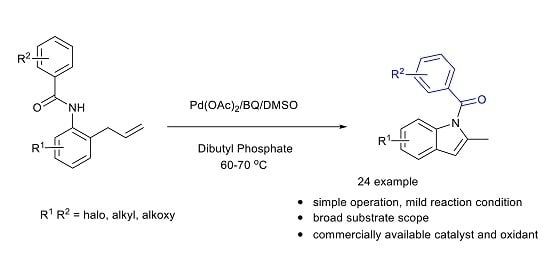
Synthesis of Functionalized Indoles via Palladium-Catalyzed Cyclization of N-(2-allylphenyl) Benzamide: A Method for Synthesis of Indomethacin Precursor
Abstract
1. Introduction
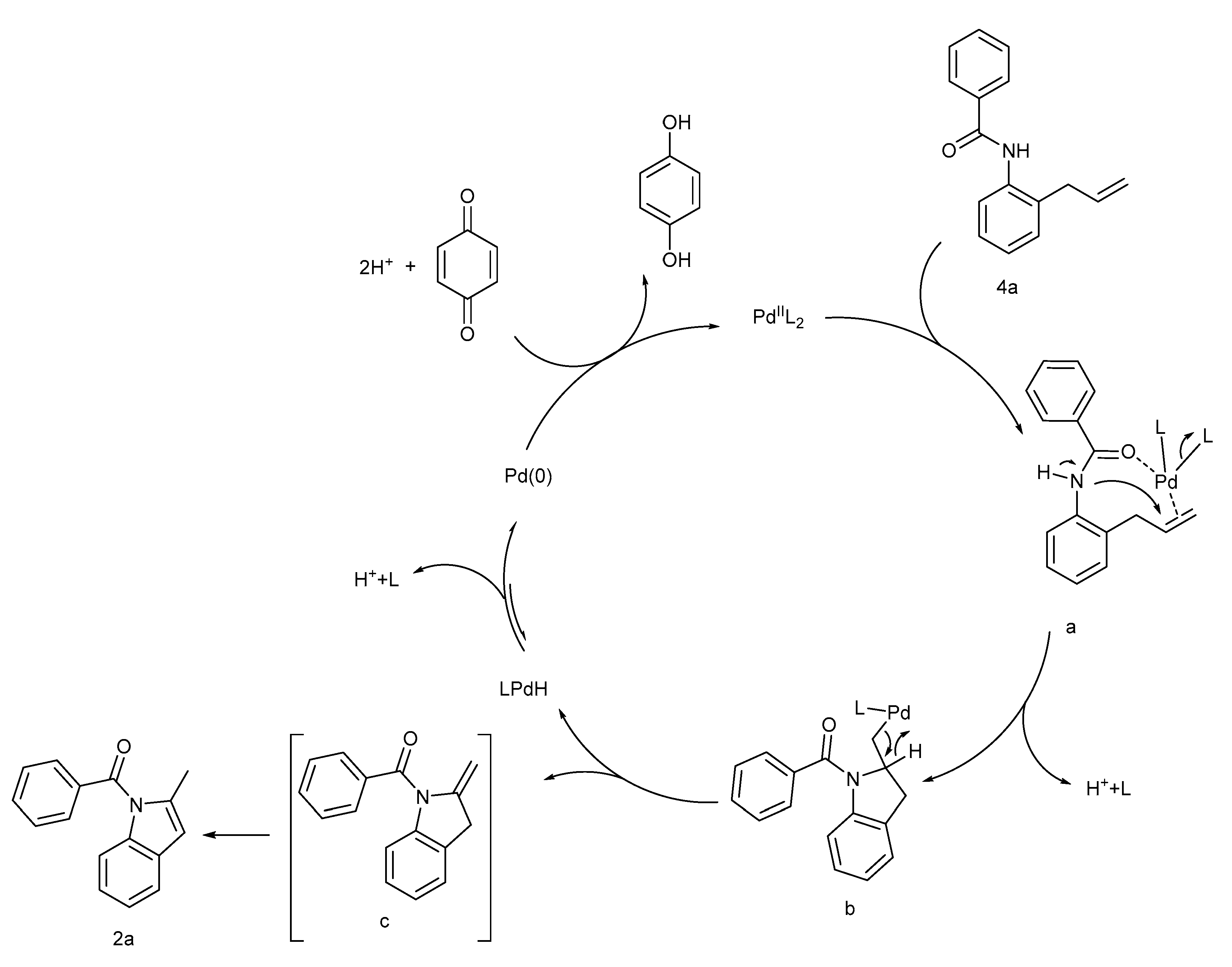
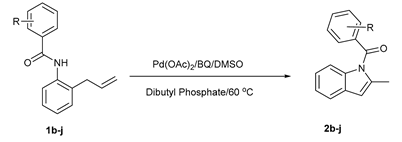
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Somei, M.; Yamada, F. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2004, 21, 278–311. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Higuchi, K. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2005, 22, 761–793. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Randriambola, L.; Quirion, J.C.; Kan-Fan, C.; Husson, H.P. Structure of goniomitine, a new type of indole alkaloid. Tetrahedron Lett. 1987, 28, 2123–2126. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Fischer, E.; Jourdan, F. Ueber die Hydrazine der Brenztraubensä ure. Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K. Synthesis of Indoles Via Palladium-Catalyzed Heteroannulation of Internal Alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K.; Refvik, M.D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1998, 63, 7652–7662. [Google Scholar] [CrossRef]
- Wagaw, S.; Yang, B.H.; Buchwald, S.L. A palladium-catalyzed strategy for the preparation of indoles: A novel entry into the Fischer indole synthesis. J. Am. Chem. Soc. 1998, 120, 6621–6622. [Google Scholar] [CrossRef]
- Wagaw, S.; Yang, B.H.; Buchwald, S.L. A palladium-catalyzed method for the preparation of indoles via the Fischer indole synthesis. J. Am. Chem. Soc. 1999, 121, 10251–10263. [Google Scholar] [CrossRef]
- Rutherford, J.L.; Rainka, M.P.; Buchwald, S.L. An annulative approach to highly substituted indoles: Unusual effect of phenolic additives on the success of the arylation of ketone enolates. J. Am. Chem. Soc. 2002, 124, 15168–15169. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, L.S.; Allen, G.F.; Waterman, E.L. Palladium assisted intramolecular amination of olefins. A new synthesis of indoles. J. Am. Chem. Soc. 1976, 98, 2674–2676. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Allen, G.F.; Bozell, J.J.; Waterman, E.L. Palladium-assisted intramolecular amination of olefins. Synthesis of nitrogen heterocycles. J. Am. Chem. Soc. 1978, 100, 5800–5807. [Google Scholar] [CrossRef]
- Bozell, J.J.; Hegedus, L.S. Palladium-Assisted Functionalization of Olefins-a New Amination of Electron-Deficient Olefins. J. Org. Chem. 1981, 46, 2561–2563. [Google Scholar] [CrossRef]
- Louis, S.H. Transition Metals in the Synthesis and Functionaiization of Indoles. Angew. Chem. Int. Ed. 1988, 27, 1113–1126. [Google Scholar] [CrossRef]
