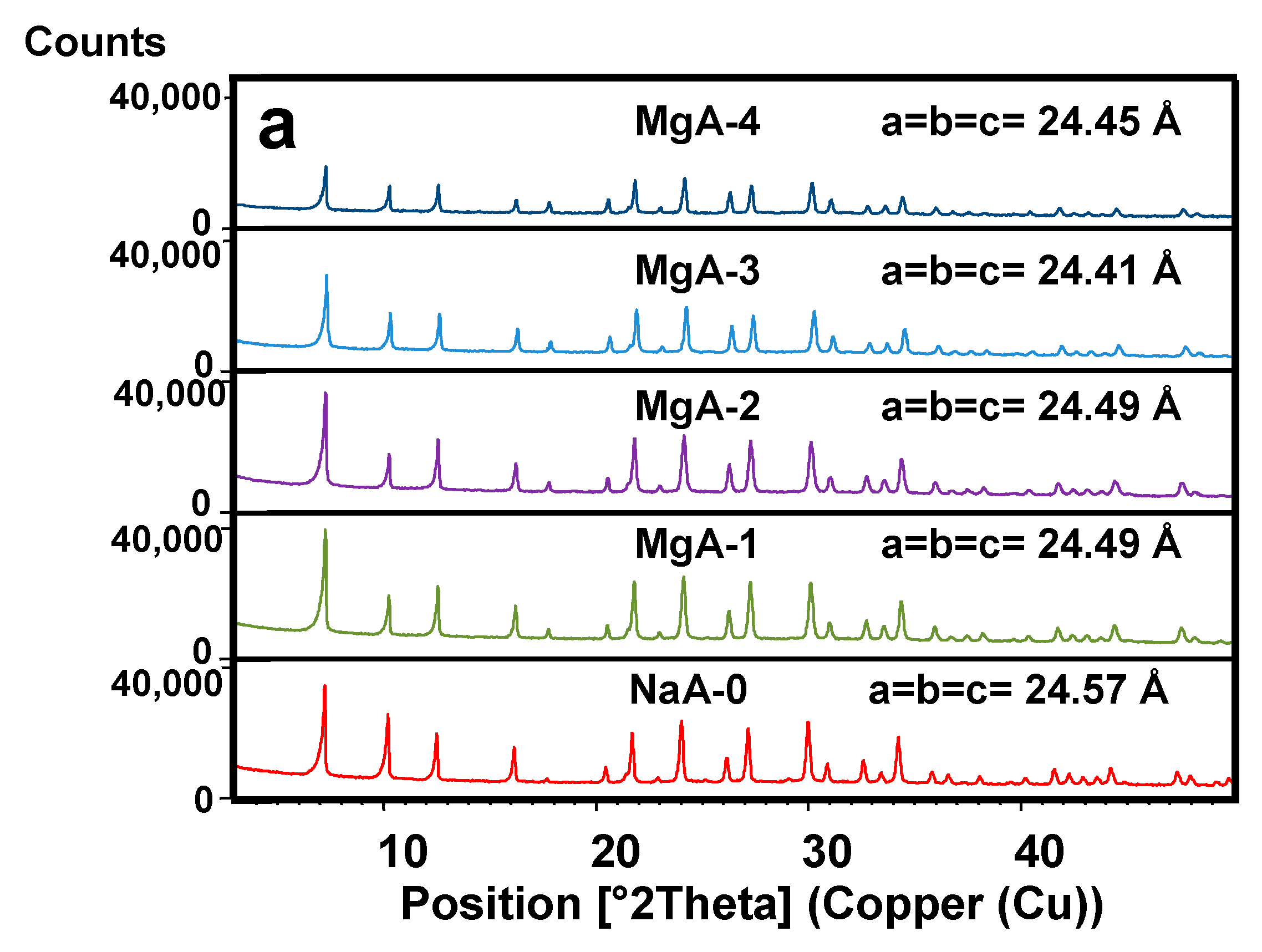
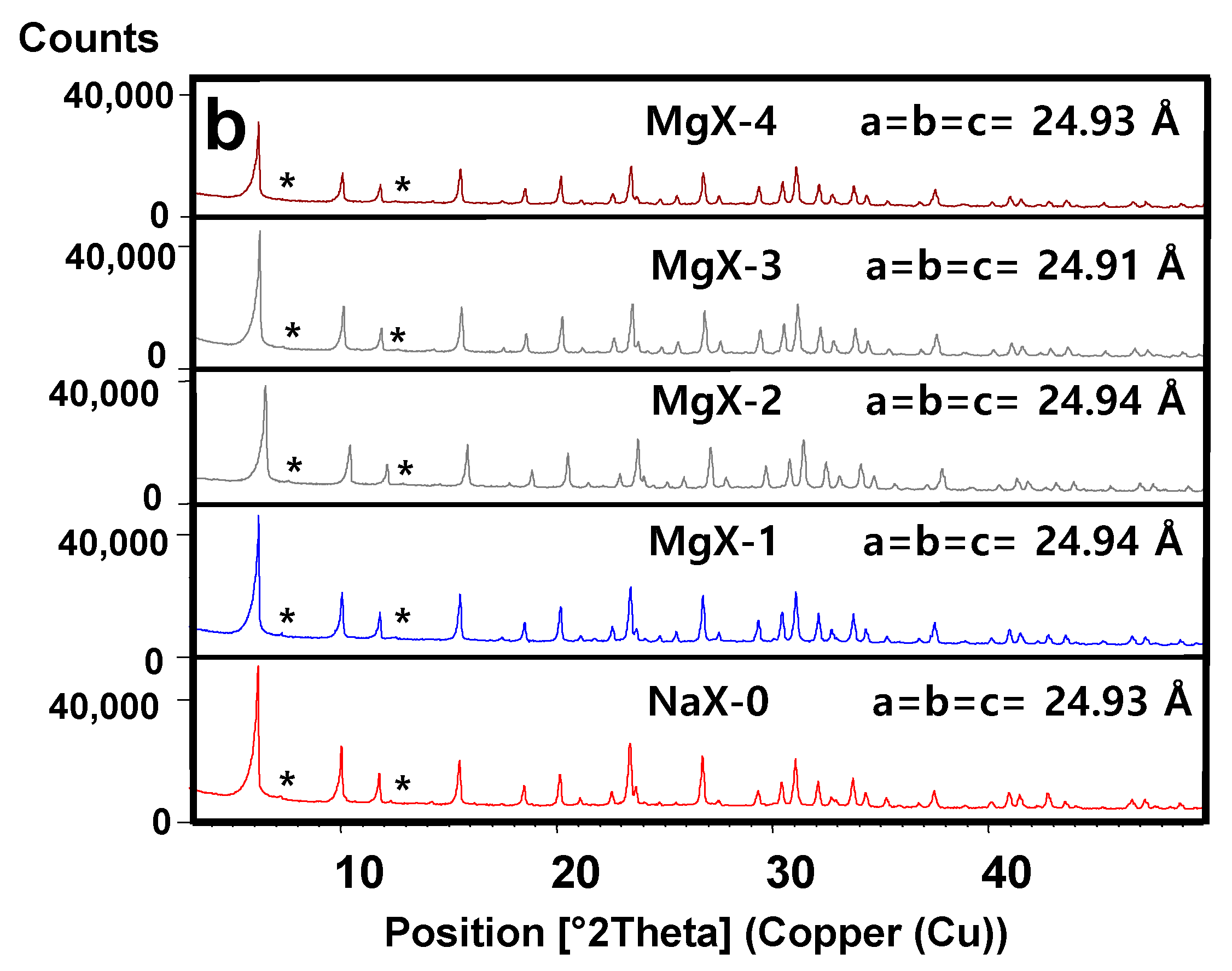
2.2. X-ray Diffraction
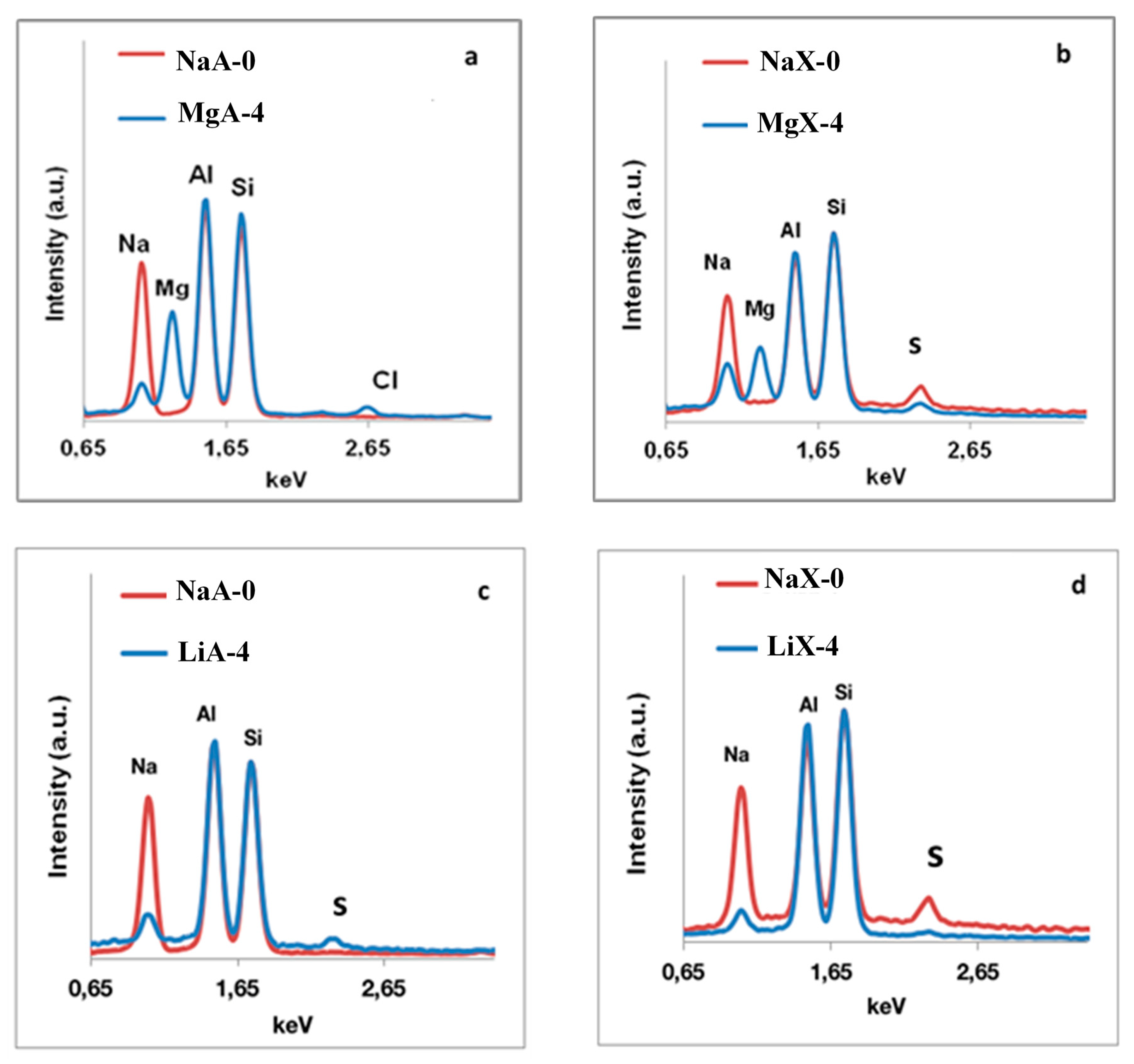
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-rays Spectroscopy (EDX) Characterization
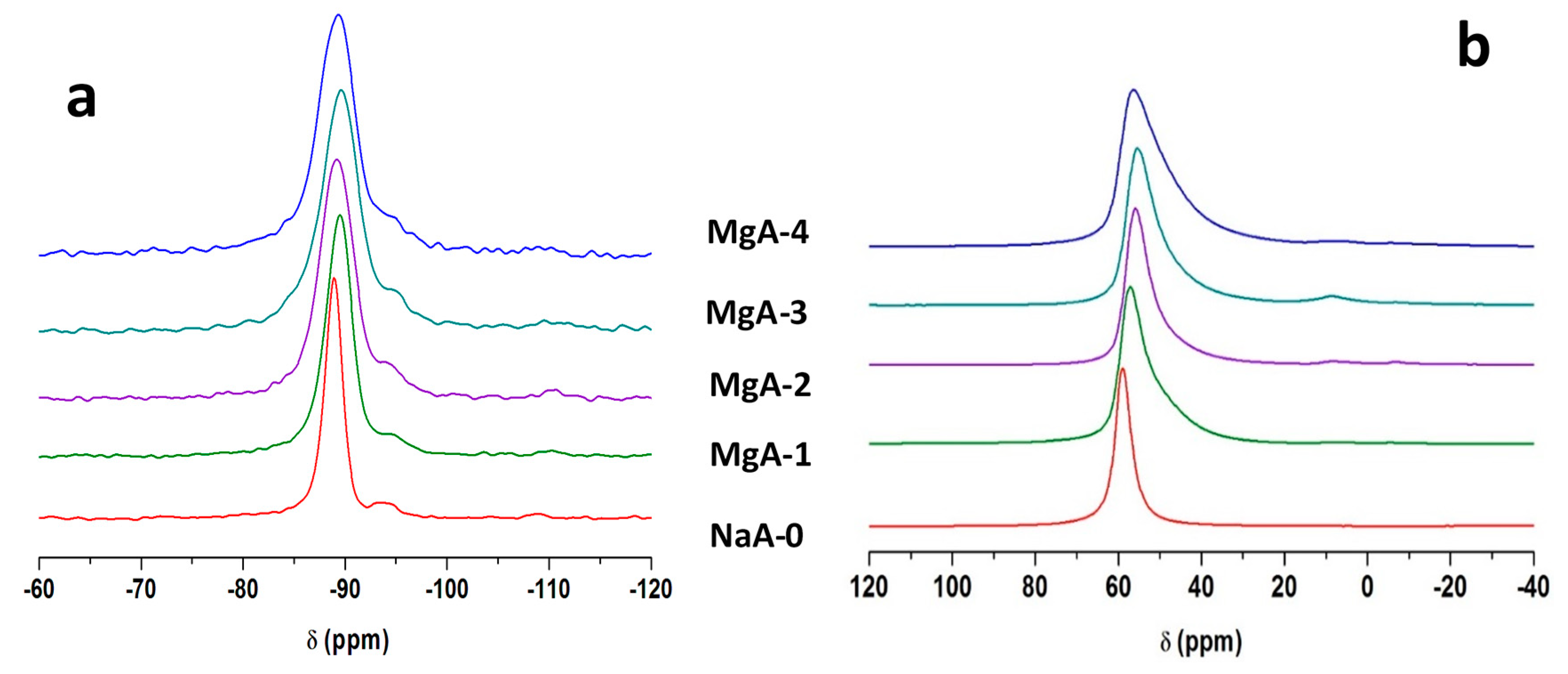
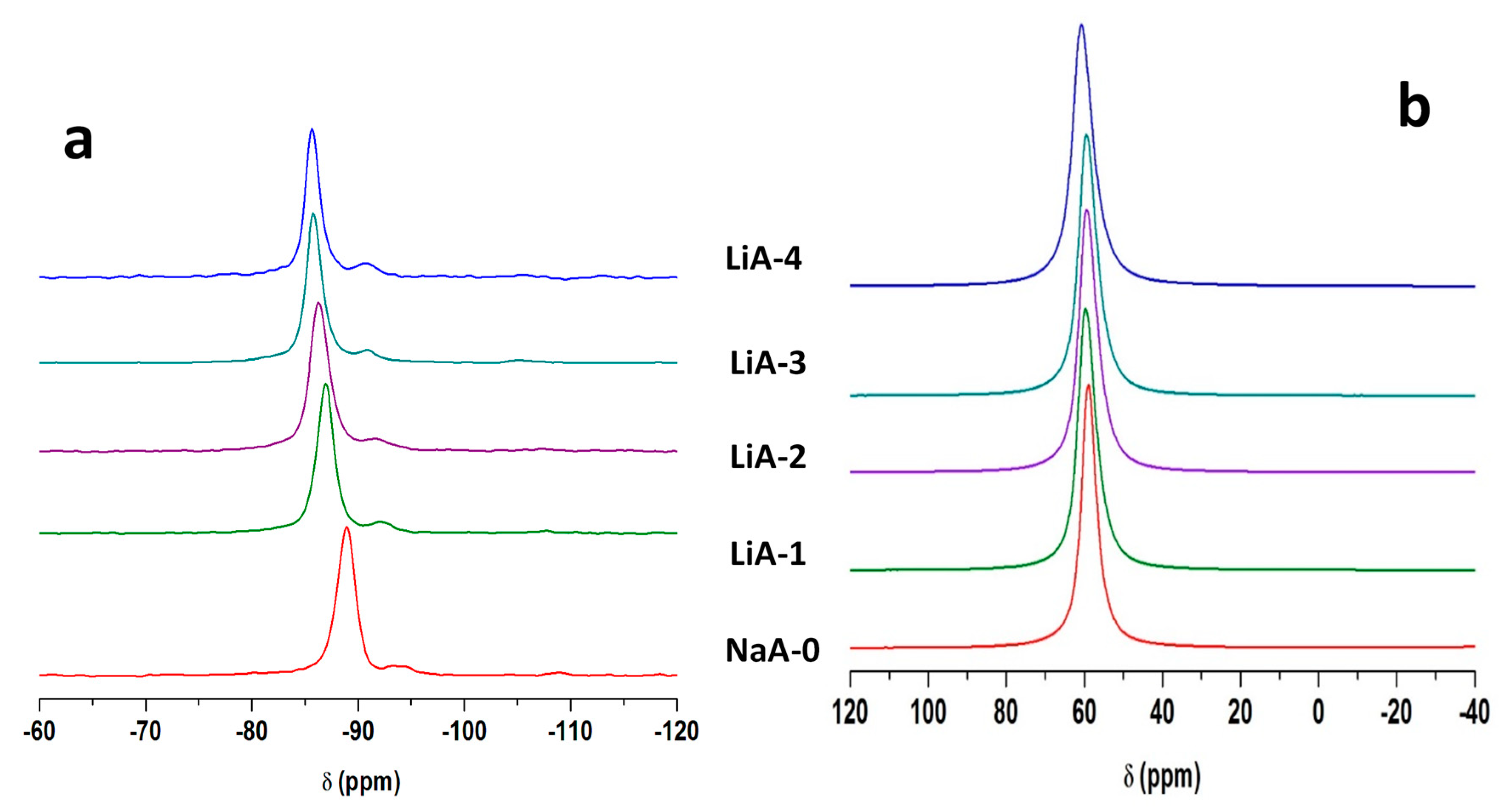
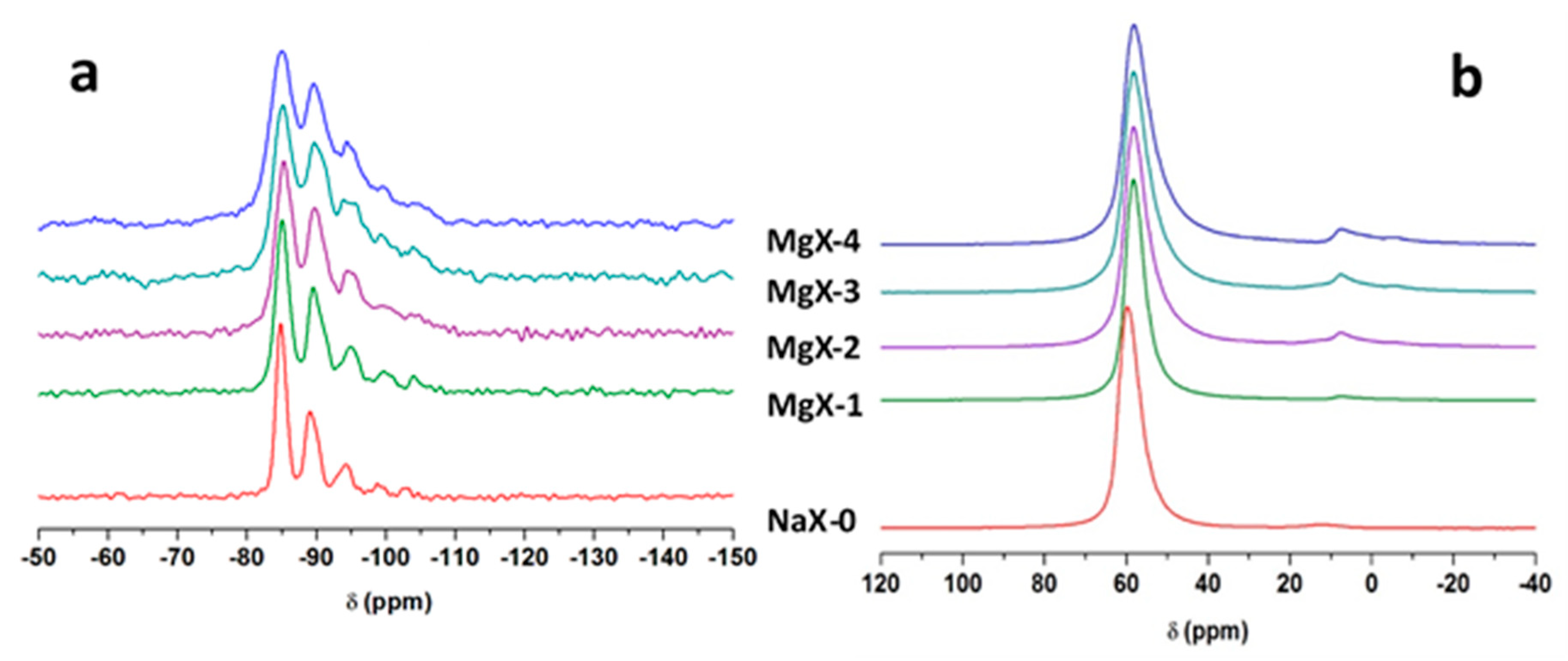
2.4. Solid-State Nuclear Magnetic Resonance (NMR)
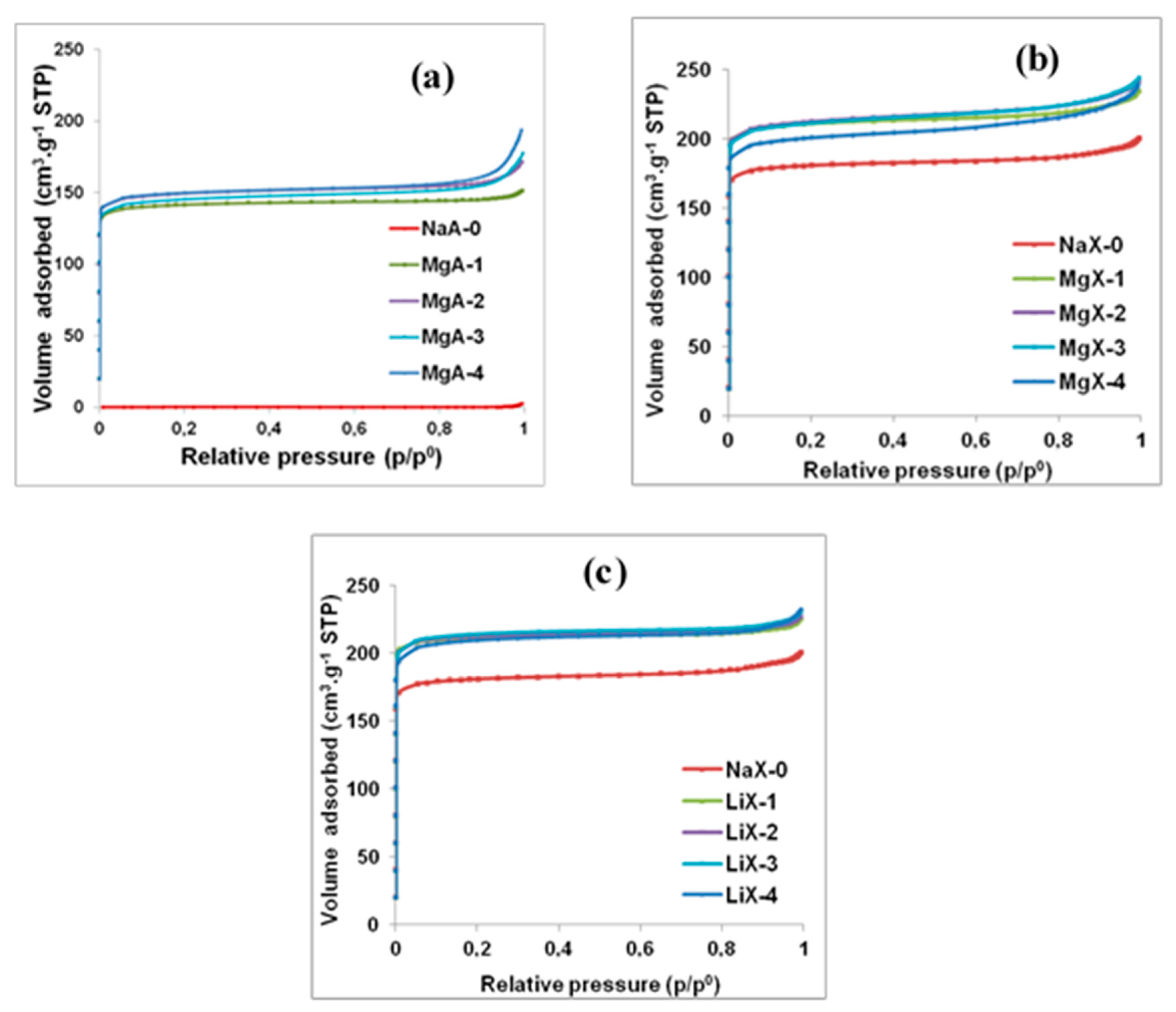
2.5. N2 Adsorption-Desorption Isotherms Characterization
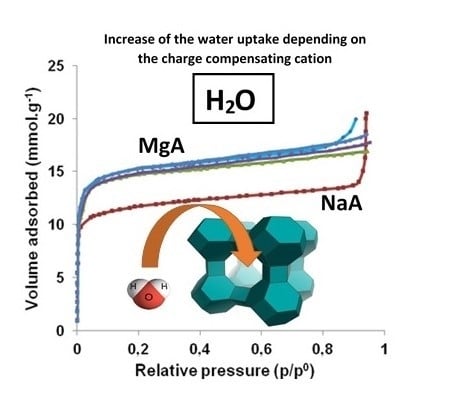
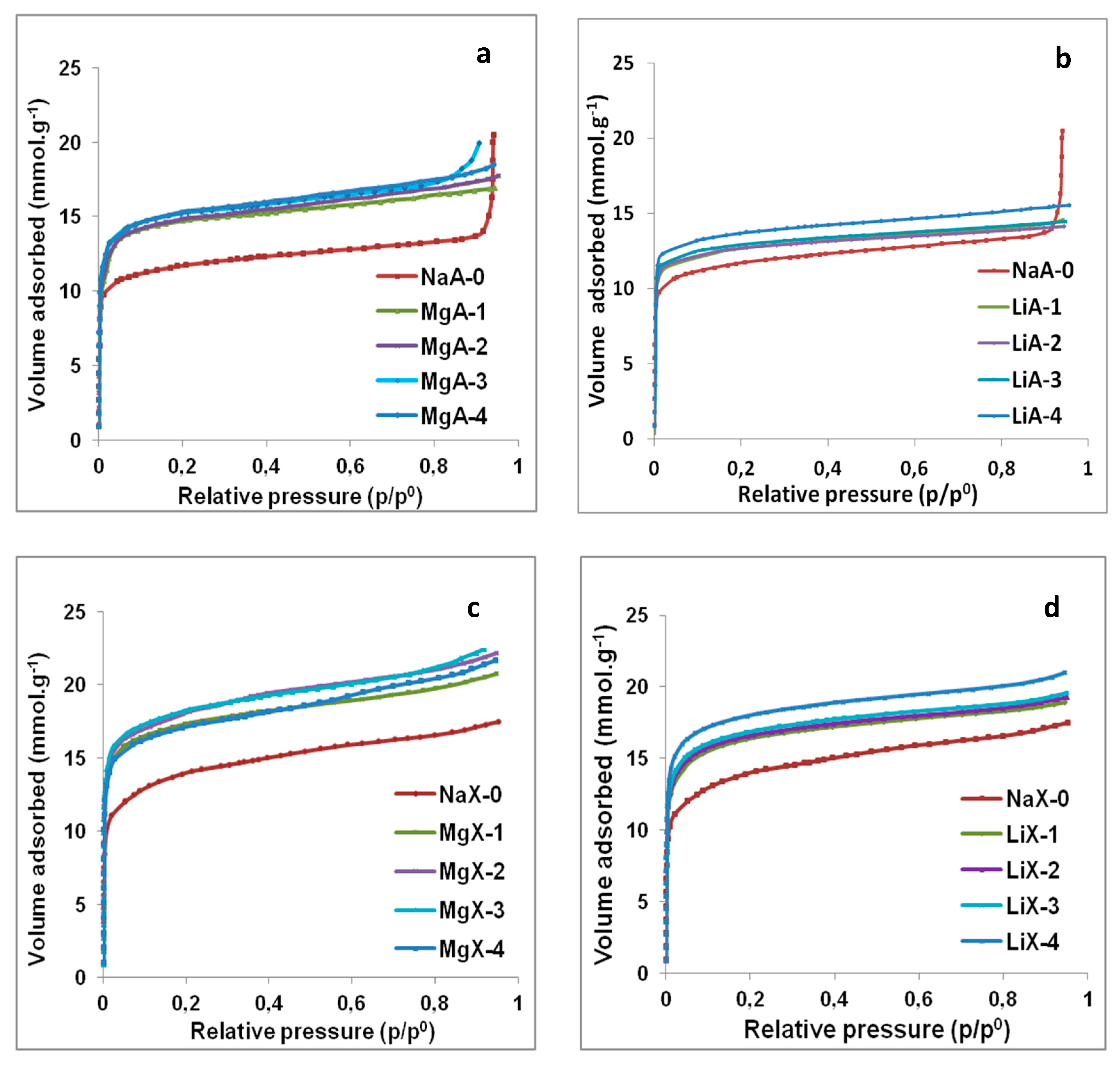
2.6. H2O Adsorption Isotherms Characterization
3. Materials and Methods
3.1. Raw Materials
3.2. Cation Exchange
3.3. Characterization Techniques
3.3.1. X-ray Fluorescence (XRF)
3.3.2. Inductive Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
3.3.3. X-ray Diffraction (XRD)
3.3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-rays Spectroscopy (EDX)
3.3.5. Solid-State Nuclear Magnetic Resonance (Solid-State NMR)
3.3.6. N2 Adsorption-Desorption Measurements
3.3.7. Water Adsorption Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Douss, N.; Meunier, F.E.; Sun, L.M. Predictive model and experimental results for a two-adsorber solid adsorption heat pump. Ind. Eng. Chem. Res. 1988, 27, 310–316. [Google Scholar] [CrossRef]
- Restuccia, G.; Recupero, V.; Cacciola, G.; Rothmeyer, M. Zeolite heat pump for domestic heating. Energy 1988, 13, 333–342. [Google Scholar] [CrossRef]
