High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art
Abstract
1. Introduction
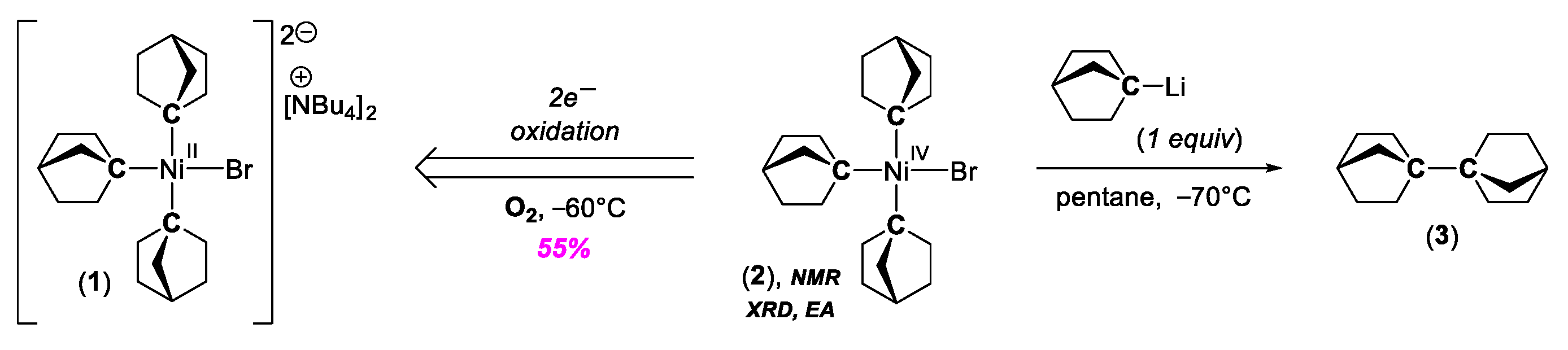
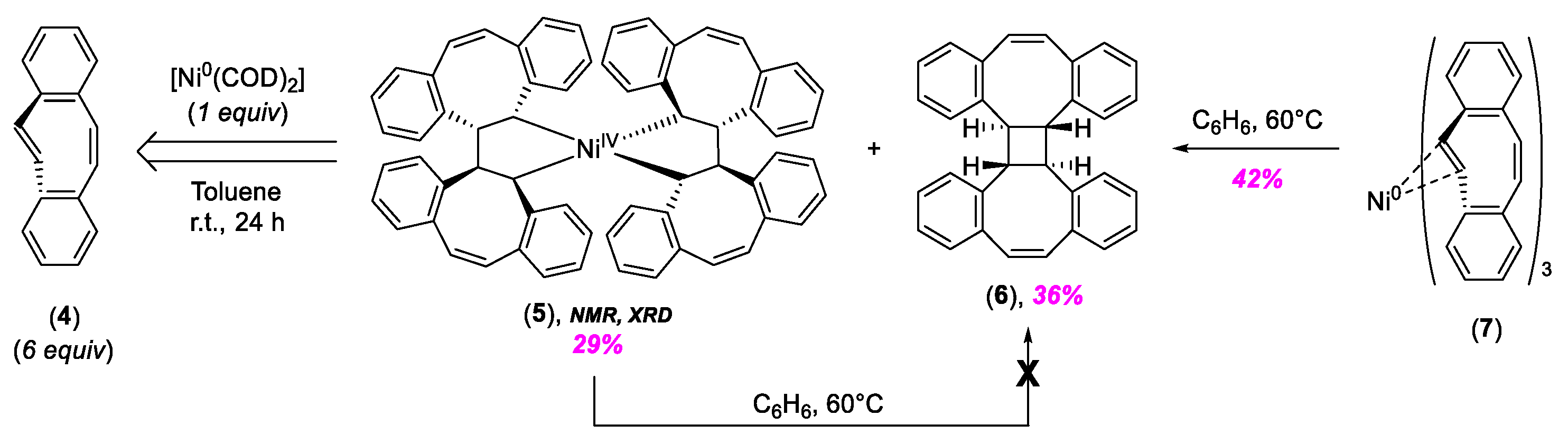
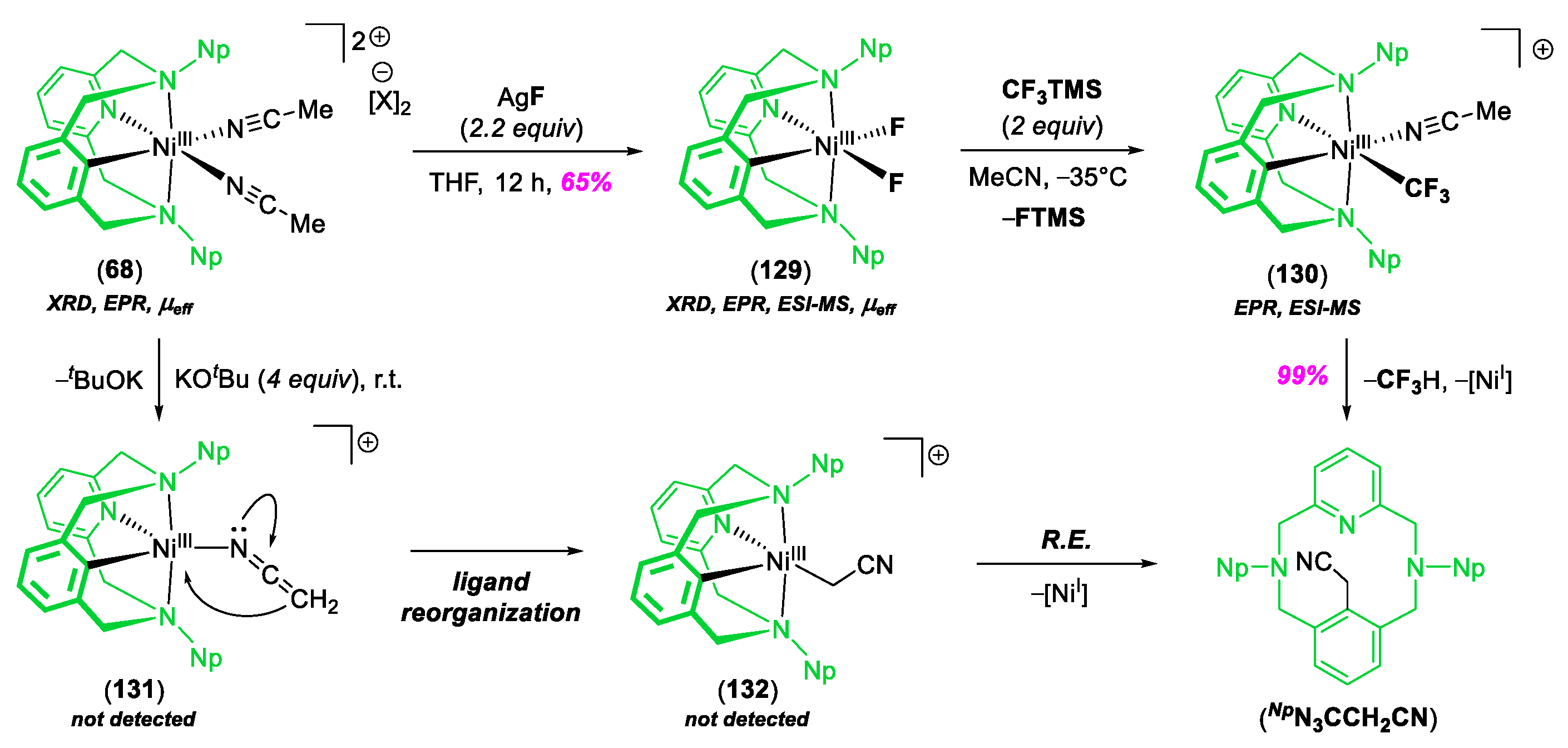
2. C–C Bond Forming Reactions Mediated by High-Valent NiIII and NiIV
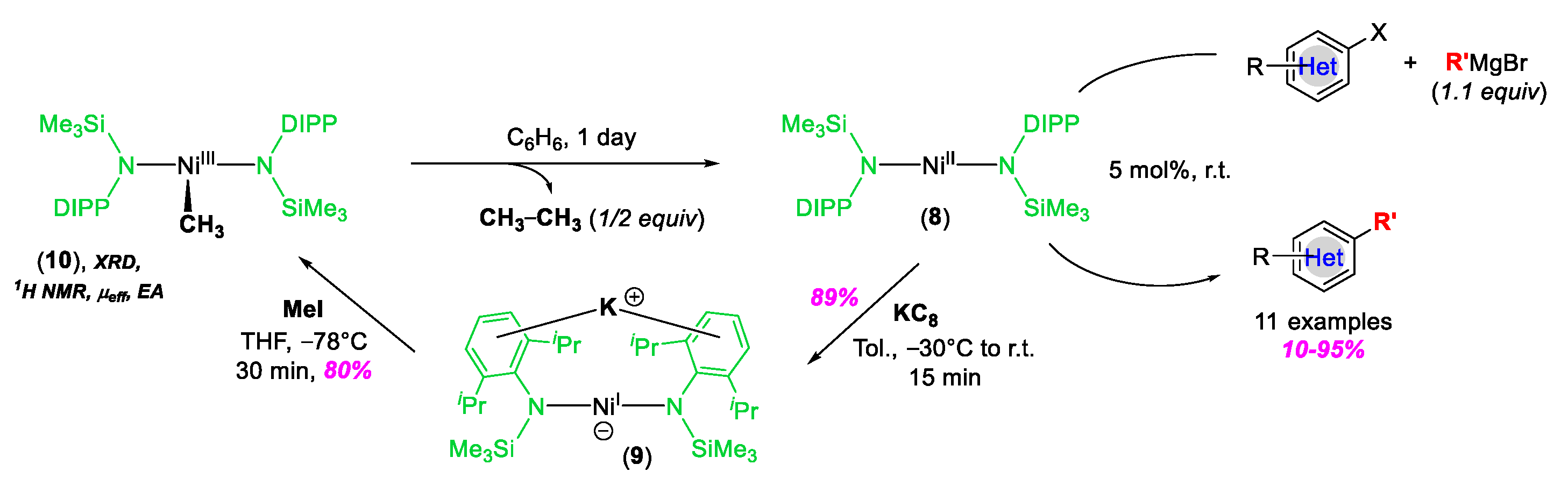
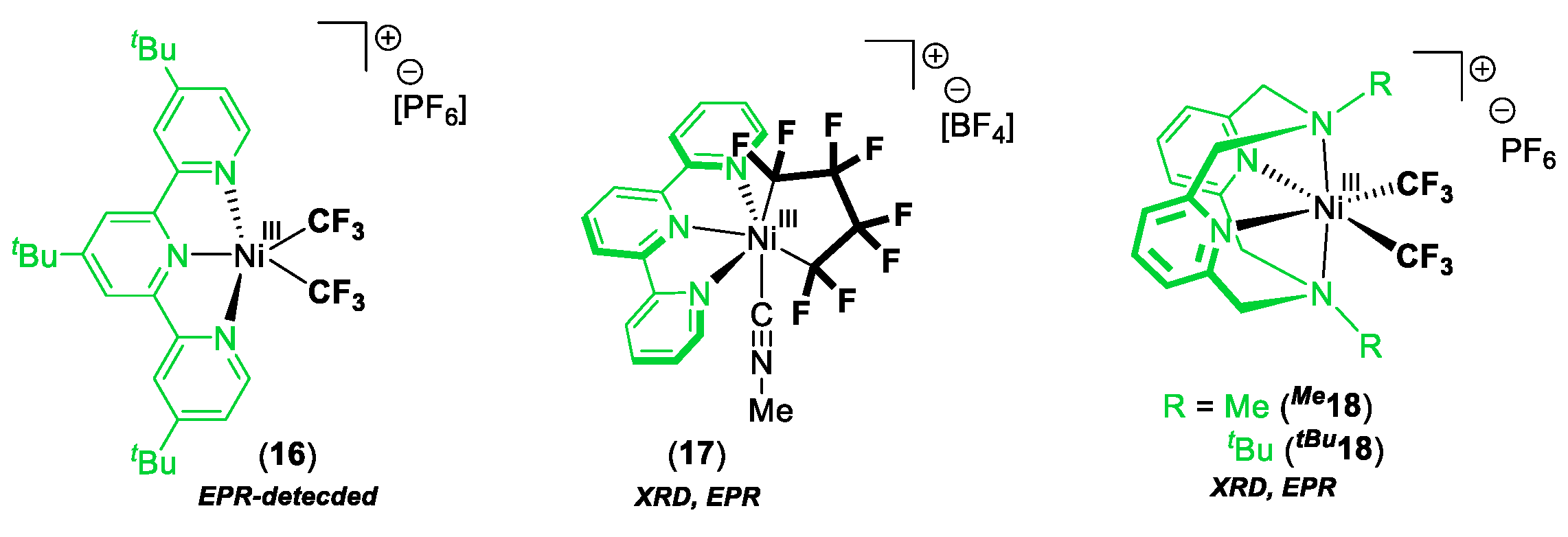
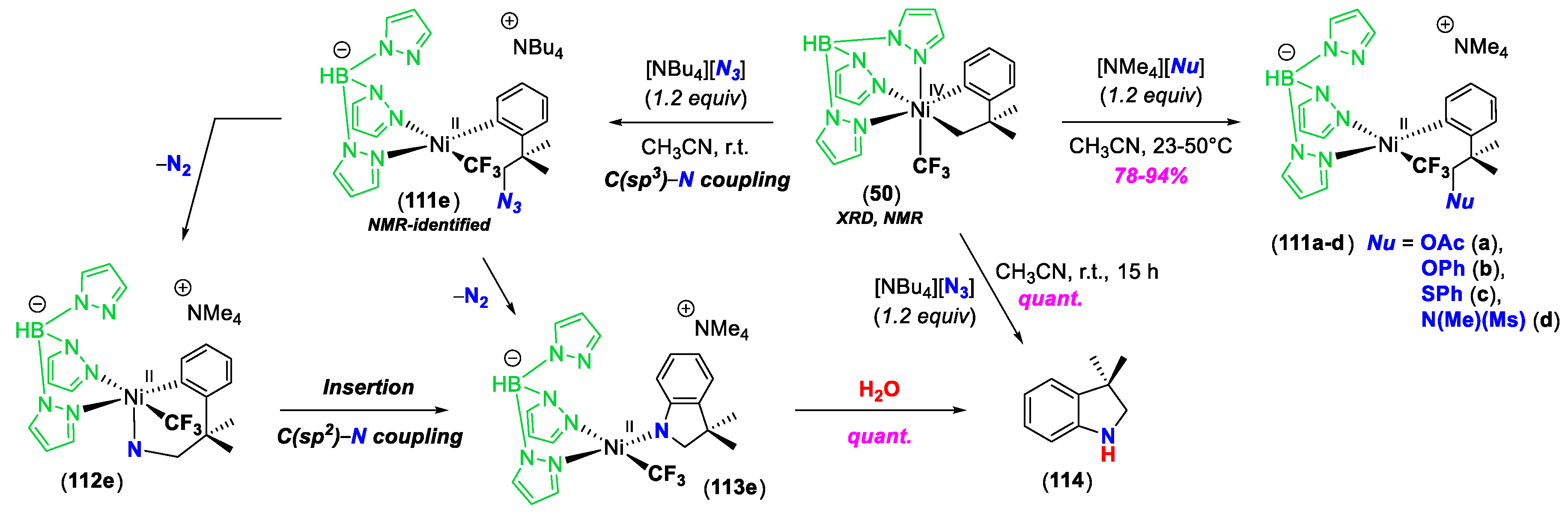
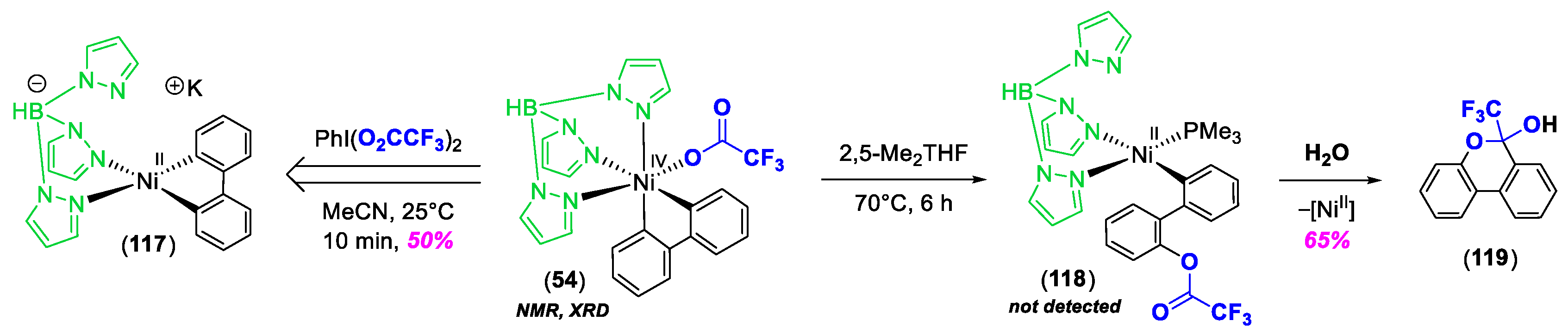
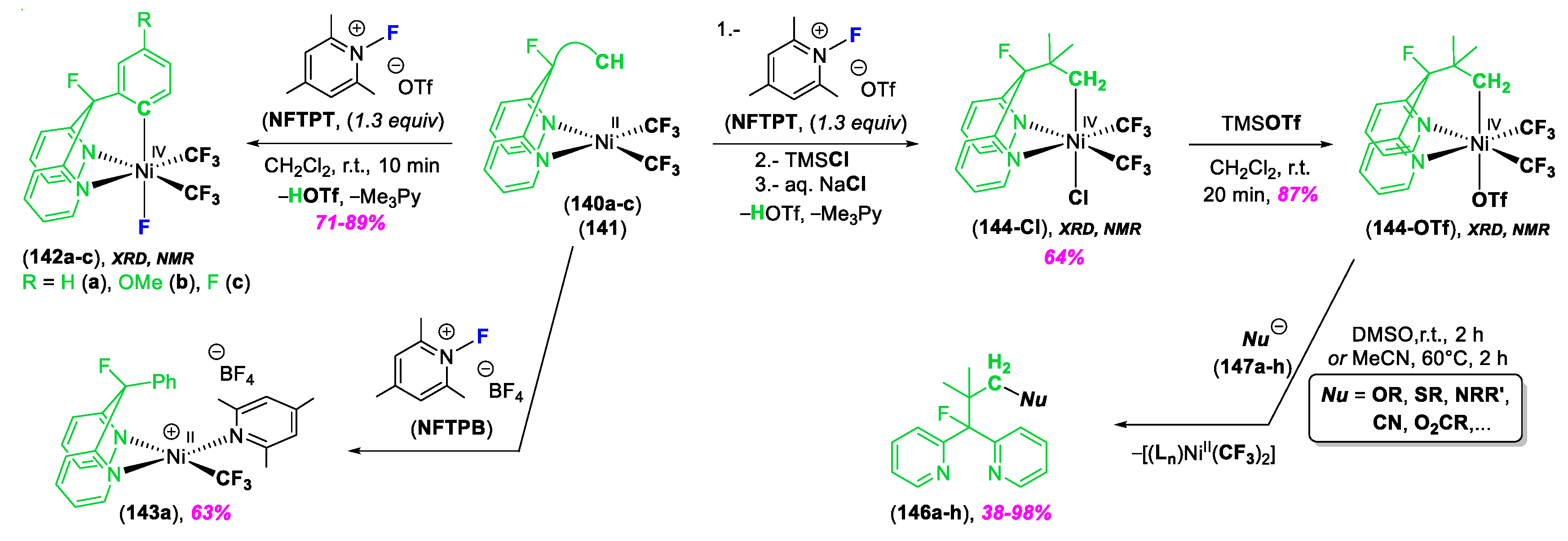
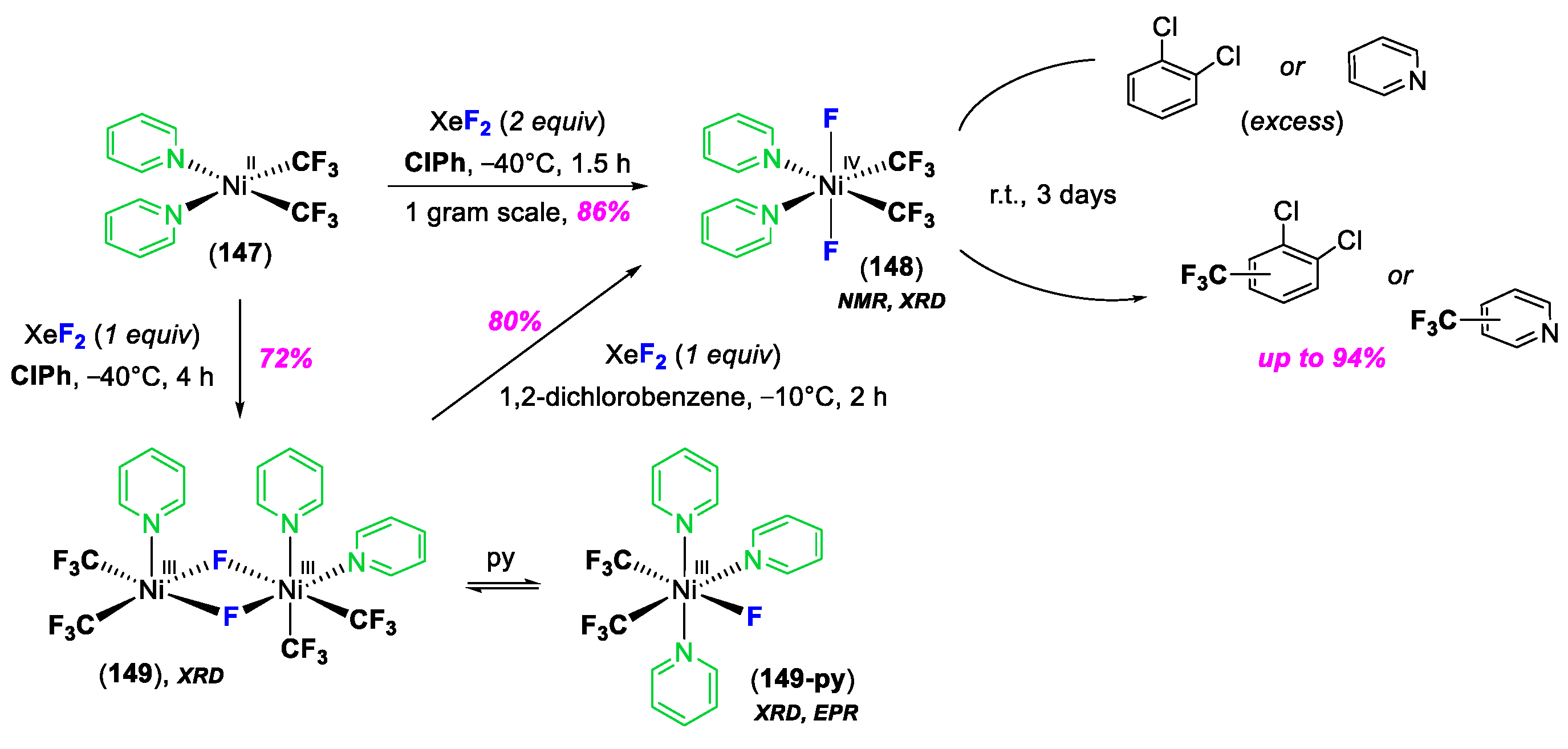
3. C–Heteroatom Bond Formation Mediated by High-Valent NiIII and NiIV
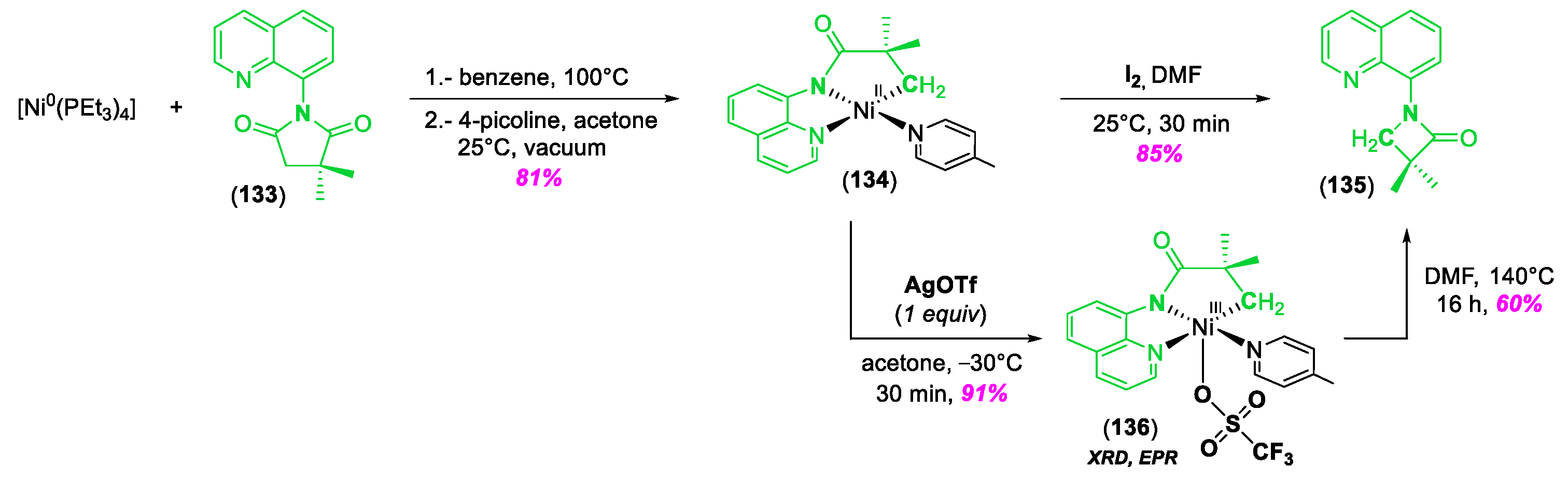
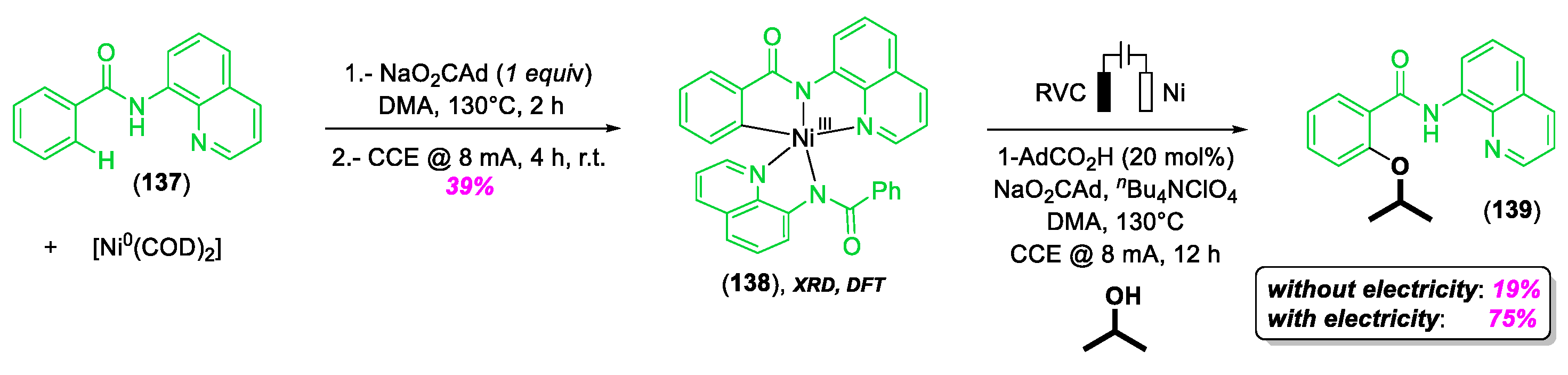
4. C–H Bond Activation and/or Functionalization Enabled by High-Valent NiIII and NiIV
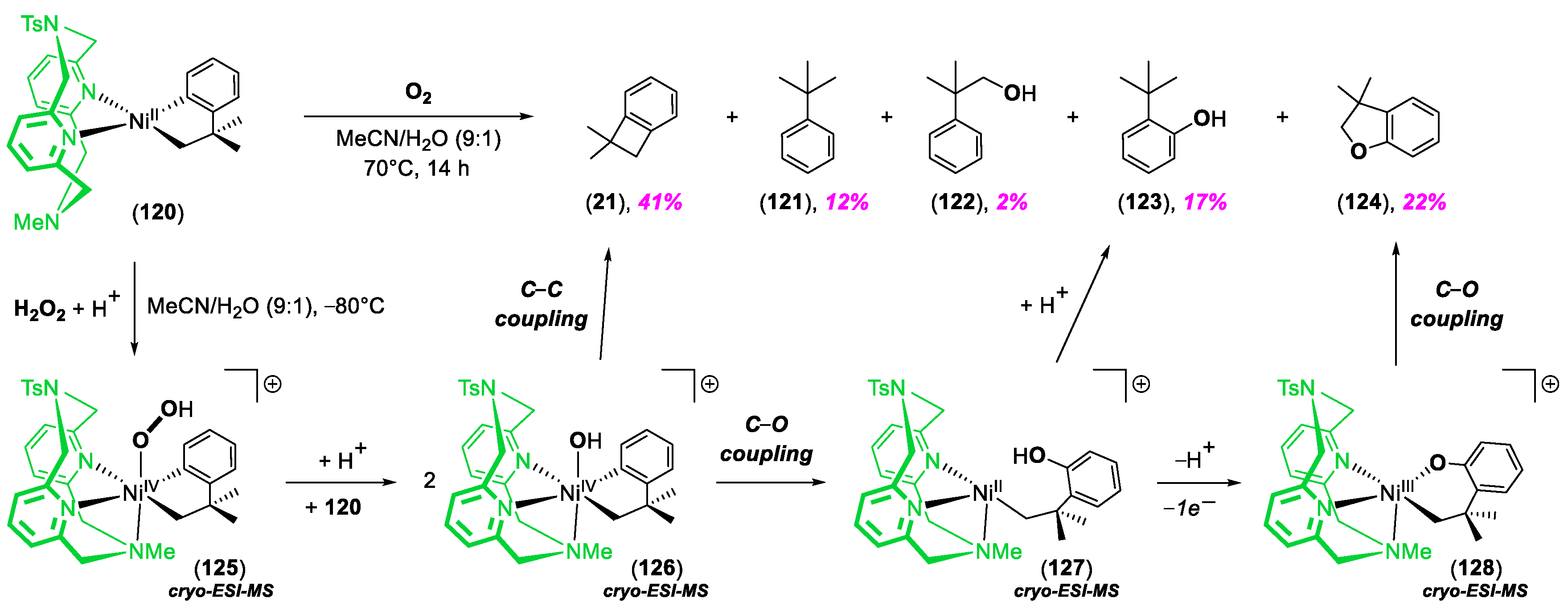
5. Miscellaneous
6. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Gildner, P.G.; Colacot, T.J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34, 5497–5508. [Google Scholar] [CrossRef]
- Li, H.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, Beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, C.M.; Stradiotto, M. Bisphosphines: A Prominent Ancillary Ligand Class for Application in Nickel-Catalyzed C–N Cross-Coupling. ACS Catal. 2018, 8, 7228–7250. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Vicic, D.A.; Klein, A. Exploring Mechanisms in Ni Terpyridine Catalyzed C–C Cross-Coupling Reactions—A Review. Inorganics 2018, 6, 18. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Hu, X. Nickamine and Analogous Nickel Pincer Catalysts for Cross-Coupling of Alkyl Halides and Hydrosilylation of Alkenes. Acc. Chem. Res. 2019, 52, 1471–1483. [Google Scholar] [CrossRef]
- Cornella, J.; Zarate, C.; Martin, R. Metal-Catalyzed Activation of Ethers via C–O Bond Cleavage: A New Strategy for Molecular Diversity. Chem. Soc. Rev. 2014, 43, 8081–8097. [Google Scholar] [CrossRef]
- Borjesson, M.; Moragas, T.; Gallego, D.; Martin, R. Metal-Catalyzed Carboxylation of Organic (Pseudo)halides with CO2. ACS Catal. 2016, 6, 6739–6749. [Google Scholar] [CrossRef]
- Baba, S.; Negishi, E. A Novel Stereospecific Alkenyl–Alkenyl Cross-Coupling by a Palladium- or Nickel-Catalyzed Reaction of Alkenylalanes with Alkenyl Halides. J. Am. Chem. Soc. 1976, 98, 6729–6731. [Google Scholar] [CrossRef]
- Negishi, E.-I.; King, A.O.; Okukado, N. Selective Carbon–Carbon Bond Formation via Transition Metal Catalysis. A Highly Selective Synthesis of Unsymmetrical Biaryls and Diarylmethanes by the Nickel- or Palladium-Catalyzed Reaction of Aryl- and Benzylzinc Derivatives with Aryl Halides. J. Org. Chem. 1977, 42, 1821–1823. [Google Scholar] [CrossRef]
- Hu, X. Nickel-Catalyzed Cross Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci. 2011, 2, 1867–1886. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Aihara, Y.; Chatani, N. Nickel-Catalyzed Direct Arylation of C(sp3)–H Bonds in Aliphatic Amides via Bidentate-Chelation Assistance. J. Am. Chem. Soc. 2014, 136, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Yamaguchi, M.; Chatani, N. Phenyltrimethylammonium Salts as Methylation Reagents in the Nickel-Catalyzed Methylation of C–H Bonds. Angew. Chem. Int. Ed. 2016, 55, 3162–3165. [Google Scholar] [CrossRef]
- Ruan, Z.; Lackner, S.; Ackermann, L. A General Strategy for the Nickel-Catalyzed C–H Alkylation of Anilines. Angew. Chem. Int. Ed. 2016, 55, 3153–3157. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Samanta, R.C.; Sauermann, N.; Ackermann, L. Nickel-Catalyzed Electrooxidative C–H Amination: Support for Nickel(IV). Chem. Eur. J. 2018, 24, 19166–19170. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, L.; Shen, J.; Chai, K.; Shen, C.; Zhang, P. Nickel(II)-Catalyzed Site-Selective C–H Bond Trifluoromethylation of Arylamine in Water through a Coordinating Activation Strategy. Org. Lett. 2017, 19, 5661–5664. [Google Scholar] [CrossRef]
- Liu, X.; Mao, G.; Qiao, J.; Xu, C.; Liu, H.; Ma, J.; Sun, Z.; Chu, W. Nickel-catalyzed C–H Bond Trifluoromethylation of 8-Aminoquinoline Derivatives by Acyl-directed Functionalization. Org. Chem. Front. 2019, 6, 1189–1193. [Google Scholar] [CrossRef]