- Stuart, D.R.; Bertrand-Laperle, M.; Burgess, K.M.; Fagnou, K. Indole synthesis via rhodium catalyzed oxidative coupling of acetanilides and internal alkynes. J. Am. Chem. Soc. 2008, 130, 16474–16475. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Copper-catalyzed C-C bond formation through C-H functionalization: Synthesis of multisubstituted indoles from N-aryl enaminones. Angew. Chem. Int. Ed. 2009, 48, 8078–8081. [Google Scholar] [CrossRef]
- Guan, Z.H.; Yan, Z.Y.; Ren, Z.H.; Liu, X.Y.; Liang, Y.M. Preparation of indoles via iron catalyzed direct oxidative coupling. Chem. Commun. 2010, 46, 2823–2825. [Google Scholar] [CrossRef]
- Stuart, D.R.; Alsabeh, P.; Kuhn, M.; Fagnou, K. Rhodium(III)-catalyzed arene and alkene C-H bond functionalization leading to indoles and pyrroles. J. Am. Chem. Soc. 2010, 132, 18326–18339. [Google Scholar] [CrossRef]
- Tan, Y.; Hartwig, J.F. Palladium-catalyzed amination of aromatic C-H bonds with oxime esters. J. Am. Chem. Soc. 2010, 132, 3676–3677. [Google Scholar] [CrossRef]
- Huestis, M.P.; Chan, L.; Stuart, D.R.; Fagnou, K. The vinyl moiety as a handle for regiocontrol in the preparation of unsymmetrical 2,3-aliphatic-substituted indoles and pyrroles. Angew. Chem. Int. Ed. 2011, 50, 1338–1341. [Google Scholar] [CrossRef]
- Neumann, J.J.; Rakshit, S.; Droge, T.; Wurtz, S.; Glorius, F. Exploring the oxidative cyclization of substituted N-aryl enamines: Pd-catalyzed formation of indoles from anilines. Chem. Eur. J. 2011, 17, 7298–7303. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, L.; Zhang, L. Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes. Chem. Commun. 2011, 47, 7815–7817. [Google Scholar] [CrossRef]
- Wei, Y.; Deb, I.; Yoshikai, N. Palladium-catalyzed aerobic oxidative cyclization of N-aryl imines: Indole synthesis from anilines and ketones. J. Am. Chem. Soc. 2012, 134, 9098–9101. [Google Scholar] [CrossRef]
- Song, W.; Ackermann, L. Nickel-catalyzed alkyne annulation by anilines: Versatile indole synthesis by C-H/N-H functionalization. Chem. Commun. 2013, 49, 6638–6640. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Fang, Y.; Huang, Y. General and efficient synthesis of indoles through triazene-directed C-H annulation. Angew. Chem. Int. Ed. 2013, 52, 5795–5798. [Google Scholar] [CrossRef]
- Tremont, S.J.; Rahman, H.U. Ortho-Alkylation of Acetanilides Using Alkyl-Halides and Palladium Acetate. J. Am. Chem. Soc. 1984, 106, 5759–5760. [Google Scholar] [CrossRef]
- Fix, S.R.; Brice, J.L.; Stahl, S.S. Efficient intramolecular oxidative amination of olefins through direct dioxygen-coupled palladium catalysis. Angew. Chem. Int. Ed. 2002, 41, 164–166. [Google Scholar] [CrossRef]