- Netusil, M.; Ditl, P. Comparison of three methods for natural gas dehydration. J. Nat. Gas Chem. 2011, 20, 471–476. [Google Scholar] [CrossRef]
- Ghiasi, M.M.; Esmaeili-Jaghdan, Z.; Halali, M.A.; Lee, M.; Abbas, A.; Bahadori, A. Development of soft computing methods to predict moisture content of natural gases. J. Taiwan Inst. Chem. E 2015, 55, 36–41. [Google Scholar] [CrossRef]
- Sijbesma, H.; Nymeijer, K.; van Marwijk, R.; Heijboer, R.; Potreck, J.; Wessling, M. Flue gas dehydration using polymer membranes. J. Membr. Sci. 2008, 313, 263–276. [Google Scholar] [CrossRef]
- Karimi, A.; Abdi, M.A. Selective dehydration of high-pressure natural gas using supersonic nozzles. Chem. Eng. Process. 2009, 48, 560–568. [Google Scholar] [CrossRef]
- Farag, H.A.A.; Ezzat, M.M.; Amer, H.; Nashed, A.W. Natural gas dehydration by desiccant materials. Alex. Eng. J. 2011, 50, 431–439. [Google Scholar] [CrossRef]
- Gandhidasan, P.; Al-Farayedhi, A.A.; Al-Mubarak, A.A. Dehydration of natural gas using solid desiccants. Energy 2001, 26, 855–868. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, H.; Hendrix, M.M.R.M.; Lousberg, N.J.H.G.M.; de With, G.; Esteves, A.C.C.; Xin, J.H. Temperature-Triggered Collection and Release of Water from Fogs by a Sponge-Like Cotton Fabric. Adv. Mater. 2013, 25, 1150–1154. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.H.; Kim, W.S.; Lee, J.S.; Kim, J.T.; Suh, J.K.; Lee, J.M. Adsorption Equilibria of Water Vapor on Alumina, Zeolite 13X, and a Zeolite X/Activated Carbon Composite. J. Chem. Eng. Data 2003, 48, 137–141. [Google Scholar] [CrossRef]