- Tsou, T.T.; Kochi, J.K. Reductive Coupling of Organometals Induced by Oxidation-Detection of Metastable Paramagnetic Intermediates. J. Am. Chem. Soc. 1978, 100, 1634–1635. [Google Scholar] [CrossRef]
- Lee, C.M.; Chuang, Y.L.; Chiang, C.Y.; Lee, G.-H.; Liaw, W.-F. Mononuclear NiIII Complexes [NiIII(L)(P(C6H3-3-SiMe3-2-S)3)]0/1- (L = Thiolate, Selenolate, CH2CN, Cl, PPh3): Relevance to the Nickel Site of [NiFe] Hydrogenases. Inorg. Chem. 2006, 45, 10895–10904. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.W.; Liaw, W.F. Mononuclear Nickel(III) Complexes [NiIII(OR)(P(C6H3-3-SiMe3-2-S)3)]-(R = Me, Ph) Containing the Terminal Alkoxide Ligand: Relevance to the Nickel Site of Oxidized-Form [NiFe] Hydrogenases. Inorg. Chem. 2008, 47, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Chen, C.H.; Liao, F.X.; Hu, C.-H.; Lee, G.-H. Mononuclear NiIII-Alkyl Complexes (Alkyl = Me and Et): Relevance to the Acetyl-CoA Synthase and Methyl-CoM Reductase. J. Am. Chem. Soc. 2010, 132, 9256–9258. [Google Scholar] [CrossRef] [PubMed]
- Kuwamura, N.; Kitano, K.; Hirotsu, M.; Nishioka, T.; Teki, Y.; Santo, R.; Ichimura, A.; Hashimoto, H.; Wright, J.; Kinoshita, I. Redox-Controlled, Reversible Rearrangement of a Tris(2-pyridylthio)methyl Ligand on Nickel to an Isomer with an “N,S-confused” 2-Pyridylthiolate Arm. Chem. Eur. J. 2011, 17, 10708–10715. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, F.F.; Heims, F.; Kundu, S.; Mebs, S.; Ray, K. Spectroscopic Capture and Reactivity of S = 1/2 Nickel(III)–Oxygen Intermediates in the Reaction of a NiII-Salt with mCPBA. Chem. Commun. 2012, 48, 3730–3732. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.K.; Roy, S.; Barman, S.K.; Maji, R.C.; Olmstead, M.M.; Patra, A.K. Shuttling of Nickel Oxidation States in N4S2 Coordination Geometry versus Donor Strength of Tridentate N2S Donor Ligands. Inorg. Chem. 2012, 51, 7625–7635. [Google Scholar] [CrossRef]
- Baucom, E.I.; Drago, R.S. Nickel(II) and Nickel(IV) Complexes of 2,6-Diacetylpyridine Dioxime. J. Am. Chem. Soc. 1971, 93, 6469–6475. [Google Scholar] [CrossRef]
- Robbins, J.L.; Edelstein, N.; Spencer, B.; Smart, J.C. Syntheses and Electronic-Structures of Decamethylmetallocenes. J. Am. Chem. Soc. 1982, 104, 1882–1893. [Google Scholar] [CrossRef]
- Kölle, U.; Khouzami, F.; Lueken, H. Permethylmetallocenes III. Decamethylnickelocene: The Neutral Sandwich Complex, the Monocation, the Dication, and Their Addition Reactions. Chem. Ber. 1982, 115, 1178–1196. [Google Scholar] [CrossRef]
- Klein, H.-F.; Bickelhaupt, A.; Jung, T.; Cordier, G. Syntheses and Properties of the First Octahedral Diorganonickel(IV) Compounds. Organometallics 1994, 13, 2557–2559. [Google Scholar] [CrossRef]
- Klein, H.F.; Bickelhaupt, A.; Hammerschmitt, B. Ligand-Induced Fragmentation of MethylNickel Phenolates Containing a 2-Aldehyde Function-Structure of (3-Tert-butyl-5-methyl-2-oxobenzoyl)-tris(trimethylphosphine)nickel. Organometallics 1994, 13, 2944–2950. [Google Scholar] [CrossRef]
- Klein, H.-F.; Bickelhaupt, A.; Lemke, M.; Jung, T.; Röhr, C. Synthesis and Structure of Octahedral Nickel(IV) Complexes Containing Two Chelating Acylphenolato Ligands. Chem. Lett. 1995, 24, 467–468. [Google Scholar] [CrossRef]
- Klein, H.-F.; Bickelhaupt, A.; Lemke, M.; Sun, H.; Brand, A.; Jung, T.; Röhr, C.; Flörke, U.; Haupt, H.-J. Trimethylphosphine Complexes of Diorganonickel(IV) Moieties. Organometallics 1997, 16, 668–676. [Google Scholar] [CrossRef]
- Shimada, S.; Rao, M.L.N.; Tanaka, M. Reaction of 1,2-Disilylbenzene with Bis[1,2-bis(dimethylphosphino)ethane]nickel(0). Isolation and Characterization of the First Silylnickel(IV) Complex. Organometallics 1999, 18, 291–293. [Google Scholar] [CrossRef]
- Patra, A.K.; Mukherjee, R. Bivalent, Trivalent, and Tetravalent Nickel Complexes with a Common Tridentate Deprotonated Pyridine Bis-Amide Ligand. Molecular Structures of Nickel(II) and Nickel(IV) and Redox Activity. Inorg. Chem. 1999, 38, 1388–1393. [Google Scholar] [CrossRef]
