Thermal Stability of Chromium-Iron Oxidation Mixture Cermet-Based Solar Selective Absorbing Coatings
Abstract
:1. Introduction
2. Materials and Methods
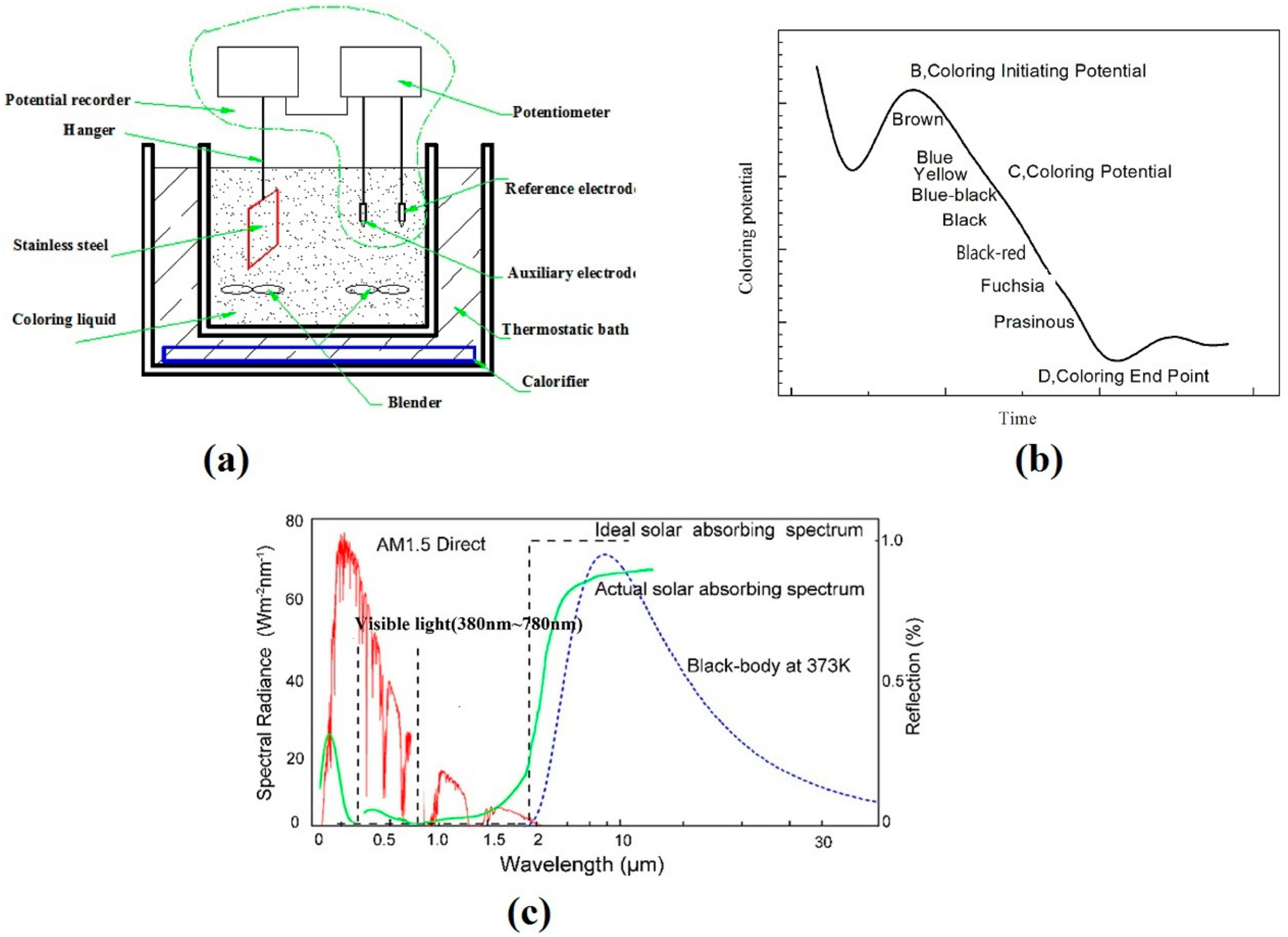
2.1. Coloring Process
2.2. Metal-Dielectric Composite Coatings
2.3. Coloring Curve
2.4. Characterization