- Huggahalli, M.; Fair, J.R. Prediction of Equilibrium Adsorption of Water onto Activated Carbon. Ind. Eng. Chem. Res. 1996, 35, 2071–2074. [Google Scholar] [CrossRef]
- Ryu, Y.K.; Lee, S.J.; Kim, J.W.; Leef, C.H. Adsorption equilibrium and kinetics of H2O on zeolite 13×. Korean J. Chem. Eng. 2001, 18, 525–530. [Google Scholar] [CrossRef]
- Ng, K.C. Recent Developments in Heat-Driven Silica Gel-Water Adsorption Chillers. Heat Transf. Eng. 2003, 24, 1–3. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schmidt, F.P.; Henning, H.M. Water adsorption characteristics of novel materials for heat transformation applications. Appl. Therm. Eng. 2010, 30, 1692–1702. [Google Scholar] [CrossRef]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Water and methanol adsorption on MOFs for cycling heat transformation processes. New J. Chem. 2014, 38, 1846–1852. [Google Scholar] [CrossRef]
- Moïse, J.C.; Bellat, J.P.; Méthivier, A. Adsorption of water vapor on X and Y zeolites exchanged with barium. Microporous Mesoporous Mater. 2001, 43, 91–101. [Google Scholar] [CrossRef]
- Bhatia, S. Zeolite Catalysis: Principles and Applications; CRC Press: London, UK, 1990. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Guisnet, M.; Gilson, J.P. Zeolites for Cleaner Technologies; Imperial College Press: London, UK, 2002; Volume 3, p. 388. [Google Scholar]