- Chen, W.Z.; Shimada, S.; Tanaka, M.; Kobayashi, Y.; Saigo, K. Reaction of [2-(SiH3)C6H4]2SiH2 with Ni(Et2PCH2CH2PEt2)(PEt3)2: Characterization of η2-(Si–H)Ni and NiIV–H Complexes. J. Am. Chem. Soc. 2004, 126, 8072–8073. [Google Scholar] [CrossRef]
- Dimitrov, V.; Linden, A. A Pseudotetrahedral, High-Oxidation-State Organonickel Compound: Synthesis and Structure of Bromotris(1-norbornyl)nickel(IV). Angew. Chem. Int. Ed. 2003, 42, 2631–2633. [Google Scholar] [CrossRef]
- Klein, H.-F.; Kraikivskii, P. Unexpected Formation of a Molecular Tetraalkyl Nickel Complex from an Olefin/Nickel(0) System. Angew. Chem. Int. Ed. 2009, 48, 260–261. [Google Scholar] [CrossRef]
- Carnes, M.; Buccella, D.; Chen, J.Y.-C.; Ramirez, A.P.; Turro, N.J.; Nuckolls, C.; Steigerwald, M. A Stable Tetraalkyl Complex of Nickel(IV). Angew. Chem. Int. Ed. 2009, 48, 290–294. [Google Scholar] [CrossRef]
- Lipschutz, M.I.; Yang, X.; Chatterjee, R.; Tilley, T.D. A Structurally Rigid Bis(amido) Ligand Framework in Low-Coordinate NiI, NiII, and NiIII Analogues Provides Access to a NiIII Methyl Complex via Oxidative Addition. J. Am. Chem. Soc. 2013, 135, 15298–15301. [Google Scholar] [CrossRef]
- Lipschutz, M.I.; Tilley, T.D. Carbon–Carbon Cross-Coupling Reactions Catalyzed by a Two-Coordinate Nickel(II)-Bis(amido) Complex via Observable NiI, NiII, and NiIII Intermediates. Angew. Chem. Int. Ed. 2014, 53, 7290–7294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Tang, F.; Luo, J.; Schultz, J.W.; Rath, N.P.; Mirica, L.P. Organometallic Nickel(III) Complexes Relevant to Cross-Coupling and Carbon–Heteroatom Bond Formation Reactions. J. Am. Chem. Soc. 2014, 136, 6499–6504. [Google Scholar] [CrossRef] [PubMed]
- Tomashenko, O.A.; Grushin, V.V. Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev. 2011, 111, 4475–4521. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Martínez de Marigorta, E.; Rubiales, G.; Palacios, F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for Fluorination and Trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E.; Henefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green Chemistry: Science and Politics of Change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef]
- Dubinina, G.G.; Brennessel, W.W.; Miller, J.L.; Vicic, D.A. Exploring Trifluoromethylation Reactions at Nickel: A Structural and Reactivity Study. Organometallics 2008, 27, 3933–3938. [Google Scholar] [CrossRef]
- Jover, J.; Miloserdov, F.M.; Benet-Buchholz, J.; Grushin, V.V.; Maseras, F. On the Feasibility of Nickel-Catalyzed Trifluoromethylation of Aryl Halides. Organometallics 2014, 33, 6531–6543. [Google Scholar] [CrossRef]
- Bour, J.R.; Roy, P.; Canty, A.J.; Kampf, J.W.; Sanford, M.S. Oxidatively Induced Aryl–CF3 Coupling at Diphosphine Nickel Complexes. Organometallics 2020, 39, 3–7. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Wang, H.; Klein, A.; Biewer, C.; Stirnat, K.; Yamaguchi, Y.; Xu, L.; Gomez-Benitez, V.; Vicic, D.A. A Five-Coordinate Nickel(II) Fluoroalkyl Complex as a Precursor to a Spectroscopically Detectable Ni(III) Species. J. Am. Chem. Soc. 2013, 135, 8141–8144. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Dudkina, Y.; Wang, H.; Kholin, K.V.; Kadirov, M.K.; Budnikova, Y.H.; Vicic, D.A. Accessing Perfluoroalkyl Nickel(II), (III), and (IV) Complexes Bearing a Readily Attached [C4F8] ligand. Dalton Trans. 2015, 44, 19443–19446. [Google Scholar] [CrossRef]
- Tang, F.; Rath, N.P.; Mirica, L.M. Stable Bis(trifluoromethyl)nickel(III) Complexes. Chem. Commun. 2015, 51, 3113–3116. [Google Scholar] [CrossRef]
- Camasso, N.M.; Sanford, M.S. Design, Synthesis, and Carbon–Heteroatom Coupling Reactions of Organometallic Nickel(IV) Complexes. Science 2015, 347, 1218–1220. [Google Scholar] [CrossRef]
- Riordan, C.G. Catalysis by Nickel in its High Oxidation State. Science 2015, 347, 1203–1204. [Google Scholar] [CrossRef]
- Mitra, R.; Pörschke, K.-P. Organonickel(IV) Chemistry: A New Catalyst? Angew. Chem. Int. Ed. 2015, 54, 7488–7490. [Google Scholar] [CrossRef]
- Hull, K.L.; Anani, W.Q.; Sanford, M.S. Palladium-Catalyzed Fluorination of Carbon–Hydrogen Bonds. J. Am. Chem. Soc. 2006, 128, 7134–7135. [Google Scholar] [CrossRef]