3. Results and Discussion
3.1. Chemical Potential
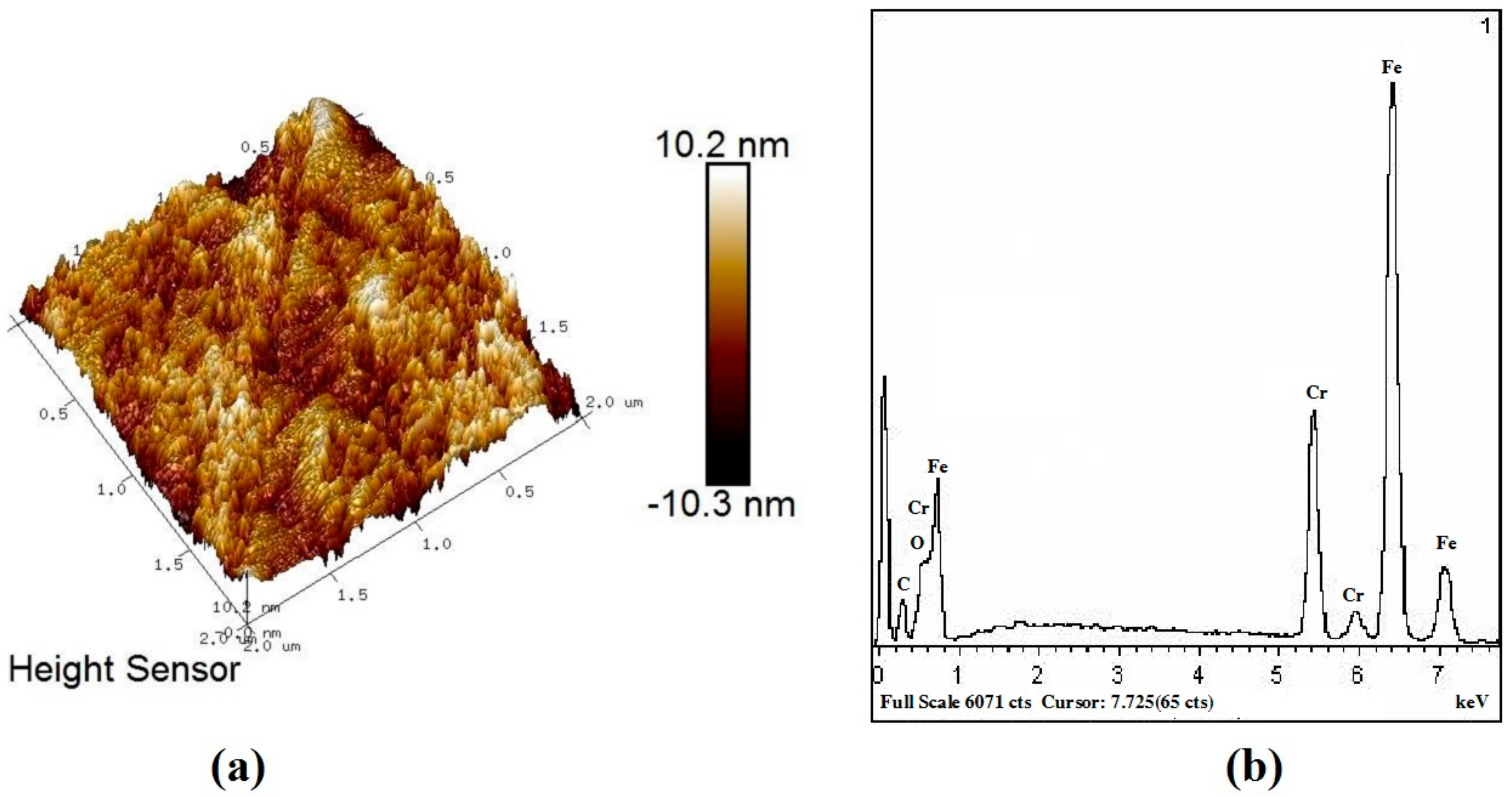
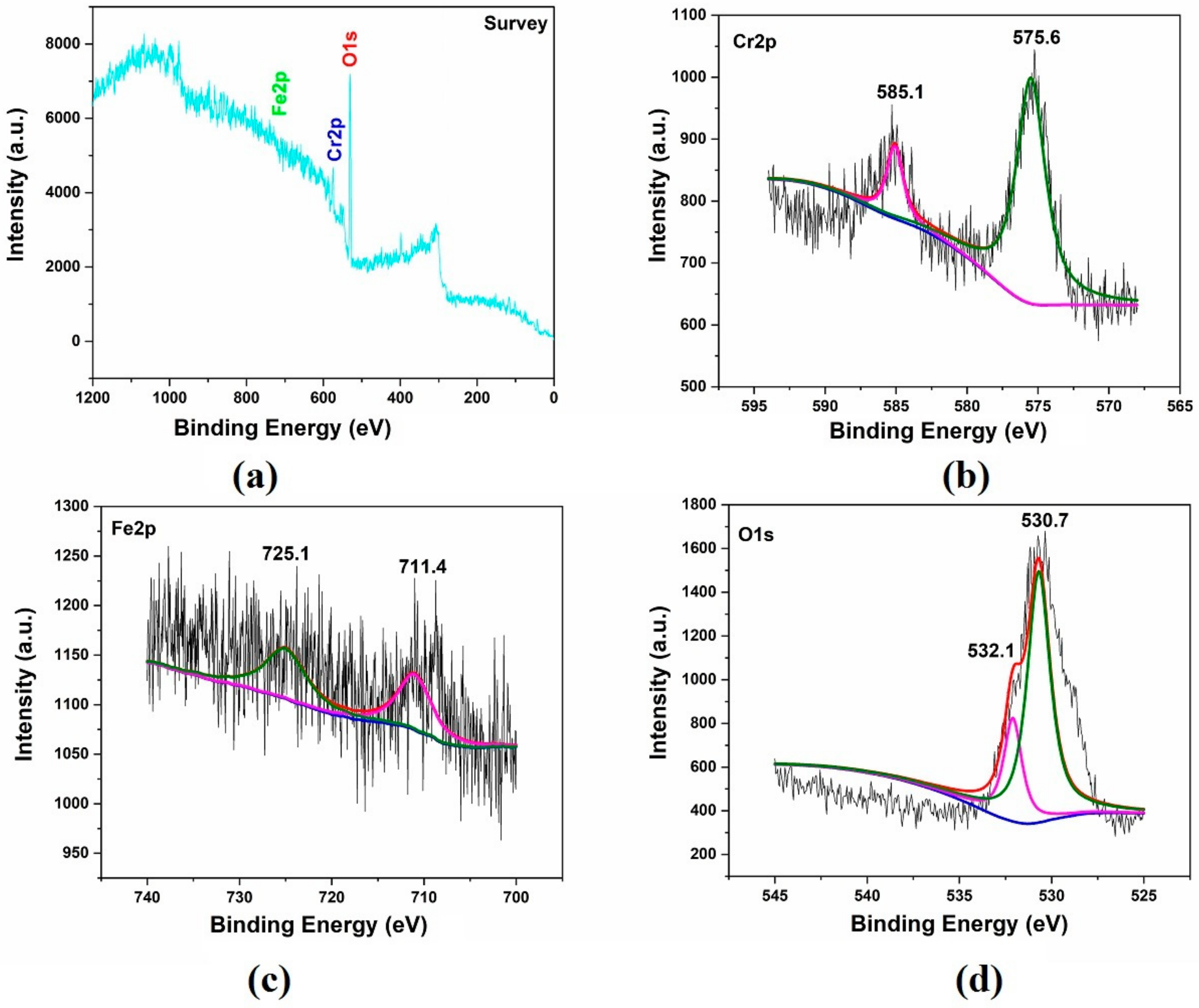
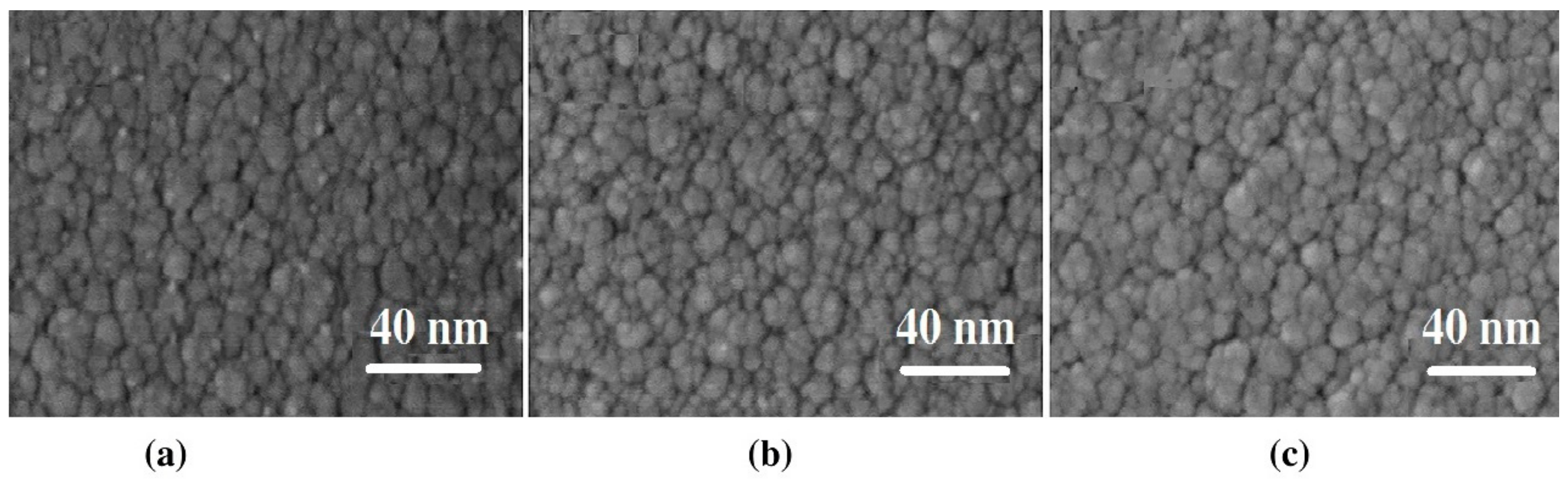
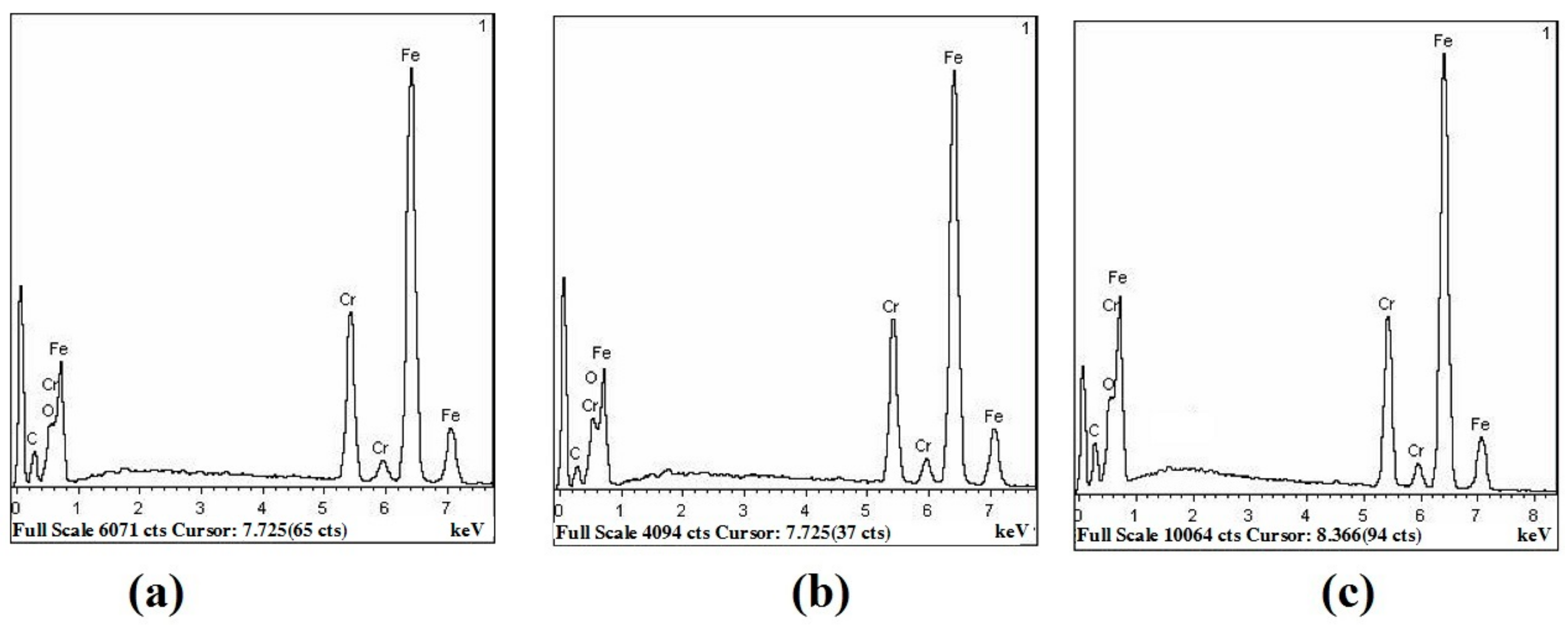
3.2. Analysis of Compositions and Pattern
3.3. Thermal Shock Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonhôte, P.; Eperon, Y.; Renaud, P. Unglazed coloured solar absorbers on façade: Modelling and performance evaluation. Sol. Energy 2009, 83, 799–811. [Google Scholar] [CrossRef]
- Joly, M.; Antonetti, Y.; Python, M.; Gonzalez, M.; Gascou, T.; Scartezzini, J.-L.; Schüler, A. Novel black selective coating for tubular solar absorbers based on a sol-gel method. Sol. Energy 2013, 94, 233–239. [Google Scholar] [CrossRef]
- Payback, S. Solar heat for industry–Solar Payback. 2017. Available online: https://www.solarpayback.com/wp-content/uploads/2017/07/Solar-Heat-for-Industry-SolarPayback-April-2017.pdf.