- Cheetham, A.K.; Day, P. Solid State Chemistry: Compounds; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Wojcik, A.M.W.; Jansen, J.C.; Maschmeyer, T. Regarding pressure in the adsorber of an adsorption heat pump with thin synthesized zeolite layers on heat exchangers. Microporous Mesoporous Mater. 2001, 43, 313–317. [Google Scholar] [CrossRef]
- Llano-Restrepo, M.; Mosquera, M.A. Accurate correlation, thermochemistry, and structural interpretation of equilibrium adsorption isotherms of water vapor in zeolite 3A by means of a generalized statistical thermodynamic adsorption model. Fluid Phase Equilib. 2009, 283, 73–88. [Google Scholar] [CrossRef]
- Hauer, A. Evaluation of adsorbent materials for heat pump and thermal energy storage applications in open systems. Adsorption 2007, 13, 399–405. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, Q.L.; Xiong, Y.; Zhu, A.M.; Chen, Y. Molecular Simulation of Water/Alcohol Mixtures’ Adsorption and Diffusion in Zeolite 4A Membranes. J. Phys. Chem. B 2009, 113, 4267–4274. [Google Scholar] [CrossRef]
- Benaliouche, F.; Hidous, N.; Guerza, M.; Zouad, Y.; Boucheffa, Y. Characterization and water adsorption properties of Ag- and Zn-exchanged A zeolites. Microporous Mesoporous Mater. 2015, 209, 184–188. [Google Scholar] [CrossRef]
- Kim, K.M.; Oh, H.T.; Lim, S.J.; Ho, K.; Park, Y.; Lee, C.H. Adsorption Equilibria of Water Vapor on Zeolite 3A, Zeolite 13X, and Dealuminated Y Zeolite. J. Chem. Eng. Data 2016, 61, 1547–1554. [Google Scholar] [CrossRef]
- Wang, Y.; LeVan, M.D. Adsorption Equilibrium of Carbon Dioxide and Water Vapor on Zeolites 5A and 13X and Silica Gel: Pure Components. J. Chem. Eng. Data 2009, 54, 2839–2844. [Google Scholar] [CrossRef]