- Nallagonda, R.; Rehan, M.; Ghorai, P. Synthesis of functionalized indoles via palladium-catalyzed aerobic oxidative cycloisomerization of o-allylanilines. Org. Lett. 2014, 16, 4786–4789. [Google Scholar] [CrossRef]
- Ning, X.S.; Wang, M.M.; Qu, J.P.; Kang, Y.B. Synthesis of Functionalized Indoles via Palladium-Catalyzed Aerobic Cycloisomerization of o-Allylanilines Using Organic Redox Cocatalyst. J. Org. Chem. 2018, 83, 13523–13529. [Google Scholar] [CrossRef]
- Savvidou, A.; IoannisTzaras, D.; Koutoulogenis, G.S.; Theodorou, A.; Kokotos, C.G. Synthesis of Benzofuran and Indole Derivatives Catalyzed by Palladium on Carbon. Eur. J. Org. Chem. 2019, 2019, 3890–3897. [Google Scholar] [CrossRef]
- Cajaraville, A.; Lopez, S.; Varela, J.A.; Saa, C. Rh(III)-catalyzed tandem C-H allylation and oxidative cyclization of anilides: A new entry to indoles. Org. Lett. 2013, 15, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.; Sharma, S.; Han, S.; Han, S.H.; Kwak, J.H.; Jung, Y.H.; Kim, I.S. Synthesis and C2-functionalization of indoles with allylic acetates under rhodium catalysis. Org. Biomol. Chem. 2013, 11, 7427–7434. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.-J.; Cheng, W.-M.; Su, W.; Xiao, B.; Fu, Y. Synthesis of indoles through Rh(III)-catalyzed C–H cross-coupling with allyl carbonates. Tetrahedron Lett. 2014, 55, 1859–1862. [Google Scholar] [CrossRef]
- Andries-Ulmer, A.; Brunner, C.; Rehbein, J.; Gulder, T. Fluorine as a Traceless Directing Group for the Regiodivergent Synthesis of Indoles and Tryptophans. J. Am. Chem. Soc. 2018, 140, 13034–13041. [Google Scholar] [CrossRef]
- Xia, H.D.; Zhang, Y.D.; Wang, Y.H.; Zhang, C. Water-Soluble Hypervalent Iodine(III) Having an I-N Bond. A Reagent for the Synthesis of Indoles. Org. Lett. 2018, 20, 4052–4056. [Google Scholar] [CrossRef]
- McDonald, R.I.; Stahl, S.S. Modular synthesis of 1,2-diamine derivatives by palladium-catalyzed aerobic oxidative cyclization of allylic sulfamides. Angew. Chem. Int. Ed. 2010, 49, 5529–5532. [Google Scholar] [CrossRef]
- Strambeanu, I.I.; White, M.C. Catalyst-controlled C-O versus C-N allylic functionalization of terminal olefins. J. Am. Chem. Soc. 2013, 135, 12032–12037. [Google Scholar] [CrossRef]
- Osberger, T.J.; White, M.C. N-Boc amines to oxazolidinones via Pd(II)/bis-sulfoxide/Bronsted acid co-catalyzed allylic C-H oxidation. J. Am. Chem. Soc. 2014, 136, 11176–11181. [Google Scholar] [CrossRef]
- CCDC 1894284 contains the supplementary crystallographic data for 2r. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.czm.ac.uk/datarequest/cif.