- Fernandes, J.C.S.; Nunes, A.; Carvalho, M.J.; Diamantino, T.C. Degradation of selective solar absorber surfaces in solar thermal collectors—An EIS study. Sol. Energy Mater. Sol. Cells 2017, 160, 149–163. [Google Scholar] [CrossRef]
- Khamlich, S.; McCrindle, R.; Nuru, Z.Y.; Cingo, N.; Maaza, M. Annealing effect on the structural and optical properties of Cr/α-Cr2O3 monodispersed particles based solar absorbers. Appl. Surf. Sci. 2013, 265, 745–749. [Google Scholar] [CrossRef]
- Khatibani, A.B. Spray pyrolytically grown NiAlOx cermets for solar thermal selective absorbers spectral properties and thermal stability. Indian Acad. Sci. 2016, 39, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sun, Z.-Q.; Wang, H. Design and characterization of solar absorbing multilayer stack based on Al/ Cr-N-O/SiO2 layers. Sol. Energy Mater. Sol. Cells 2018, 188, 18–26. [Google Scholar] [CrossRef]
- Boubault, A.; Ho, C.K.; Hall, A.; Lambert, T.N.; Ambrosini, A. Durability of solar absorber coatings and their cost-effectiveness. Sol. Energy Mater. Sol. Cells 2017, 166, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Diamantino, T.C.; Gonçalves, R.; Nunes, A.; Páscoa, S.; Carvalho, M.J. Durability of different selective solar absorber coatings in environments with different corrosivity. Sol. Energy Mater. Sol. Cells 2017, 166, 27–38. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, J. Aqueous solution-chemical derived NiAl2O3 solar selective absorbing coatings. 2. Wetting agents and spreading of aqueous solutions on aluminum substrate. Appl. Surf. Sci. 2013, 268, 231–236. [Google Scholar] [CrossRef]
- Ma, P.; Geng, Q.; Gao, X.; Yang, S.; Liu, G. Aqueous chemical solution deposition of spinel Cu1.5Mn1.5O4 single layer films for solar selective absorber. Rsc Adv. 2016, 6, 54820–54829. [Google Scholar] [CrossRef]