- Stach, H.; Mugele, J.; Jänchen, J.; Weiler, E. Influence of Cycle Temperatures on the Thermochemical Heat Storage Densities in the Systems Water/Microporous and Water/Mesoporous Adsorbents. Adsorption 2005, 11, 393–404. [Google Scholar] [CrossRef]
- Ghodhbene, M.; Bougie, F.; Fongarland, P.; Iliuta Maria, C. Hydrophilic zeolite sorbents for In-situ water removal in high temperature processes. Can. J. Chem. Eng. 2017, 95, 1842–1849. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.S.; Wang, R.Z. Recent progress on desiccant materials for solid desiccant cooling systems. Energy 2014, 74, 280–294. [Google Scholar] [CrossRef]
- Bergerhoff, G.; Baur, W.H.N.; Nowacki, W. Uber die Kristallstrukturen des Faujasits. Neues Jahrb Min. Monat. 1958, 193–200. [Google Scholar]
- Xu, R. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons (Asia): Singapore, 2007. [Google Scholar]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Tounsi, H.; Mseddi, S.; Djemel, S. Preparation and characterization of Na-LTA zeolite from Tunisian sand and aluminum scrap. Phys. Procedia 2009, 2, 1065–1074. [Google Scholar] [CrossRef]
- Alby, D.; Salles, F.; Fullenwarth, J.; Zajac, J. On the use of metal cation-exchanged zeolites in sorption thermochemical storage: Some practical aspects in reference to the mechanism of water vapor adsorption. Sol. Energy Mater. Sol. C 2018, 179, 223–230. [Google Scholar] [CrossRef]