Sample Availability: Samples of the compounds are not available from the authors. |



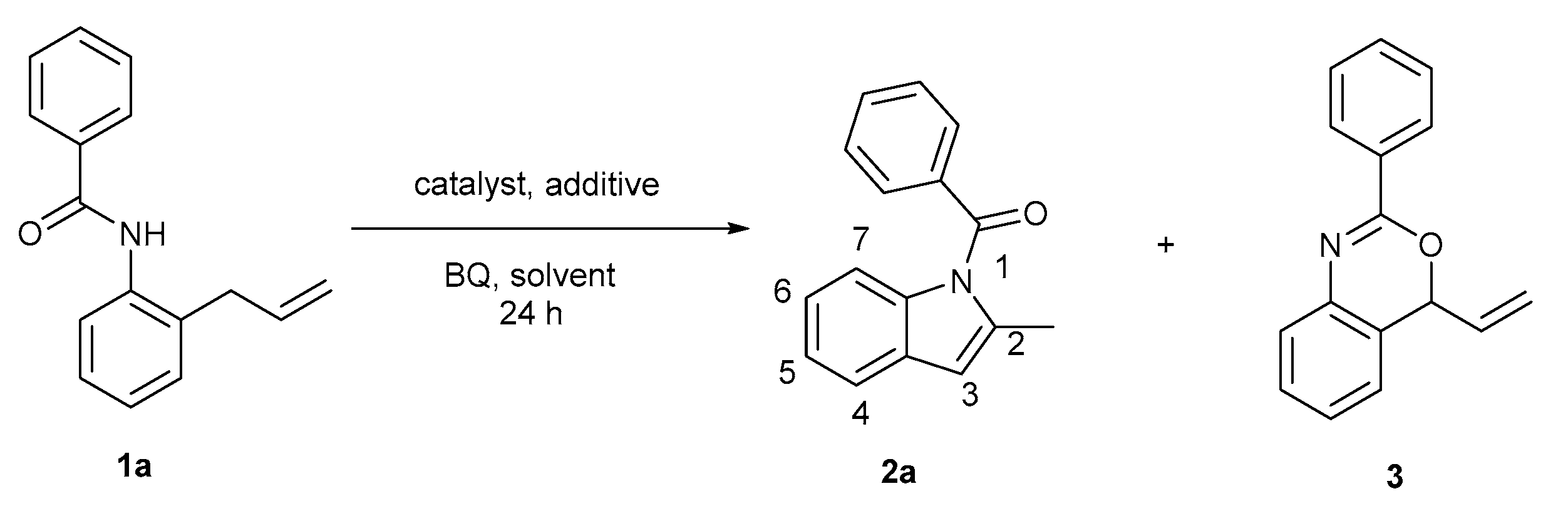
| Entry | Catalyst | Additive | Solvent | T [°C] | Yield b (2a:3) c |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | - | CH3CN | RT | 8 (1:1) |
| 2 | Pd(OAc)2 | - | CH3CN | 45 | 26 (2:1) |
| 3 | Pd(OAc)2 | - | CH3CN | 60 | 33 (4:1) |
| 4 | Pd(OAc)2 | AcOH | CH3CN | 60 | 12 (10:1) |
| 5 | Pd(OAc)2 | PhCOOH | CH3CN | 60 | 22 (10:1) |
| 6 | Pd(OAc)2 | TFA | CH3CN | 60 | 26 (9:1) |
| 7 | Pd(OAc)2 | Ph2PO2H | CH3CN | 60 | 36 (15:1) |
| 8 | Pd(OAc)2 | (BuO)2PO2H | CH3CN | 60 | 71 (>20:1) |
| 9 | PdCl2 | (BuO)2PO2H | CH3CN | 60 | 23 (>20:1) |
| 10 | White catalyst d | (BuO)2PO2H | CH3CN | 60 | 9 (>20:1) |
| 11 | Pd(OAc)2 | (BuO)2PO2H | DMSO | 60 | 77 (>20:1) |
| 12 | Pd(OAc)2 | (BuO)2PO2H | dioxane | 60 | 52 (>20:1) |
| 13 | Pd(OAc)2 | (BuO)2PO2H | THF | 60 | 45 (>20:1) |
| 14 | Pd(OAc)2 | (BuO)2PO2H | DMF | 60 | 36 (>20:1) |
| 15 | Pd(OAc)2 | (BuO)2PO2H | DMSO | 60 | 81 (>20:1) |
 |
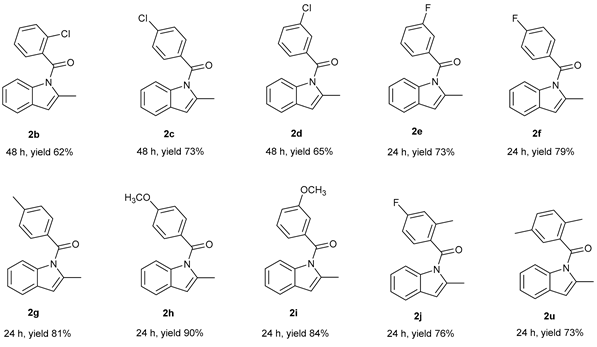 |
 |
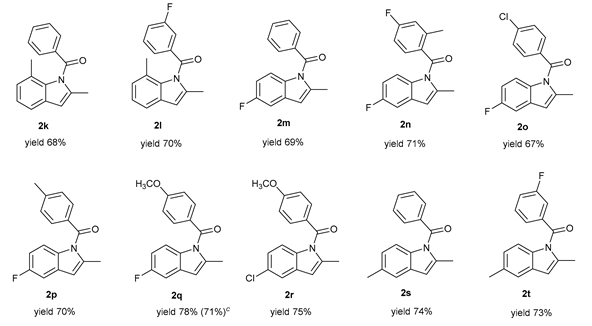 |
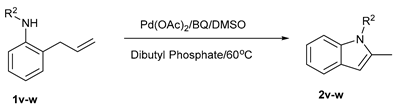 |