- Meng, J.-P.; Guo, R.-R.; Li, H.; Zhao, L.-M.; Liu, X.-P.; Li, Z. Microstructure and thermal stability of Cu/Zr0.3Al0.7N/Zr0.2Al0.8N/Al34O60N6 cermet-based solar selective absorbing coatings. Appl. Surf. Sci. 2018, 440, 932–938. [Google Scholar] [CrossRef]
- Rodríguez-Palomo, A.; Céspedes, E.; Hernández-Pinilla, D.; Prieto, C. High-temperature air-stable solar selective coating based on MoSi2–Si3N4 composite. Sol. Energy Mater. Sol. Cells 2018, 174, 50–55. [Google Scholar] [CrossRef]
- Köhl, M.; Heck, M.; Brunold, S.; Frei, U.; Carlsson, B.; Möller, K. Advanced procedure for the assessment of the lifetime of solar absorber coatings. Sol. Energy Mater. Sol. Cells 2004, 84, 275–289. [Google Scholar] [CrossRef]
- Song, P.; Wu, Y.; Wang, L.; Sun, Y.; Ning, Y.; Zhang, Y.; Dai, B.; Tomasella, E.; Bousquet, A.; Wang, C. The investigation of thermal stability of Al/NbMoN/NbMoON/SiO2 solar selective absorbing coating. Sol. Energy Mater. Sol. Cells 2017, 171, 253–257. [Google Scholar] [CrossRef]
- Soum-Glaude, A.; Le Gal, A.; Bichotte, M.; Escape, C.; Dubost, L. Optical characterization of TiAlN x /TiAlN y /Al 2 O 3 tandem solar selective absorber coatings. Sol. Energy Mater. Sol. Cells 2017, 170, 254–262. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhang, X.; Li, Y.; Cheng, X. Effect of multilayered CoO-CoAl2O4 films on improving solar absorptance of Co-WC solar selective absorbing coatings. Vacuum 2018, 155, 185–192. [Google Scholar] [CrossRef]
- Ding, D.; Wu, H.; Yu, X. Air-stable NiFeCrOx selective absorber for mid-to-high temperature application. Sol. Energy 2015, 113, 43–47. [Google Scholar] [CrossRef]
- Bayón, R.; San Vicente, G.; Morales, Á. Durability tests and up-scaling of selective absorbers based on copper–manganese oxide deposited by dip-coating. Sol. Energy Mater. Sol. Cells 2010, 94, 998–1004. [Google Scholar] [CrossRef]