- Furuya, T.; Ritter, T. Carbon–Fluorine Reductive Elimination from a High-Valent Palladium Fluoride. J. Am. Chem. Soc. 2008, 130, 10060–10061. [Google Scholar] [CrossRef]
- Furuya, T.; Benitez, D.; Tkatchouk, E.; Strom, A.E.; Tang, P.; Goddard, W.A., III; Ritter, T. Mechanism of C–F Reductive Elimination from Palladium(IV) Fluorides. J. Am. Chem. Soc. 2010, 132, 3793–3807. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.D.; Sanford, M.S. Synthesis and Reactivity of a Mono-σ-Aryl Palladium(IV) Fluoride Complex. J. Am. Chem. Soc. 2009, 131, 3796–3797. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kamlet, A.S.; Powers, D.C.; Neumann, C.N.; Boursalian, G.B.; Furuya, T.; Choi, D.C.; Hooker, J.M.; Ritter, T. A Fluoride-Derived Electrophilic Late-Stage Fluorination Reagent for PET Imaging. Science 2011, 334, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.R.; Lee, E.; Boursalian, G.B.; Ritter, T. Mechanism of Electrophilic Fluorination with PdIV: Fluoride Capture and Subsequent Oxidative Fluoride Transfer. Chem. Sci. 2014, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ball, N.D.; Kampf, J.W.; Sanford, M.S. Oxidation of a Cyclometalated Pd(II) Dimer with “CF3+”: Formation and Reactivity of a Catalytically Competent Monomeric PdIV Aquo Complex. J. Am. Chem. Soc. 2010, 132, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Lee, E.; Ariafard, A.; Sanford, M.S.; Yates, B.F.; Canty, A.J.; Ritter, T. Connecting Binuclear PdIII and Mononuclear PdIV Chemistry by Pd–Pd Bond Cleavage. J. Am. Chem. Soc. 2012, 134, 12002–12009. [Google Scholar] [CrossRef] [PubMed]
- Bour, J.R.; Camasso, N.M.; Sanford, M.S. Oxidation of NiII to NiIV with Aryl Electrophiles Enables Ni-Mediated Aryl–CF3 Coupling. J. Am. Chem. Soc. 2015, 137, 8034–8037. [Google Scholar] [CrossRef]
- Steen, J.S.; Knizia, G.; Klein, J.E.M.N. σ-Noninnocence: Masked Phenyl-Cation Transfer at Formal NiIV. Angew. Chem. Int. Ed. 2019, 58, 13133–13139. [Google Scholar] [CrossRef]
- Bour, J.R.; Camasso, N.M.; Meucci, E.A.; Kampf, J.W.; Canty, A.J.; Sanford, M.S. Carbon–Carbon Bond-Forming Reductive Elimination from Isolated Nickel(III) Complexes. J. Am. Chem. Soc. 2016, 138, 16105–16111. [Google Scholar] [CrossRef]
- Watson, M.B.; Rath, N.P.; Mirica, L.M. Oxidative C–C Bond Formation Reactivity of Organometallic NiII, NiIII, and NiIV Complexes. J. Am. Chem. Soc. 2017, 139, 35–38. [Google Scholar] [CrossRef]
- Tellis, J.C.; Primer, D.N.; Molander, G.A. Single-Electron Transmetalation in Organoboron Cross-Coupling by Photoredox/Nickel Dual Catalysis. Science 2014, 345, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.W.; Ahneman, D.T.; Chu, L.L.; Terrett, J.A.; Doyle, A.G.; MacMillan, D.W.C. Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S.Z.; Jamison, T.F. Highly Regioselective Indoline Synthesis under Nickel/Photoredox Dual Catalysis. J. Am. Chem. Soc. 2015, 137, 9531–9534. [Google Scholar] [CrossRef] [PubMed]
- Terrett, J.A.; Cuthbertson, J.D.; Shurtleff, V.W.; MacMillan, D.W.C. Switching on Elusive Organometallic Mechanisms with Photoredox Catalysis. Nature 2015, 524, 330–334. [Google Scholar] [CrossRef]
- Schultz, J.W.; Fuchigami, K.; Zheng, B.; Rath, N.P.; Mirica, L.M. Isolated Organometallic Nickel(III) and Nickel(IV) Complexes Relevant to Carbon–Carbon Bond Formation Reactions. J. Am. Chem. Soc. 2016, 138, 12928–12934. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Rath, N.P.; Mirica, L.M. Axial Donor Effects on Oxidatively Induced Ethane Formation from Nickel-Dimethyl Complexes. Organometallics 2019, 38, 3602–3609. [Google Scholar] [CrossRef]
- Smith, S.M.; Planas, O.; Gomez, L.; Rath, N.P.; Ribas, X.; Mirica, L.M. Aerobic C–C and C–O Bond Formation Reactions Mediated by High-Valent Nickel Species. Chem. Sci. 2019, 10, 10366–10372. [Google Scholar] [CrossRef]
- Camasso, N.M.; Canty, A.J.; Ariafard, A.; Sanford, M.S. Experimental and Computational Studies of High-Valent Nickel and Palladium Complexes. Organometallics 2017, 36, 4382–4393. [Google Scholar] [CrossRef]
- Roberts, C.C.; Camasso, N.M.; Bowes, E.G.; Sanford, M.S. Impact of Oxidation State on Reactivity and Selectivity Differences between Nickel(III) and Nickel(IV) Alkyl Complexes. Angew. Chem. Int. Ed. 2019, 58, 9104–9108. [Google Scholar] [CrossRef]