 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Ma, T.; Zhang, Y.; Dong, Z.; Zhao, H.; Zhao, D. Synthesis of Functionalized Indoles via Palladium-Catalyzed Cyclization of N-(2-allylphenyl) Benzamide: A Method for Synthesis of Indomethacin Precursor. Molecules 2020, 25, 1233. https://doi.org/10.3390/molecules25051233
Chang Z, Ma T, Zhang Y, Dong Z, Zhao H, Zhao D. Synthesis of Functionalized Indoles via Palladium-Catalyzed Cyclization of N-(2-allylphenyl) Benzamide: A Method for Synthesis of Indomethacin Precursor. Molecules. 2020; 25(5):1233. https://doi.org/10.3390/molecules25051233
Chicago/Turabian StyleChang, Zhe, Tong Ma, Yu Zhang, Zheng Dong, Heng Zhao, and Depeng Zhao. 2020. "Synthesis of Functionalized Indoles via Palladium-Catalyzed Cyclization of N-(2-allylphenyl) Benzamide: A Method for Synthesis of Indomethacin Precursor" Molecules 25, no. 5: 1233. https://doi.org/10.3390/molecules25051233
APA StyleChang, Z., Ma, T., Zhang, Y., Dong, Z., Zhao, H., & Zhao, D. (2020). Synthesis of Functionalized Indoles via Palladium-Catalyzed Cyclization of N-(2-allylphenyl) Benzamide: A Method for Synthesis of Indomethacin Precursor. Molecules, 25(5), 1233. https://doi.org/10.3390/molecules25051233