- Sheu, H.-H.; Lu, C.-E.; Lee, H.-B.; Pu, N.-W.; Wu, P.-F.; Hsieh, S.-H.; Ger, M.-D. Electrodeposition of black chromium–cobalt alloy based on trivalent sulfate electrolyte. J. Taiwan Inst. Chem. Eng. 2016, 59, 496–505. [Google Scholar] [CrossRef]
- Cheng, Z.; Xue, Y.; Ju, H. Chemical coloring on stainless steel by ultrasonic irradiation. Ultrason. Sonochemistry 2018, 40, 558–566. [Google Scholar] [CrossRef]
- Cheng, Z.; Xue, Y.; Tang, Z.; An, L.; Tian, Y. A one-step process for chemical coloring on stainless steel. Surf. Coat. Technol. 2008, 202, 4102–4106. [Google Scholar] [CrossRef]
- Corredor, J.; Bergmann, C.P.; Pereira, M.; Dick, L.F.P. Coloring ferritic stainless steel by an electrochemical–photochemical process under visible light illumination. Surf. Coat. Technol. 2014, 245, 125–132. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Wang, H.; Chen, R. A one-step sol–gel route derived Ag–CuO film as a novel solar selective absorber. Sol. Energy Mater. Sol. Cells 2016, 144, 264–272. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Li, Q.; Min, J.; Cheng, X. Spectral properties of AlCrNO-based multi-layer solar selective absorbing coating during the initial stage of thermal aging upon exposure to air. Sol. Energy Mater. Sol. Cells 2018, 188, 81–92. [Google Scholar] [CrossRef]
- Kikuti, E.; Bocchi, N.; Pastol, J.L.; Ferreira, M.G.; Montemor, M.F.; da Cunha Belo, M.; Simões, A.M. Composition and structure of coloured oxide films on stainless steel formed by triangular current scan and cathodic hardening treatment. Corros. Sci. 2007, 49, 2303–2314. [Google Scholar] [CrossRef]
- Kim, J.-S.; Chung, W.-S.; Kim, K.; Kim, D.Y.; Paeng, K.-J.; Jo, S.M.; Jang, S.-Y. Performance optimization of polymer solar cells using electrostatically sprayed photoactive layers. Adv. Funct. Mater. 2010, 20, 3538–3546. [Google Scholar] [CrossRef]