- Meucci, E.A.; Camasso, N.M.; Sanford, M.S. An Organometalllic NiIV Complex That Participates in Competing Transmetalation and C(sp2)–O Bond-Forming Reductive Elimination Reactions. Organometallics 2017, 36, 247–250. [Google Scholar] [CrossRef]
- Rovira, M.; Roldan-Gomez, S.; Martin-Diaconescu, V.; Whiteoak, C.J.; Company, A.; Luis, J.M.; Ribas, X. Trifluoromethylation of a Well-Defined Square-Planar Aryl-NiII Complex involving NiIII/CF3● and NiIV–CF3 Intermediate Species. Chem. Eur. J. 2017, 23, 11662–11668. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, L.S.; Vogt, N.; Sandleben, A.; de Bruin, B.; Klein, A.; van der Vlugt, J.I. Nickel–Alkyl Complexes with a Reactive PNC-Pincer Ligand. Eur. J. Inorg. Chem. 2018, 2018, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rath, N.P.; Mirica, L.M. Oxidatively-Induced Aromatic Cyanation Mediated by NiIII. Dalton Trans. 2016, 45, 8693–8695. [Google Scholar] [CrossRef] [PubMed]
- The N-tBu bond cleavage was reported before by Zargarian and co-workers starting from a NiII-CNtBu complex resulting in the formation of stable NiII-CN organometallic: Lefevre, X.; Spasyuk, D.M.; Zargarian, D. New POCOP-Type Pincer Complexes of Nickel(II). J. Organomet. Chem. 2011, 696, 864–870.
- Zhou, W.; Watson, M.B.; Zheng, S.; Rath, N.P.; Mirica, L.M. Ligand Effects on the Properties of NiIII Complexes: Aerobically-Induced Aromatic Cyanation at Room Temperature. Dalton Trans. 2016, 45, 15886–15893. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, S.; Schultz, J.W.; Rath, N.P.; Mirica, L.M. Aromatic Cyanoalkylation through Double C–H Activation Mediated by NiIII. J. Am. Chem. Soc. 2016, 138, 5777–5780. [Google Scholar] [CrossRef]
- Bour, J.R.; Ferguson, D.M.; McClain, E.J.; Kampf, J.W.; Sanford, M.S. Connecting Organometallic NiIII and NiIV: Reactions of Carbon-Centered Radicals with High-Valent Organonickel Complexes. J. Am. Chem. Soc. 2019, 141, 8914–8920. [Google Scholar] [CrossRef]
- Xu, H.; Diccianni, J.B.; Katigbak, J.; Hu, C.; Zhang, Y.; Diao, T. Bimetallic C–C Bond-Forming Reductive Elimination from Nickel. J. Am. Chem. Soc. 2016, 138, 4779–4786. [Google Scholar] [CrossRef]
- Koo, K.; Hillhouse, G.L. Carbon–Nitrogen Bond Formation by Reductive Elimination from Nickel(II) Amido Alkyl Complexes. Organometallics 1995, 14, 4421–4423. [Google Scholar] [CrossRef]
- Han, R.; Hillhouse, G.L. Carbon–Oxygen Reductive-Elimination from Nickel(II) Oxametallacycles and Factors That Control Formation of Ether, Aldehyde, Alcohol, or Ester Products. J. Am. Chem. Soc. 1997, 119, 8135–8136. [Google Scholar] [CrossRef]
- Koo, K.M.; Hillhouse, G.L.; Rheingold, A.L. Oxygen-Atom Transfer from Nitrous Oxide to an Organonickel(II) Phosphine Complex. Syntheses and Reactions of New Nickel(II) Aryloxides and the Crystal Structure of [cyclic] (Me2PCH2CH2PMe2)Ni(O-o-C6H4CMe2CH2). Organometallics 1995, 14, 456–460. [Google Scholar] [CrossRef]
- Lin, B.L.; Clough, C.R.; Hillhouse, G.L. Interactions of Aziridines with Nickel Complexes: Oxidative-Addition and Reductive-Elimination Reactions that Break and Make C–N Bonds. J. Am. Chem. Soc. 2002, 124, 2890–2891. [Google Scholar] [CrossRef] [PubMed]
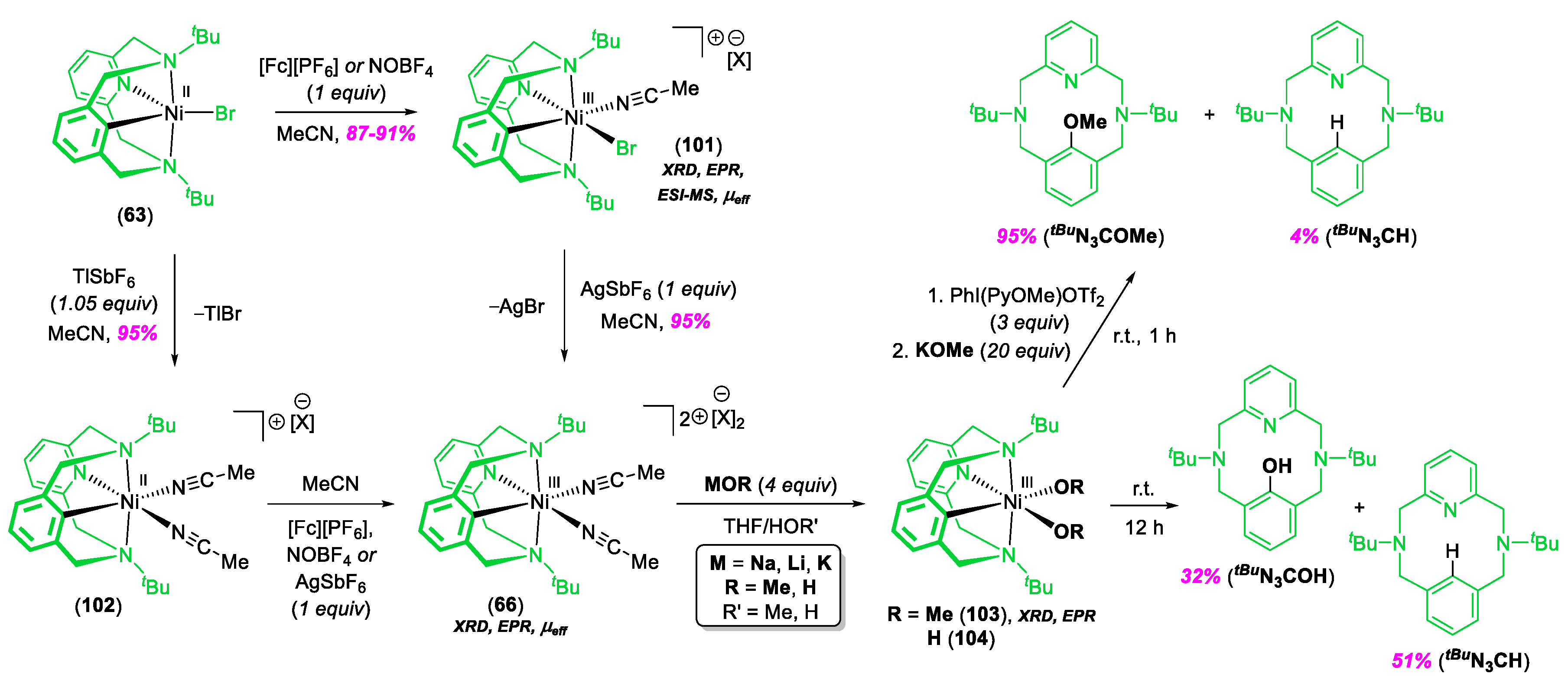
- Espinosa Martinez, G.; Ocampo, C.; Park, Y.J.; Fout, A.R. Accessing Pincer Bis(carbene) NiIV Complexes from NiII via Halogen and Halogen Surrogates. J. Am. Chem. Soc. 2016, 138, 4290–4293. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Ritter, T. Bimetallic PdIII Complexes in Palladium-Catalysed Carbon–Heteroatom Bond Formation. Nat. Chem. 2009, 1, 302–309. [Google Scholar] [CrossRef]
- Powers, D.C.; Benitez, D.; Tkatchouk, E.; Goddard, W.A.; Ritter, T. Bimetallic Reductive Elimination from Dinuclear PdIII Complexes. J. Am. Chem. Soc. 2010, 132, 14092–14103. [Google Scholar] [CrossRef]
- Powers, D.C.; Ritter, T. Bimetallic Redox Synergy in Oxidative Palladium Catalysis. Acc. Chem. Res. 2012, 45, 840–850. [Google Scholar] [CrossRef]