- Bae, T.H.; Liu, J.; Thompson, J.A.; Koros, W.J.; Jones, C.W.; Nair, S. Solvothermal deposition and characterization of magnesium hydroxide nanostructures on zeolite crystals. Microporous Mesoporous Mater. 2011, 139, 120–129. [Google Scholar] [CrossRef]
- Koh, P.Y.; Yan, J.; Ward, J.; Koros, W.J.; Teja, A.S.; Xu, B. Synthesis, deposition and characterization of magnesium hydroxide nanostructures on zeolite 4A. Mater. Res. Bull. 2011, 46, 390–397. [Google Scholar] [CrossRef]
- Koh, P.Y. Deposition and Assembly of Magnesium Hydroxide Nanostructures on Zeolite 4A Surfaces. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2010. [Google Scholar]
- Sebastian, J.; Pillai Renjith, S.; Peter, S.A.; Jasra, R.V. Sorption of N2, O2, and Ar in Mn(II)-Exchanged Zeolites A and X Using Volumetric Measurements and Grand Canonical Monte Carlo Simulation. Ind. Eng. Chem. Res. 2007, 46, 6293–6302. [Google Scholar] [CrossRef]
- Perez-Carbajo, J.; Balestra, S.R.G.; Calero, S.; Merkling, P.J. Effect of lattice shrinking on the migration of water within zeolite LTA. Microporous Mesoporous Mater. 2019, 109808. [Google Scholar] [CrossRef]
- Inamuddin; Luqman, M. Synthetic Inorganic Ion Exchange Materials. In Ion Exchange Technology I: Theory and Materials; Luqman, M., Ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- S.C. STOE GmbH. Win XPOW; S.C. STOE GmbH: Darmstadt, Germany, 2006. [Google Scholar]
- Werner, P.E.; Eriksson, L.; Westdahl, M. TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Structure of LTA-type and FAU-Type Zeolites. Available online: http://www.iza-online.org/ (accessed on 24 January 2018).