- Amri, A.; Jiang, Z.-T.; Pryor, T.; Yin, C.-Y.; Xie, Z.; Mondinos, N. Optical and mechanical characterization of novel cobalt-based metal oxide thin films synthesized using sol–gel dip-coating method. Surf. Coat. Technol. 2012, 207, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Nie, X.; Zhao, J.; Han, Y.; Shrotriya, P.; Wang, Y.; Guo, J. Coloring stability analysis of nanosecond pulsed laser induced surface coloring on stainless steel. Opt. Laser Technol. 2020, 123, 105936. [Google Scholar] [CrossRef]
Sample Availability: Samples of the solar selective absorber coatings are available from the authors. |
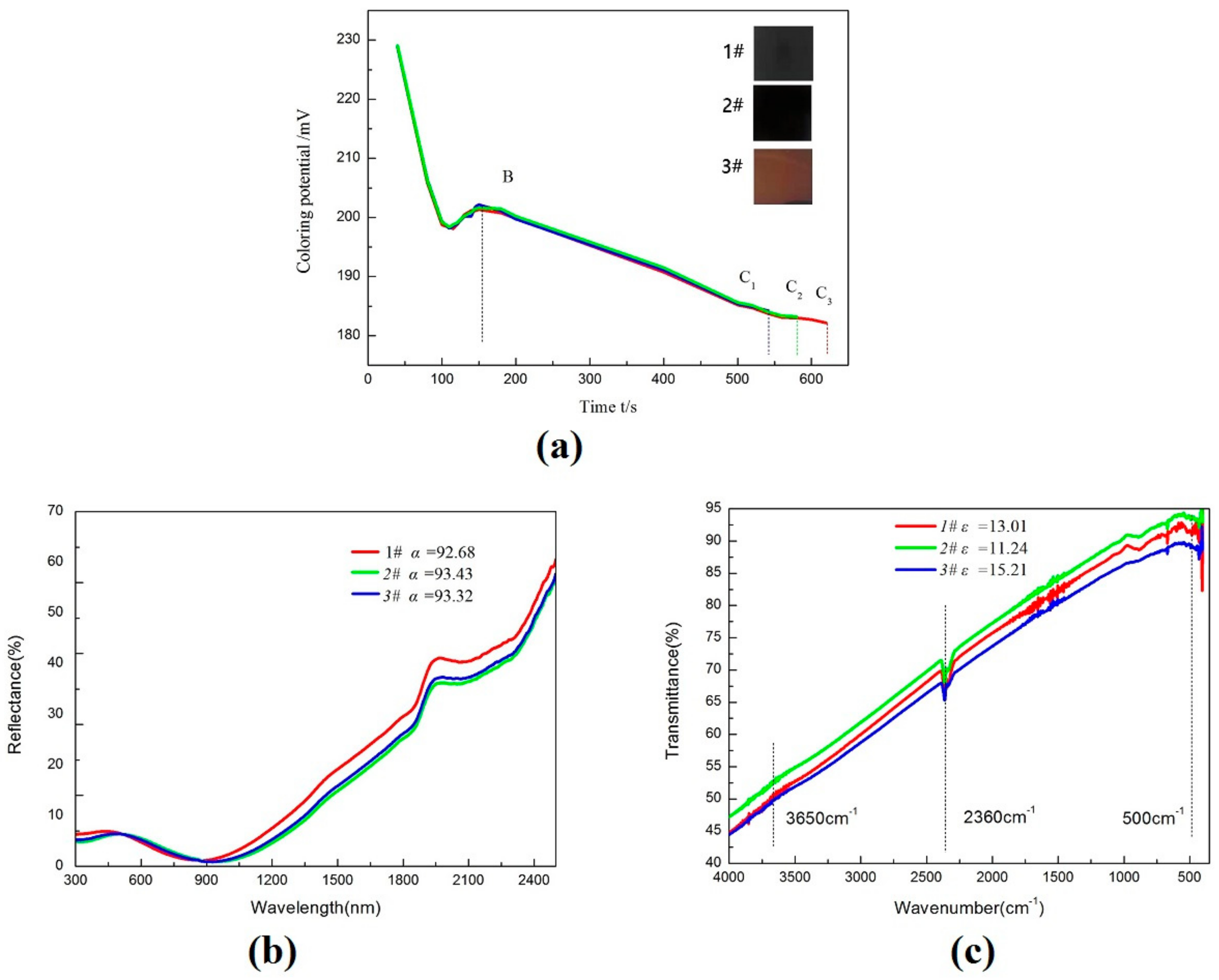

| Sample | Color Traditional | Coloring Time (s) | Chemical Potential ΔE/mV | Absorptance (AM1.5) | Emittance (90 °C) | S |
|---|---|---|---|---|---|---|
| 1# | Black-blue | 560 | 17.9 | 0.9268 | 0.130 | 7.13 |
| 2# | Black | 580 | 18.4 | 0.9343 | 0.112 | 8.34 |
| 3# | Black-red | 620 | 19.1 | 0.9332 | 0.152 | 6.14 |
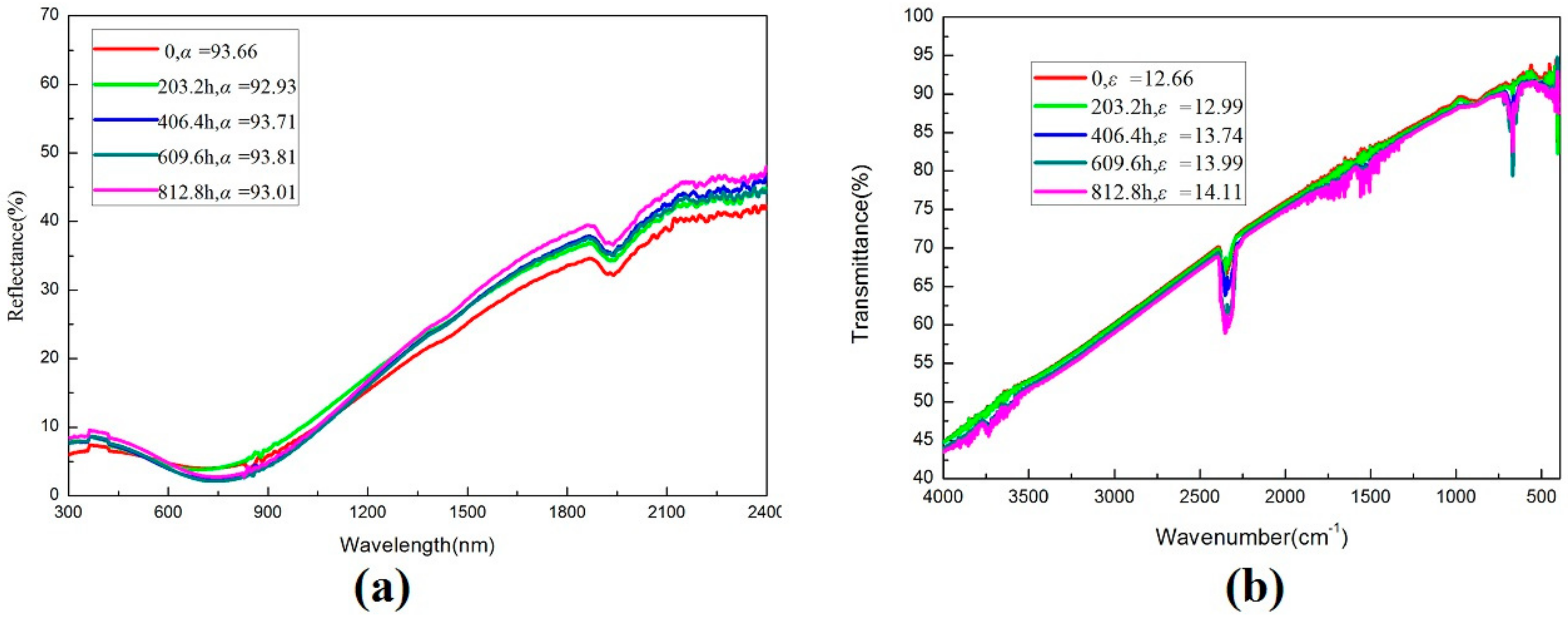
| Test Time (h) | Absorptance (AM1.5) | Emittance (90 °C) | PC |
|---|---|---|---|
| 203.2 | 92.93 | 12.99 | 0.00895 |
| 406.4 | 93.71 | 13.74 | 0.0049 |
| 609.6 | 93.81 | 13.99 | 0.00515 |
| 812.8 | 93.01 | 14.11 | 0.01375 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, J.; Zhang, Q.; Pang, W.; Yan, H.; Li, G. Thermal Stability of Chromium-Iron Oxidation Mixture Cermet-Based Solar Selective Absorbing Coatings. Molecules 2020, 25, 1178. https://doi.org/10.3390/molecules25051178
Yu H, Li J, Zhang Q, Pang W, Yan H, Li G. Thermal Stability of Chromium-Iron Oxidation Mixture Cermet-Based Solar Selective Absorbing Coatings. Molecules. 2020; 25(5):1178. https://doi.org/10.3390/molecules25051178
Chicago/Turabian StyleYu, Hongwen, Jinkai Li, Qian Zhang, Wei Pang, Hui Yan, and Guangyuan Li. 2020. "Thermal Stability of Chromium-Iron Oxidation Mixture Cermet-Based Solar Selective Absorbing Coatings" Molecules 25, no. 5: 1178. https://doi.org/10.3390/molecules25051178