- Diccianni, J.B.; Hu, C.; Diao, T. Binuclear, High-Valent Nickel Complexes: Ni–Ni Bonds in Aryl–Halogen Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 3635–3639. [Google Scholar] [CrossRef]
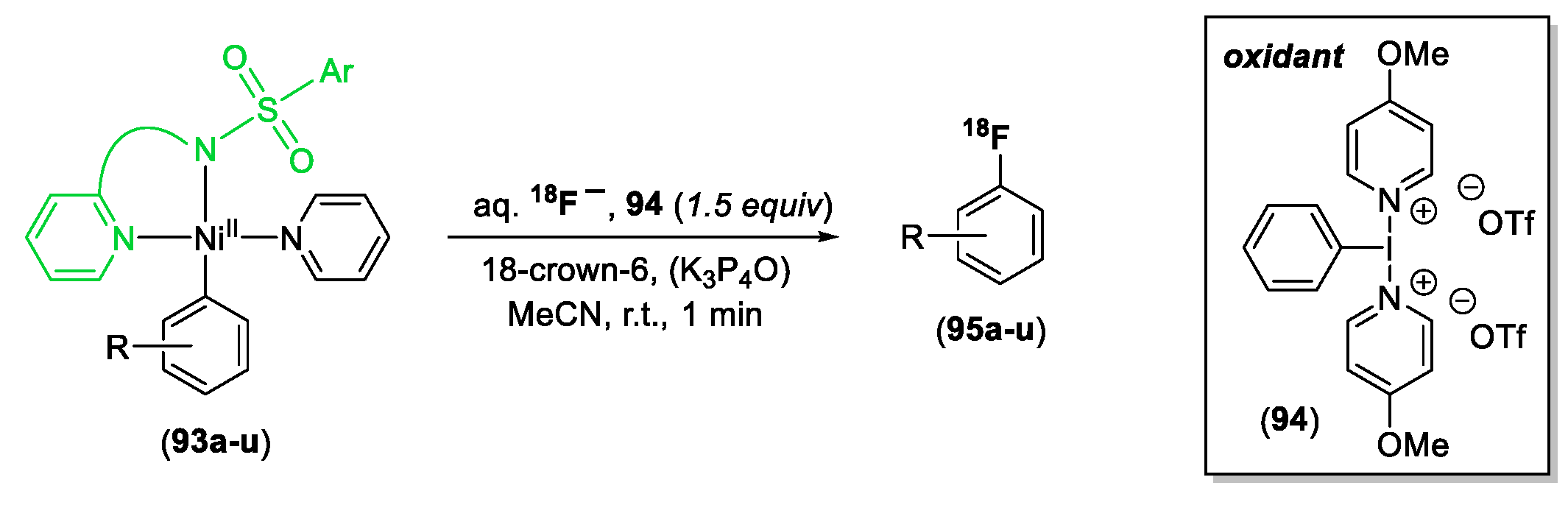
- Lee, E.; Hooker, J.M.; Ritter, T. Nickel-Mediated Oxidative Fluorination for PET with Aqueous [18F]Fluoride. J. Am. Chem. Soc. 2012, 134, 17456–17458. [Google Scholar] [CrossRef]
- Hoover, A.J.; Lazari, M.; Ren, H.; Narayanam, M.K.; Murphy, J.M.; van Dam, R.M.; Hooker, J.M.; Ritter, T. A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil from [18F]Fluoride for Human PET Imaging. Organometallics 2016, 35, 1008–1014. [Google Scholar] [CrossRef]
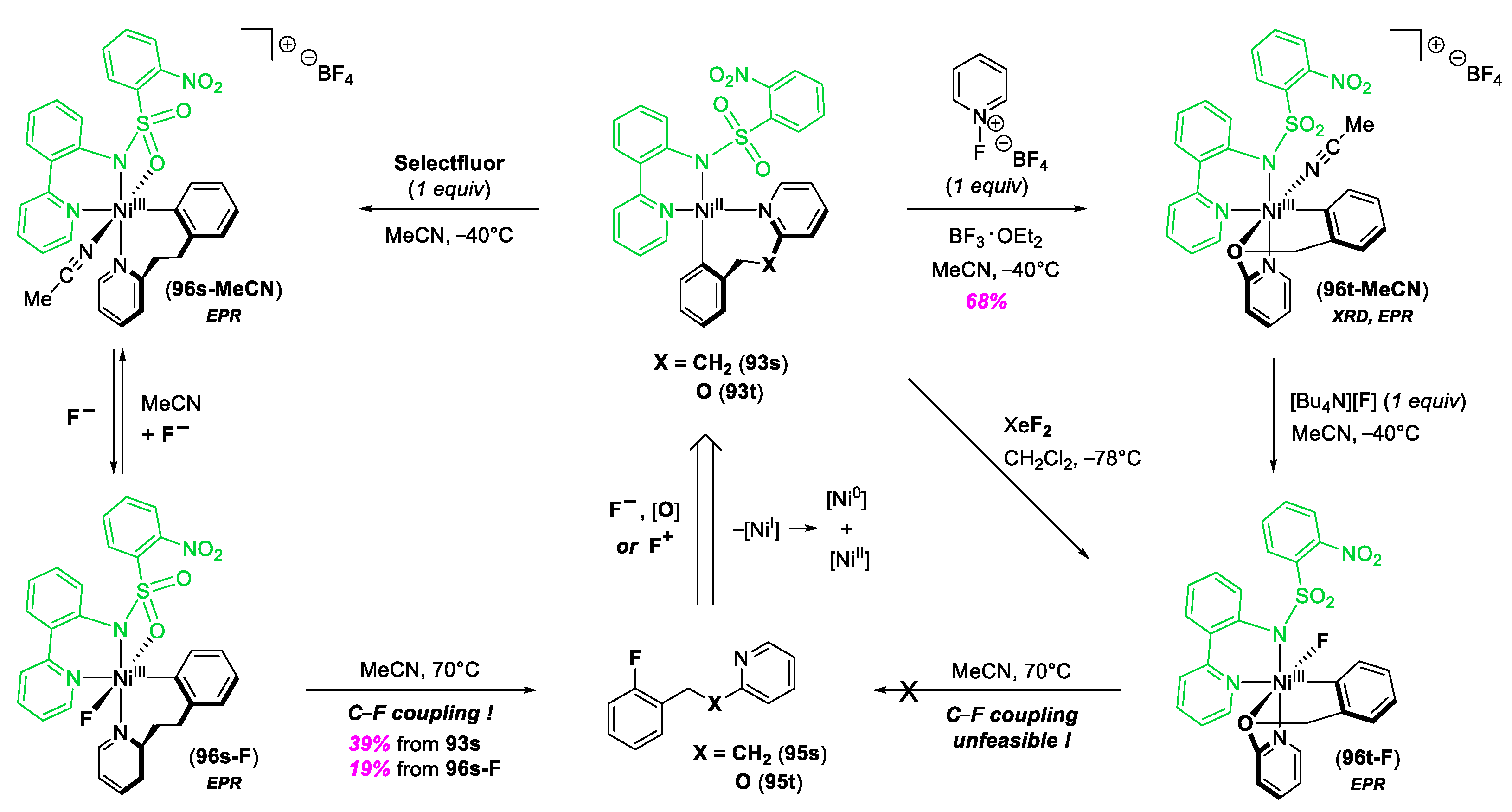
- Lee, H.; Börgel, J.; Ritter, T. Carbon–Fluorine Reductive Elimination from Nickel(III) Complexes. Angew. Chem. Int. Ed. 2017, 56, 6966–6969. [Google Scholar] [CrossRef]
- Meucci, E.A.; Ariafard, A.; Canty, A.J.; Kampf, J.W.; Sanford, M.S. Aryl–Fluoride Bond-Forming Reductive Elimination from Nickel(IV) Centers. J. Am. Chem. Soc. 2019, 141, 13261–13267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Schultz, J.W.; Rath, N.P.; Mirica, L.M. Aromatic Methoxylation and Hydroxylation by Organometallic High-Valent Nickel Complexes. J. Am. Chem. Soc. 2015, 137, 7604–7607. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.M.; Van Koten, G.; Zoet, R.; Murrall, N.W.; Welch, A.J. Unique Stable Organometallic Nickel(III) Complexes: Syntheses and the Molecular Structure of [Ni[C6H3(CH2NMe2)2-2,6]I2]. J. Am. Chem. Soc. 1983, 105, 1379–1380. [Google Scholar] [CrossRef]
- Grove, D.M.; Van Koten, G.; Mul, P.; Zoet, R.; Van der Linden, J.G.M.; Legters, J.; Schmitz, J.E.J.; Murrall, N.W.; Welch, A.J. Syntheses and Characterization of Unique Organometallic Nickel(III) Aryl Species. ESR and Electrochemical Studies and the X-Ray Molecular Study of Square-Pyramidal [Ni-{C6H3(CH2NMe2)2-o,o’}I2]. Inorg. Chem. 1988, 27, 2466–2473. [Google Scholar] [CrossRef]
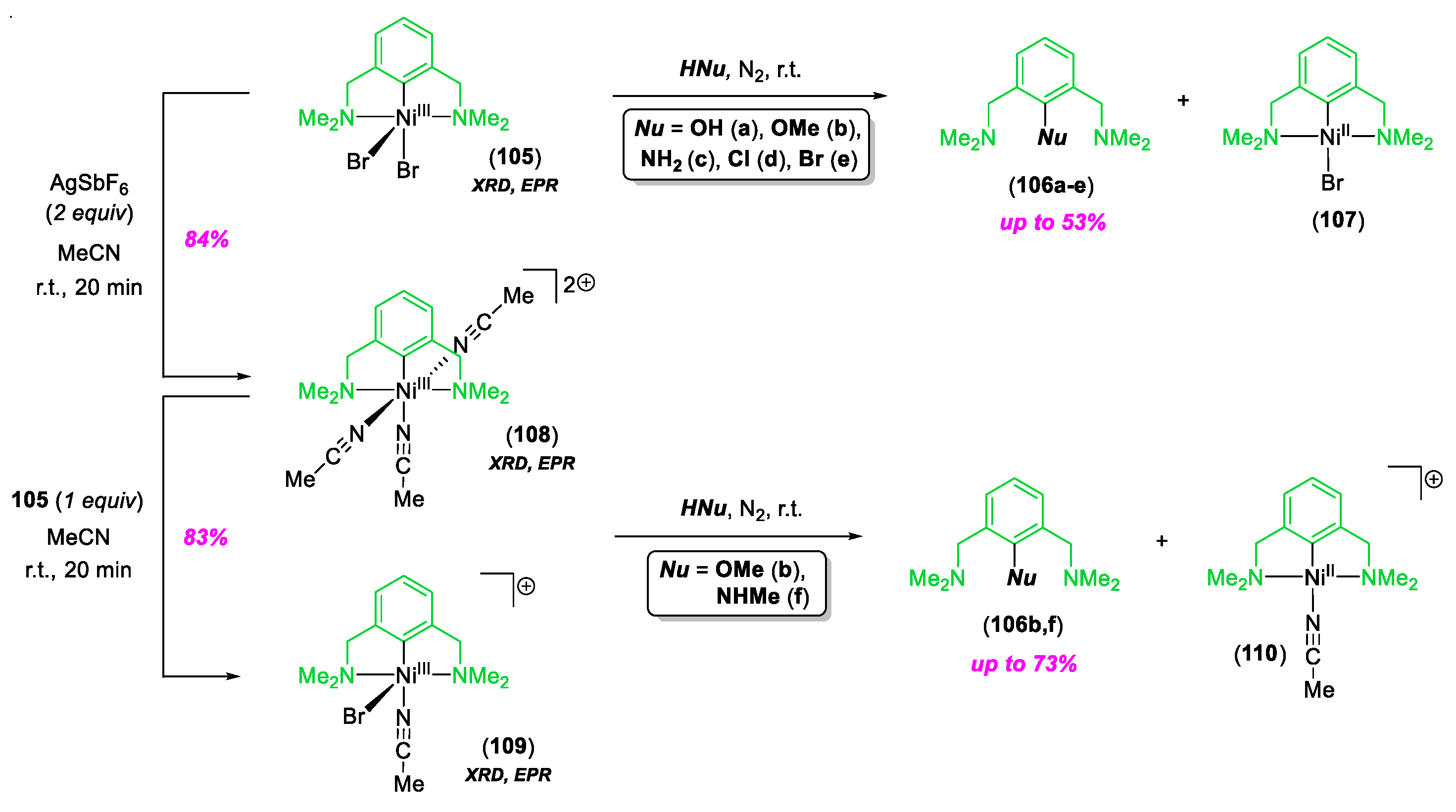
- Cloutier, J.-P.; Zargarian, D. Functionalization of the Aryl Moiety in the Pincer Complex (NCN)NiIIIBr2: Insights on NiIII-Promoted Carbon–Heteroatom Coupling. Organometallics 2018, 37, 1446–1455. [Google Scholar] [CrossRef]
- Cloutier, J.-P.; Rechignat, L.; Canac, Y.; Ess, D.H.; Zargarian, D. C–O and C–N Functionalization of Cationic, NCN-Type Pincer Complexes of Trivalent Nickel: Mechanism, Selectivity, and Kinetic Isotope Effect. Inorg. Chem. 2019, 58, 3861–3874. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition Metal-Catalyzed Direct Arylations of (Hetero)Arenes via C–H Bond Cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9827. [Google Scholar] [CrossRef]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild Metal-Catalyzed C–H Activation: Examples and Concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef]
- Ping, L.; Chung, D.S.; Bouffard, J.; Lee, S.-G. Transition Metal-Catalyzed Site- and Regio-Divergent C–H Bond Functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef]
- Castro, L.C.M.; Chatani, N. Nickel Catalysts/N,N’-Bidentate Directing Groups: An Excellent Partnership in Directed C–H Activation Reactions. Chem. Lett. 2015, 44, 410–421. [Google Scholar] [CrossRef]
- Khake, S.M.; Chatani, N. Chelation-Assisted Nickel-Catalyzed C–H Functionalizations. Trends Chem. 2019, 1, 524–539. [Google Scholar] [CrossRef]
- Li, Y.; Zou, L.; Bai, R.; Lan, Y. NiI-NiIII vs NiII-NiIV: Mechanistic Study of Ni-Catalyzed Alkylation of Benzamides with Alkyl Halides. Org. Chem. Front. 2018, 5, 615–622. [Google Scholar] [CrossRef]
- Singh, S.; Surya, K.; Sunoj, R.B. Aliphatic C(sp3)–H Bond Activation Using Nickel Catalysis: Mechanistic Insights on Regioselective Arylation. J. Org. Chem. 2017, 82, 9619–9626. [Google Scholar] [CrossRef] [PubMed]
- Omer, H.M.; Liu, P. Computational Study of Ni-Catalyzed C–H Functionalization: Factors that Control the Competition of Oxidative Addition and Radical Pathways. J. Am. Chem. Soc. 2017, 139, 9909–9920. [Google Scholar] [CrossRef] [PubMed]