- Marcus, Y. A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys. Chem. 1994, 51, 111–127. [Google Scholar] [CrossRef]
- Henao-Sierra, W.; Romero-Sáez, M.; Gracia, F.; Cacua, K.; Buitrago-Sierra, R. Water vapor adsorption performance of Ag and Ni modified 5A zeolite. Microporous Mesoporous Mater. 2018, 265, 250–257. [Google Scholar] [CrossRef]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Synthesis of 4A Zeolite and Characterization of Calcium- and Silver-Exchanged Forms. J. Miner. Mater. Charact. Eng. 2017, 5, 237–251. [Google Scholar] [CrossRef]
- Price, L.; Leung, K.M.; Sartbaeva, A. Local and Average Structural Changes in Zeolite A upon Ion Exchange. Magnetochemistry 2017, 3, 42. [Google Scholar] [CrossRef]
- Chandwadkar, A.J.; Chandwadkar, J.G.; Kulkarni, S.B. The influence of the size and concentration of alkaline earth ions on the structural and sorption properties of faujasites. J. Colloid Interface Sci. 1984, 97, 435–445. [Google Scholar] [CrossRef]
- Engelhardt, G.; Fahlke, B.; Mägi, M.; Lippmaa, E. High-resolution solid-state 29Si and 27Al n.m.r. of aluminosilicate intermediates in the synthesis of zeolite A. Part II. Zeolites 1985, 5, 49–52. [Google Scholar] [CrossRef]
- Yang, H.; Walton, R.I.; Antonijevic, S.; Wimperis, S.; Hannon, A.C. Local Order of Amorphous Zeolite Precursors from 29Si{H} CPMAS and 27Al and 23Na MQMAS NMR and Evidence for the Nature of Medium-Range Order from Neutron Diffraction. J. Phys. Chem. B 2004, 108, 8208–8217. [Google Scholar] [CrossRef]
- Magi, M.; Lippmaa, E.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Solid-state high-resolution silicon-29 chemical shifts in silicates. J. Phys. Chem. 1984, 88, 1518–1522. [Google Scholar] [CrossRef]
- Shang, Y.; Wu, J.; Zhu, J.; Wang, Y.; Meng, C. Study on adsorption of N2 and O2 by magnesium (II)-exchanged zeolite A. J. Alloys Compd. 2009, 478, L5–L7. [Google Scholar] [CrossRef]
- Pillai, R.S.; Sebastian, J.; Jasra, R.V. Grand canonical Monte Carlo simulation and volumetric equilibrium studies for adsorption of nitrogen, oxygen, and argon in cadmium (II) exchanged zeolite A. J. Porous Mater. 2012, 19, 683–693. [Google Scholar] [CrossRef]
- García, E.J.; Pérez-Pellitero, J.A.; Pirngruber, G.D.; Jallut, C.; Palomino, M.; Rey, F.; Valencia, S. Tuning the Adsorption Properties of Zeolites as Adsorbents for CO2 Separation: Best Compromise between the Working Capacity and Selectivity. Ind. Eng. Chem. Res. 2014, 53, 9860–9874. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Calero, S.; Dubbeldam, D.; Krishna, R.; Smit, B.; Vlugt, T.J.H.; Denayer, J.F.M.; Martens, J.A.; Maesen, T.L.M. Understanding the Role of Sodium during Adsorption: A Force Field for Alkanes in Sodium-Exchanged Faujasites. J. Am. Chem. Soc. 2004, 126, 11377–11386. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Dubbeldam, D.; Calero, S. Modeling Adsorption and Self-Diffusion of Methane in LTA Zeolites: The Influence of Framework Flexibility. J. Phys. Chem. C 2010, 114, 15068–15074. [Google Scholar] [CrossRef]
- Martra, G.; Damilano, N.; Coluccia, S.; Tsuji, H.; Hattori, H. Cationic mobility in MgX zeolite: An FTIR study. J. Chem. Soc., Faraday Trans. 1995, 91, 2961–2964. [Google Scholar] [CrossRef]
- Frising, T.; Leflaive, P. Extraframework cation distributions in X and Y faujasite zeolites: A review. Microporous Mesoporous Mater. 2008, 114, 27–63. [Google Scholar] [CrossRef]
- Hribar, B.; Southall, N.T.; Vlachy, V.; Dill, K.A. How ions affect the structure of water. J. Am. Chem. Soc. 2002, 124, 12302–12311. [Google Scholar] [CrossRef]
- Crupi, V.; Majolino, D.; Migliardo, P.; Venuti, V.; Mizota, T. Vibrational and diffusional dynamics of water in Mg50-A zeolites by spectroscopic investigation. Mol. Phys. 2004, 102, 1943–1957. [Google Scholar] [CrossRef]