- Haines, B.E.; Yu, J.-Q.; Musaev, D.G. The mechanism of directed NiII-catalyzed C–H Iodination with Molecular Iodine. Chem. Sci. 2018, 9, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.N.; Jain, S.; Pandey, D.K.; Gonnade, R.G.; Vanka, K.; Punji, B. Mechanistic Aspects of Pincer Nickel(II)-Catalyzed C–H Bond Alkylation of Azoles with Alkyl Halides. Organometallics 2018, 37, 1017–1025. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Zhu, L.; Liu, F.; Zhong, K.; Zhang, Y.; Bai, R.; Lan, Y. Theoretical Study of FMO Adjusted C–H Cleavage and Oxidative Addition in Nickel Catalysed C–H Arylation. Commun. Chem. 2019, 2. [Google Scholar] [CrossRef]
- Khrizanforov, M.K.; Fedorenko, S.V.; Strekalova, S.O.; Kholin, K.V.; Mustafina, A.R.; Zhilkin, M.Y.; Khrizanforova, V.K.; Osin, Y.N.; Salnikov, V.V.; Gryaznova, T.V.; et al. A NiIII Complex Stabilized by Silica Nanoparticles as an Efficient Nanoheterogeneous Catalyst for Oxidative C–H Fluoroalkylation. Dalton Trans. 2016, 45, 11976–11982. [Google Scholar] [CrossRef]
- Aihara, Y.; Chatani, N. Nickel-Catalyzed Reaction of C–H Bonds in Amides with I2: Ortho-Iodination via the Cleavage of C(sp2)–H Bonds and Oxidative Cyclization to β-lactams via the Cleavage of C(sp3)–H Bonds. ACS Catal. 2016, 6, 4323–4329. [Google Scholar] [CrossRef]
- Roy, P.; Bour, J.R.; Kampf, J.W.; Sanford, M.S. Catalytically Relevant Intermediates in the Ni-Catalyzed C(sp2)–H and C(sp3)–H Functionalization of Aminoquinoline Substrates. J. Am. Chem. Soc. 2019, 141, 17382–17387. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Struwe, J.; Hu, L.; Ackermann, L. Nickellaelectro-Catalyzed C–H Alkoxylation with Secondary Alcohols: Oxidation-Induced Reductive Elimination at Nickel(III). Angew. Chem. Int. Ed. 2020, 59, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Kampf, J.W.; Ariafard, A.; Canty, A.J.; Sanford, M.S. Oxidatively Induced C–H Activation at High Valent Nickel. J. Am. Chem. Soc. 2017, 139, 6058–6061. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.C.; Chong, E.; Kampf, J.W.; Canty, A.J.; Ariafard, A.; Sanford, M.S. Nickel(II/IV) Manifold Enables Room-Temperature C(sp3)–H Functionalization. J. Am. Chem. Soc. 2019, 141, 19513–19520. [Google Scholar] [CrossRef] [PubMed]
- D’Accriscio, F.; Borja, P.; Saffon-Merceron, N.; Fustier-Boutignon, M.; Mézailles, N.; Nebra, N. C–H Bond Trifluoromethylation of Arenes Enabled by a Robust, High-Valent Nickel(IV) Complex. Angew. Chem. Int. Ed. 2017, 56, 12898–12902. [Google Scholar] [CrossRef] [PubMed]
- Meucci, E.A.; Nguyen, S.N.; Camasso, N.M.; Chong, E.; Ariafard, A.; Canty, A.J.; Sanford, M.S. Nickel(IV)-Catalyzed C–H Trifluoromethylation of (Hetero)arenes. J. Am. Chem. Soc. 2019, 141, 12872–12879. [Google Scholar] [CrossRef]
- Corona, T.; Pfaff, F.F.; Acuña-Parés, F.; Draksharapu, A.; Whiteoak, C.J.; Martin-Diaconescu, V.; Lloret-Fillol, J.; Browne, W.R.; Ray, K.; Company, A. Reactivity of a Nickel(II) Bis(amidate) Complex with meta-Chloroperbenzoic Acid: Formation of a Potent Oxidizing Species. Chem. Eur. J. 2015, 21, 15029–15038. [Google Scholar] [CrossRef]
- Corona, T.; Draksharapu, A.; Padamati, S.K.; Gamba, I.; Martin-Diaconescu, V.; Acuña-Parés, F.; Browne, W.; Company, A. Rapid Hydrogen and Oxygen Atom Transfer by a High-Valent Nickel–Oxygen Species. J. Am. Chem. Soc. 2016, 138, 12987–12996. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takagi, Y.; Saito, T.; Ohta, T.; Ogura, T.; Tohnai, N.; Nakano, M.; Itoh, S. A Bis(μ-oxido)dinickel(III) Complex with a Triplet Ground State. Angew. Chem. Int. Ed. 2018, 57, 7640–7643. [Google Scholar] [CrossRef]
- Padamati, S.K.; Angelone, D.; Draksharapu, A.; Primi, G.; Martin, D.J.; Tromp, M.; Swart, M.; Browne, W.R. Transient Formation and Reactivity of a High-Valent Nickel(IV) Oxido Complex. J. Am. Chem. Soc. 2017, 139, 8718–8724. [Google Scholar] [CrossRef]
- Diccianni, J.B.; Hu, C.; Diao, T. N–N Bond Forming Reductive Elimination via a Mixed-Valent Nickel(II)–Nickel(III) Intermediate. Angew. Chem. Int. Ed. 2016, 55, 7534–7538. [Google Scholar] [CrossRef]
- Kosobokov, M.D.; Sandleben, A.; Vogt, N.; Klein, A.; Vicic, D.A. Nitrogen–Nitrogen Bond Formation via a Substrate-Bound Anion at a Mononuclear Nickel Platform. Organometallics 2018, 37, 521–525. [Google Scholar] [CrossRef]
- Kogut, E.; Wiencko, H.L.; Zhang, L.; Cordeau, D.E.; Warren, T.H. A Terminal NiIII-Imide with Diverse Reactivity Pathways. J. Am. Chem. Soc. 2005, 127, 11248–11249. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.M.; van Koten, G.; Verschuuren, A.H.M. New Homogeneous Catalysts in the Addition of Polyhalogenoalkanes to Olefins; Organonickel(II) Complexes [Ni{C6H3(CH2NMe2)2-o,o’}X] (X = Cl, Br, I). J. Mol. Catal. 1988, 45, 169–174. [Google Scholar] [CrossRef][Green Version]
- Grove, D.M.; Verschuuren, A.H.M.; van Koten, G.; van Beek, J.A.M. The Homogeneously Catalysed Addition Reaction of Polyhalogenoalkanes to Olefins by Divalent Arylnickel Complexes: Comparative Reactivity and Some Important Mechanistic Leads. J. Organomet. Chem. 1989, 372, C1–C6. [Google Scholar] [CrossRef][Green Version]
- Van de Kuil, L.A.; Grove, D.M.; Zwikker, J.W.; Jenneskens, L.W.; Drenth, W.; van Koten, G. New Soluble Polysiloxane Polymers Containing a Pendant Terdentate Aryldiamine Ligand Substituent Holding a Highly Catalytically Active Organometallic Nickel(II) Center. Chem. Mater. 1994, 6, 1675–1683. [Google Scholar] [CrossRef]
- Van de Kuil, L.A.; Grove, D.M.; Gossage, R.A.; Zwikker, J.W.; Jenneskens, L.W.; Drenth, W.; van Koten, G. Mechanistic Aspects of the Kharasch Addition Reaction Catalyzed by Organonickel(II) Complexes Containing the Monoanionic Terdentate Aryldiamine Ligand System [C6H2(CH2NMe2)2-2,6-R-4]−. Organometallics 1997, 16, 4985–4994. [Google Scholar] [CrossRef]
- Knapen, J.W.J.; van der Made, A.W.; de Wilde, J.C.; van Leeuwen, P.W.N.M.; Wijkens, P.; Grove, D.M.; van Koten, G. Homogeneous Catalysts Based on Silane Dendrimers Functionalized with ArylNickel(II) Complexes. Nature 1994, 372, 659–663. [Google Scholar] [CrossRef]
- Kleij, A.W.; Gossage, R.A.; Gebbink, R.J.M.K.; Brinkmann, N.; Reijerse, E.J.; Kragl, U.; Lutz, M.; Spek, A.L.; van Koten, G. A “Dendritic Effect” in Homogeneous Catalysis with Carbosilane-Supported Arylnickel(II) Catalysts: Observation of Active-Site Proximity Effects in Atom-Transfer Radical Addition. J. Am. Chem. Soc. 2000, 122, 12112–12124. [Google Scholar] [CrossRef]
- Pandarus, V.; Zargarian, D. New Pincer-Type Diphosphinito (POCOP) Complexes of NiII and NiIII. Chem. Commun. 2007, 978–980. [Google Scholar] [CrossRef]
- Pandarus, V.; Zargarian, D. New Pincer-Type Diphosphinito (POCOP) Complexes of Nickel. Organometallics 2007, 26, 4321–4334. [Google Scholar] [CrossRef]
- Spasyuk, D.M.; Zargarian, D.; van der Est, A. New POCN-Type Pincer Complexes of Nickel(II) and Nickel(III). Organometallics 2009, 28, 6531–6540. [Google Scholar] [CrossRef]
- Mirica, L.M.; Smith, S.M.; Griego, L. Organometallic Chemistry of High-Valent NiIII and NiIV Complexes. In Nickel Catalysis in Organic Synthesis: Methods and Reactions; Ogoshi, S., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 223–248. [Google Scholar]
- Diccianni, J.B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar] [CrossRef]

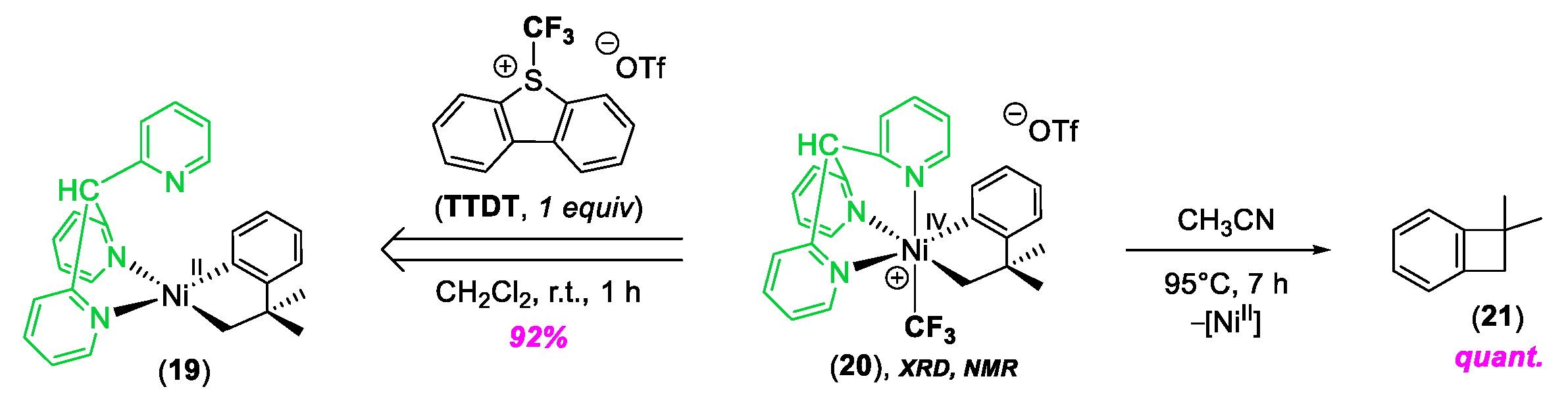
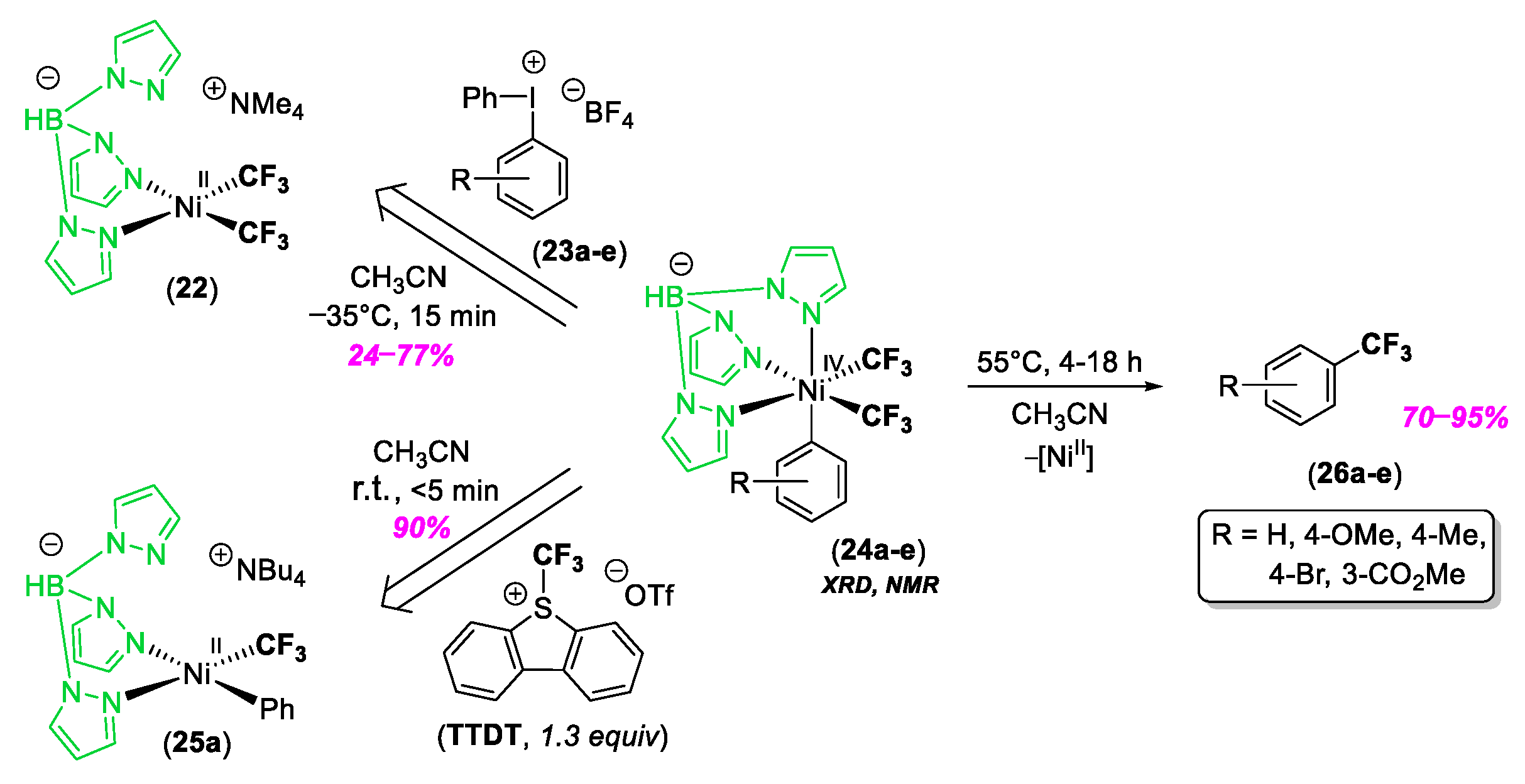

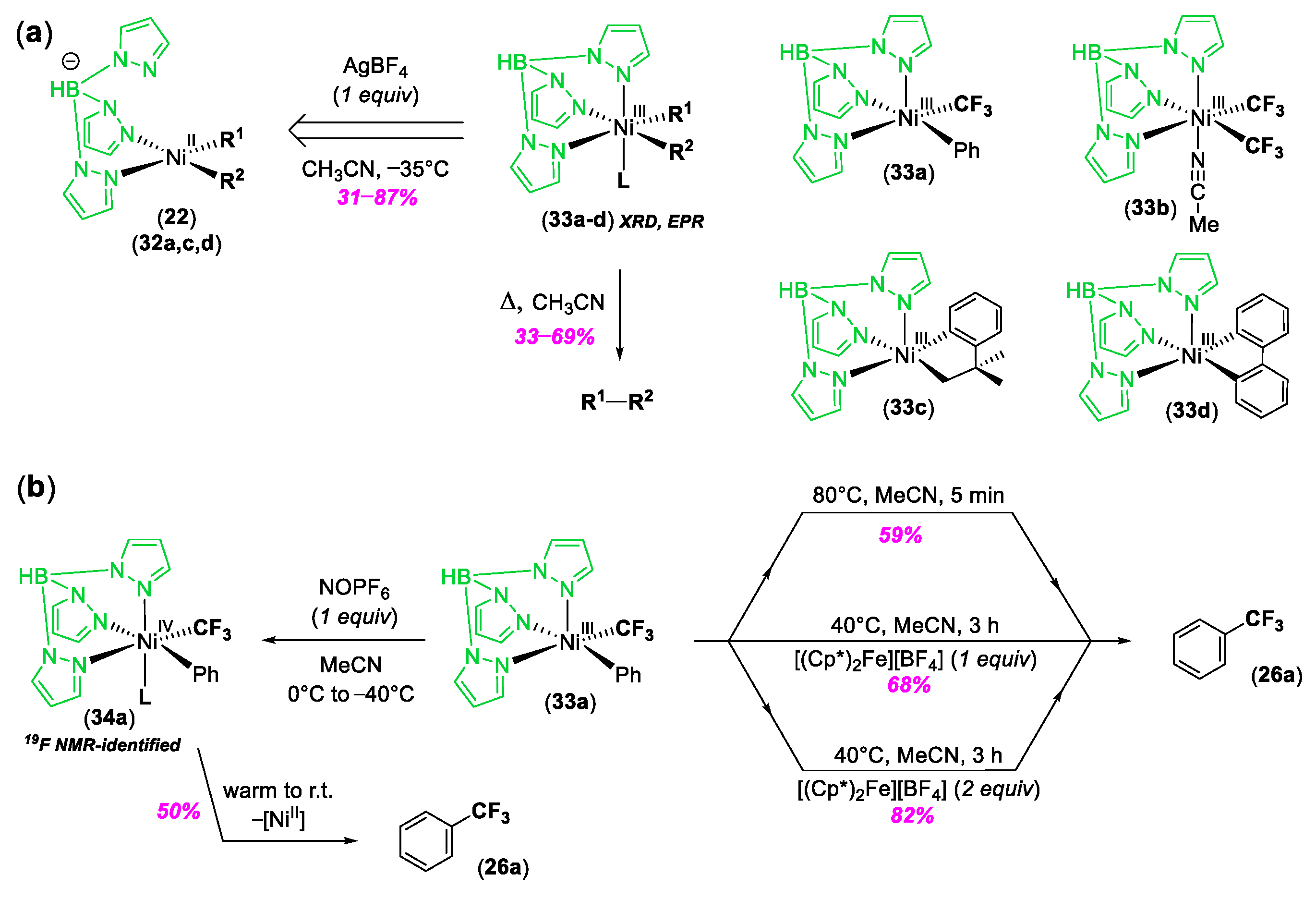
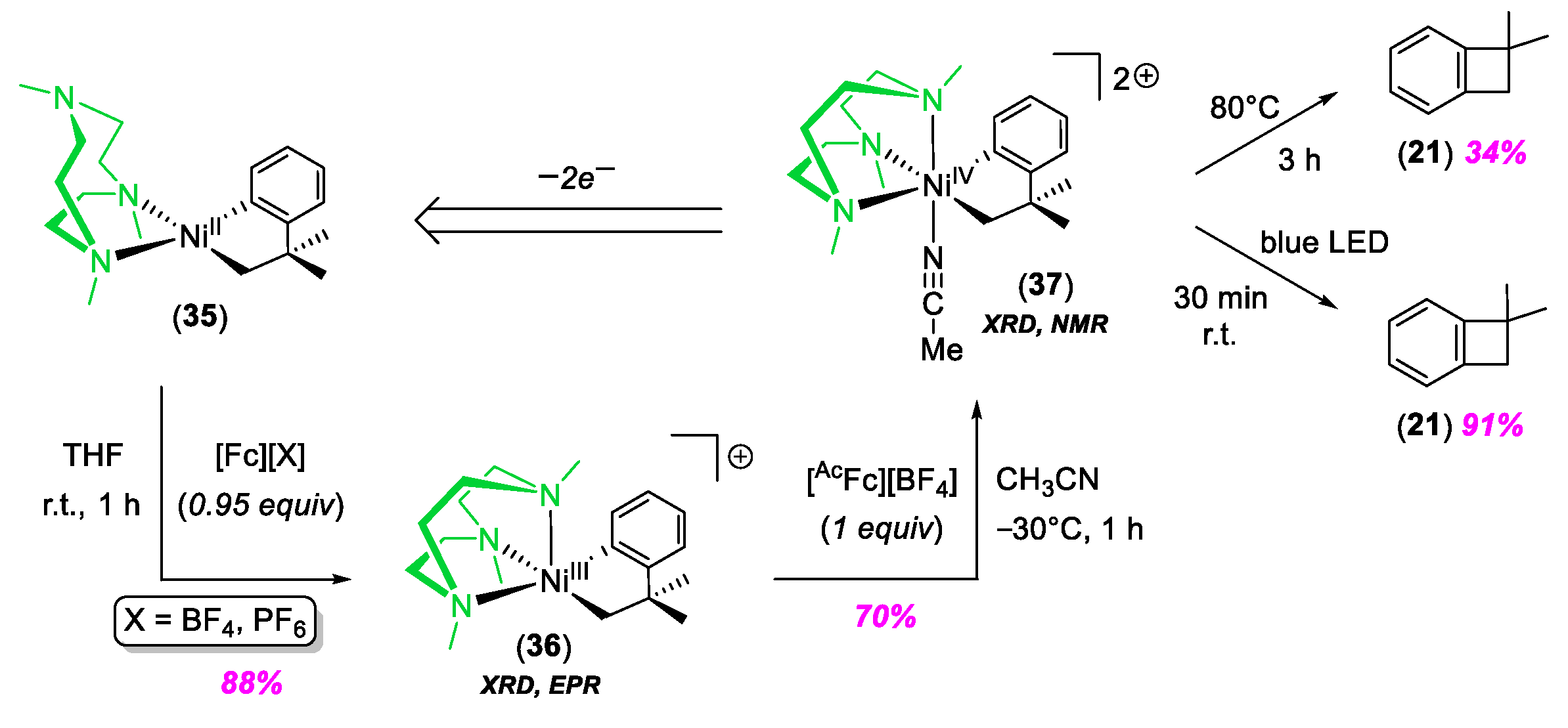

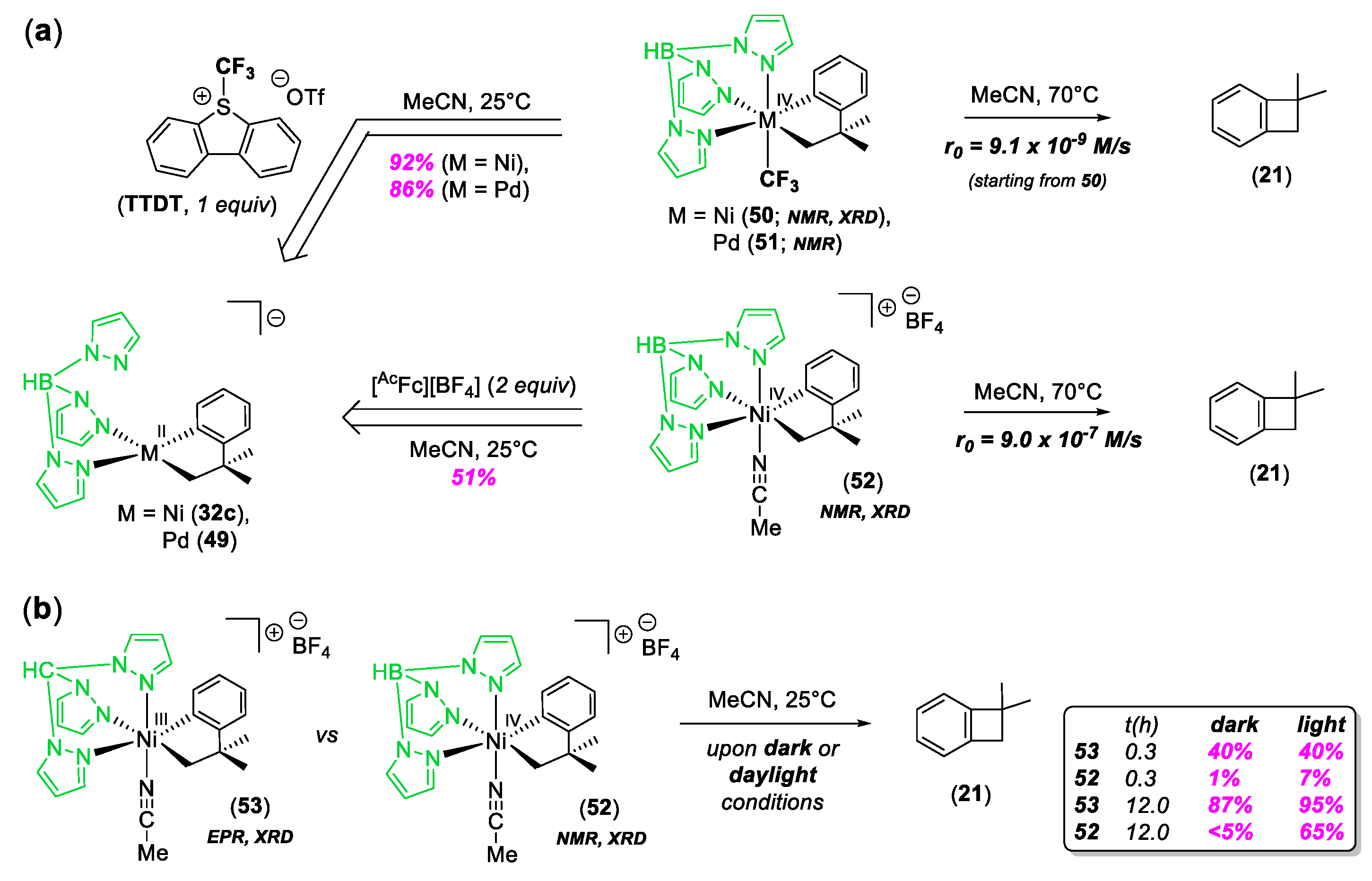
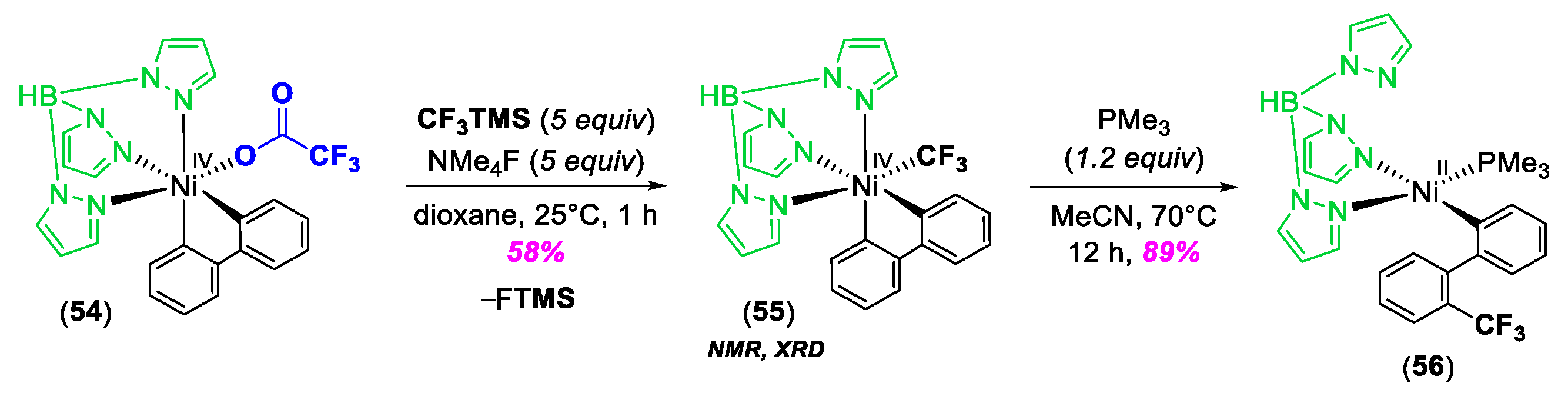
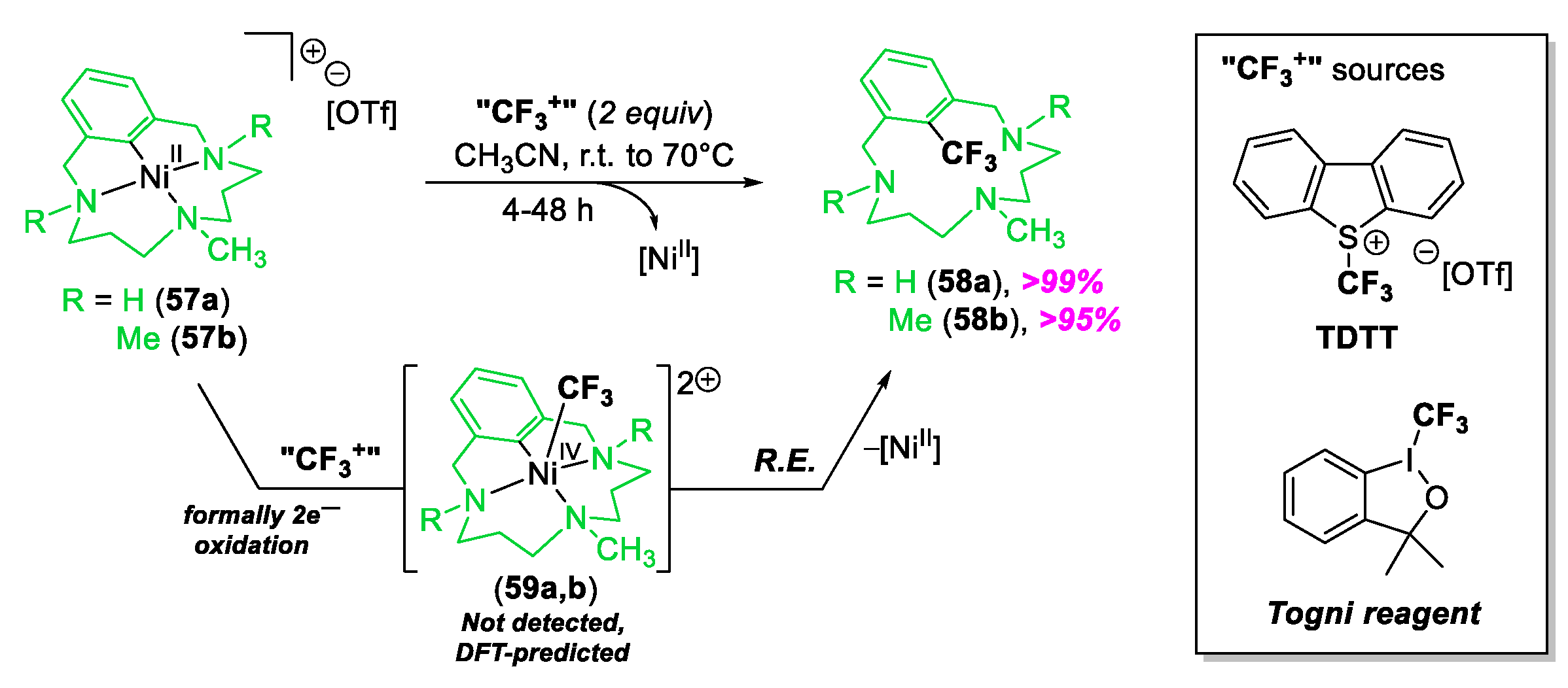
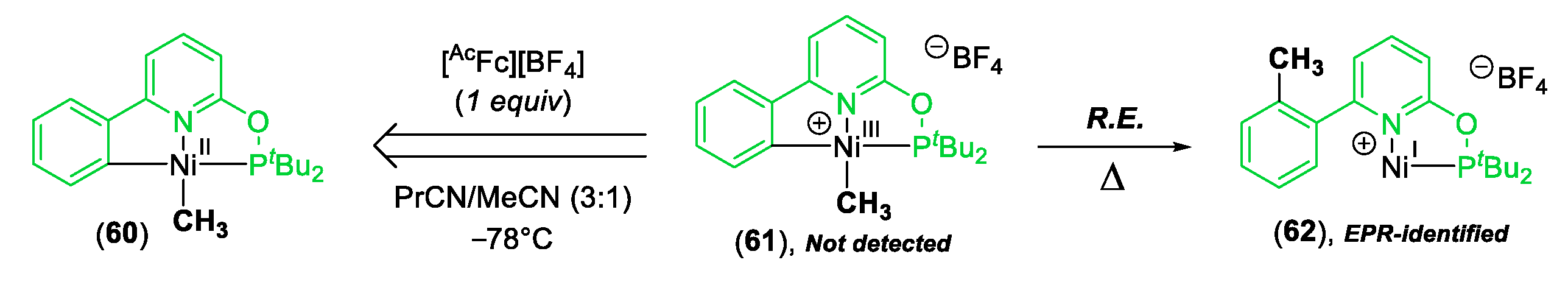

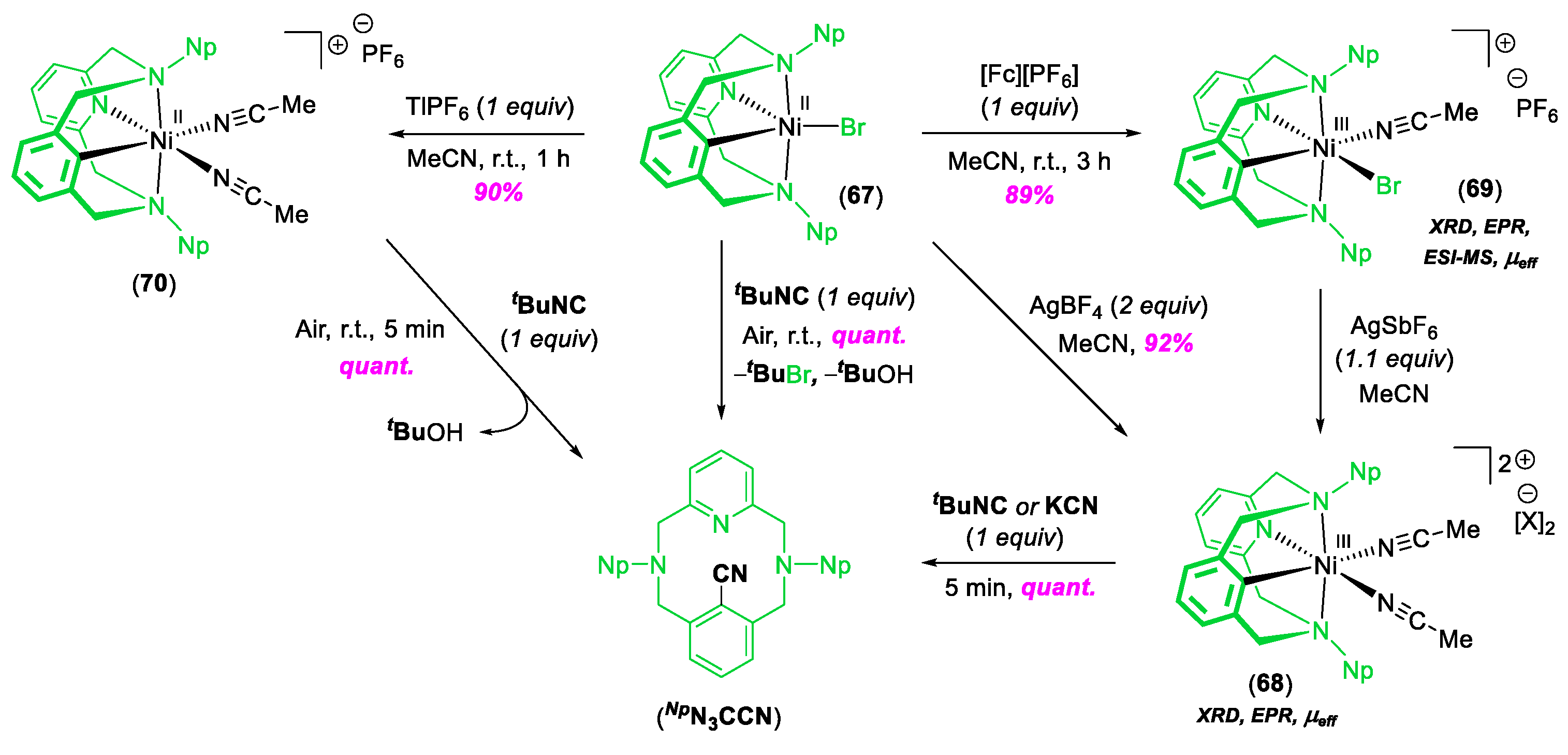
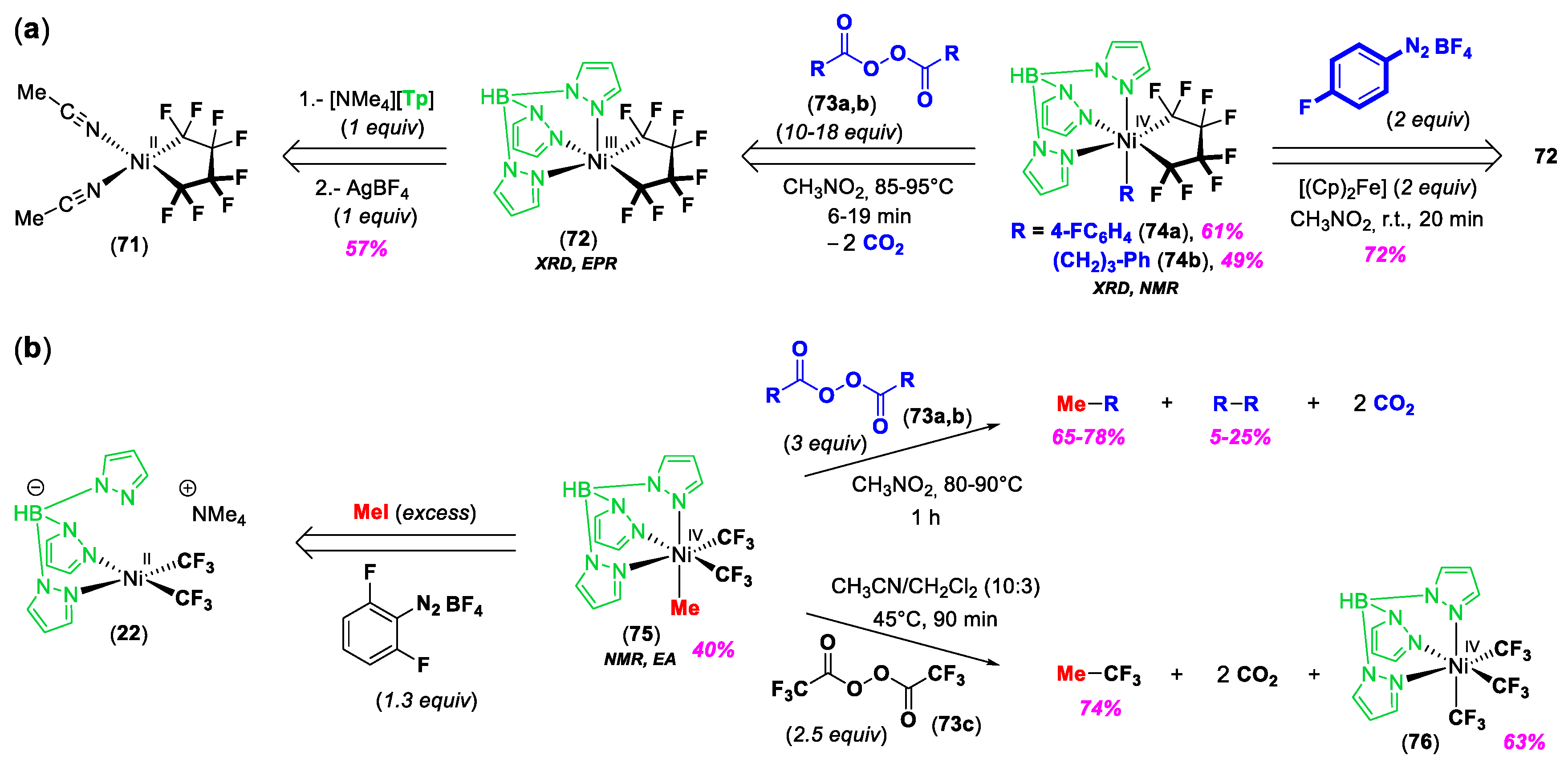


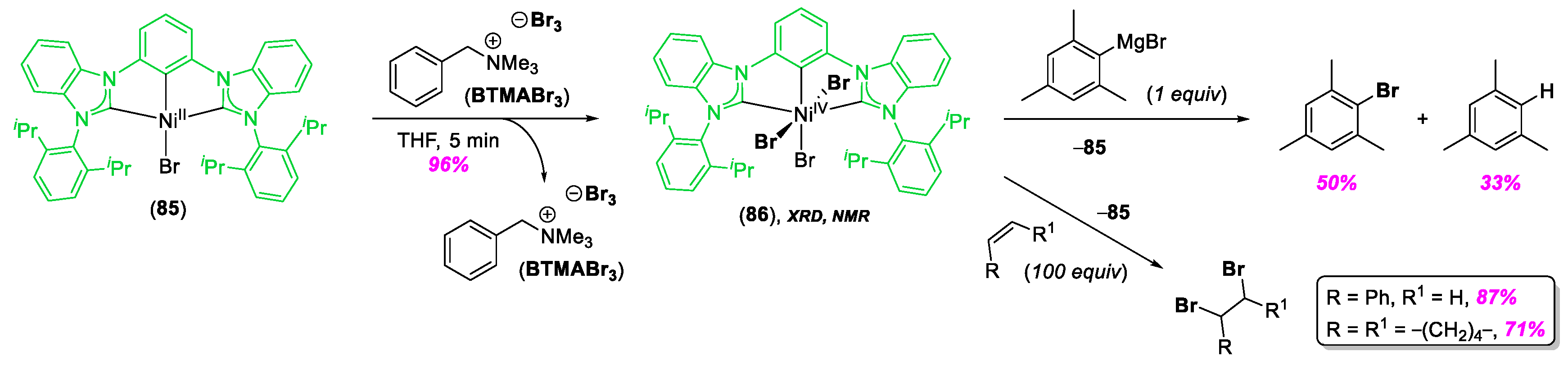
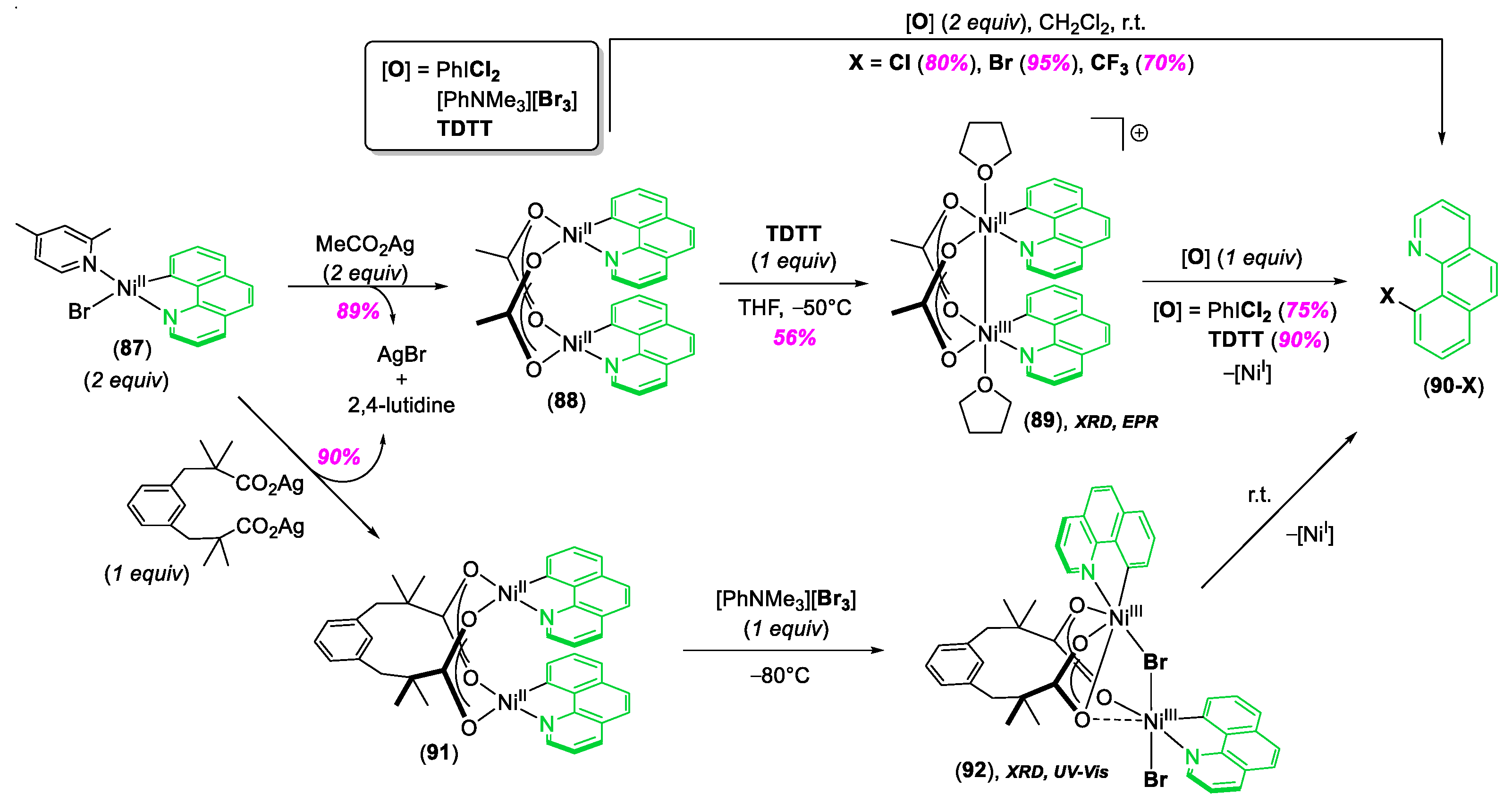






© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebra, N. High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art. Molecules 2020, 25, 1141. https://doi.org/10.3390/molecules25051141
Nebra N. High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art. Molecules. 2020; 25(5):1141. https://doi.org/10.3390/molecules25051141
Chicago/Turabian StyleNebra, Noel. 2020. "High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art" Molecules 25, no. 5: 1141. https://doi.org/10.3390/molecules25051141
APA StyleNebra, N. (2020). High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art. Molecules, 25(5), 1141. https://doi.org/10.3390/molecules25051141