- Crupi, V.; Majolino, D.; Longo, F.; Migliardo, P.; Venuti, V. FTIR/ATR study of water encapsulated in Na-A and Mg-exchanged A-zeolites. Vib. Spectrosc. 2006, 42, 375–380. [Google Scholar] [CrossRef]
Sample Availability: Samples presented in this paper are available. |





| Samples Exchanged with Magnesium | Molar Ratio 1,2 | Global Charge Ratio | |||
|---|---|---|---|---|---|
| Si/Al | Na/Al | Mg/Al | Cl/Al | (Na + 2 Mg)/Al | |
| NaA-0 | 0.97 | 1.07 | 0 | 0 | 1.07 |
| MgA-1 | 0.97 | 0.44 | 0.34 | 0.08 | 1.12 |
| MgA-2 | 1.04 | 0.36 | 0.42 | 0.07 | 1.20 |
| MgA-3 | 1.06 | 0.22 | 0.49 | 0.07 | 1.20 |
| MgA-4 | 1.03 | 0.15 | 0.50 | 0.06 | 1.15 |
| NaX-0 | 1.23 | 1.06 | 0 | 0 | 1.06 |
| MgX-1 | 1.23 | 0.42 | 0.32 | 0 | 1.06 |
| MgX-2 | 1.33 | 0.36 | 0.40 | 0 | 1.16 |
| MgX-3 | 1.34 | 0.26 | 0.48 | 0 | 1.22 |
| MgX-4 | 1.29 | 0.20 | 0.48 | 0 | 1.16 |
| Samples Exchanged with Lithium | Molar Ratio 1 | Global Charge Ratio | ||
|---|---|---|---|---|
| Si/Al | Na/Al | Li/Al | (Na + Li)/Al | |
| NaA-0 | 1.04 | 1.07 | 0 | 1.07 |
| LiA-1 | 1.11 | 0.41 | 0.37 | 0.78 |
| LiA-2 | 1.14 | 0.15 | 0.60 | 0.75 |
| LiA-3 | 1.07 | 0.13 | 0.76 | 0.89 |
| LiA-4 | 1.00 | 0.12 | 0.93 | 1.05 |
| NaX-0 | 1.25 | 0.95 | 0 | 0.95 |
| LiX-1 | 1.36 | 0.34 | 0.41 | 0.75 |
| LiX-2 | 1.33 | 0.16 | 0.71 | 0.87 |
| LiX-3 | 1.32 | 0.14 | 0.75 | 0.89 |
| LiX-4 | 1.27 | 0.11 | 0.84 | 0.95 |
| Samples | Q4 Si(Al)4 (%) | Q4 Si(Al)3 (%) | Q4 Si(Al)2 (%) | Q4 Si(Al)1 (%) | Q4 Si(Al)0 (%) | Si/Al 1 |
|---|---|---|---|---|---|---|
| NaX-0 | 50 | 30 | 14 | 3 | 3 | 1.25 |
| MgX-1 | 43 | 38 | 8 | 8 | 3 | 1.29 |
| MgX-2 | 37 | 38 | 16 | 6 | 3 | 1.28 |
| MgX-3 | 43 | 30 | 13 | 7 | 7 | 1.36 |
| MgX-4 | 49 | 22 | 18 | 6 | 5 | 1.27 |
| Samples | Q4 Si(Al)4 (%) | Q4 Si(Al)3 (%) | Q4 Si(Al)2 (%) | Q4 Si(Al)1 (%) | Q4 Si(Al)0 (%) | Si/Al 1 |
|---|---|---|---|---|---|---|
| NaX-0 | 50 | 30 | 14 | 3 | 3 | 1.25 |
| LiX-1 | 47 | 34 | 12 | 5 | 2 | 1.25 |
| LiX-2 | 45 | 36 | 12 | 5 | 2 | 1.26 |
| LiX-3 | 42 | 37 | 14 | 5 | 2 | 1.28 |
| LiX-4 | 48 | 32 | 14 | 3 | 3 | 1.25 |
| Samples | Sodium Cation Exchange Rate (%) | SBET 3 (m2·g−1) | Vm 4 (cm3·g−1) | Water Adsorption Capacity 5 (mmol g−1) | Water Adsorption Capacity 6 (Wt.%) |
|---|---|---|---|---|---|
| NaA-0 | 0 | x | x | 11.7 | 21.1 |
| MgA-1 | 59 1 | 577 | 0.22 | 14.7 | 26.5 |
| MgA-2 | 66 1 | 605 | 0.22 | 14.9 | 26.8 |
| MgA-3 | 79 1 | 583 | 0.21 | 15.2 | 27.4 |
| MgA-4 | 86 1 | 605 | 0.22 | 15.3 | 27.5 |
| NaX-0 | 0 | 738 | 0.27 | 14.0 | 25.3 |
| MgX-1 | 60 1 | 863 | 0.31 | 17.3 | 31.2 |
| MgX-2 | 66 1 | 862 | 0.31 | 18.1 | 32.6 |
| MgX-3 | 75 1 | 854 | 0.31 | 18.2 | 32.8 |
| MgX-4 | 81 1 | 805 | 0.30 | 17.2 | 31.0 |
| LiA-1 | 61 2 | x | x | 12.7 | 22.8 |
| LiA-2 | 86 2 | x | x | 12.7 | 22.9 |
| LiA-3 | 88 2 | x | x | 12.9 | 23.3 |
| LiA-4 | 89 2 | x | x | 13.7 | 24.7 |
| LiX-1 | 64 2 | 877 | 0.33 | 16.4 | 29.5 |
| LiX-2 | 83 2 | 862 | 0.33 | 16.6 | 29.9 |
| LiX-3 | 85 2 | 866 | 0.33 | 16.9 | 30.4 |
| LiX-4 | 88 2 | 841 | 0.32 | 18.0 | 32.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahraoui, Z.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. https://doi.org/10.3390/molecules25040944
Tahraoui Z, Nouali H, Marichal C, Forler P, Klein J, Daou TJ. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules. 2020; 25(4):944. https://doi.org/10.3390/molecules25040944
Chicago/Turabian StyleTahraoui, Zakaria, Habiba Nouali, Claire Marichal, Patrice Forler, Julien Klein, and T. Jean Daou. 2020. "Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites" Molecules 25, no. 4: 944. https://doi.org/10.3390/molecules25040944
APA StyleTahraoui, Z., Nouali, H., Marichal, C., Forler, P., Klein, J., & Daou, T. J. (2020). Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules, 25(4), 944. https://doi.org/10.3390/molecules25040944