Response Surface Optimization of an Extraction Method for the Simultaneous Detection of Sulfamethoxazole and 17β-Estradiol in Soil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Conditions for the HPLC Detection Method
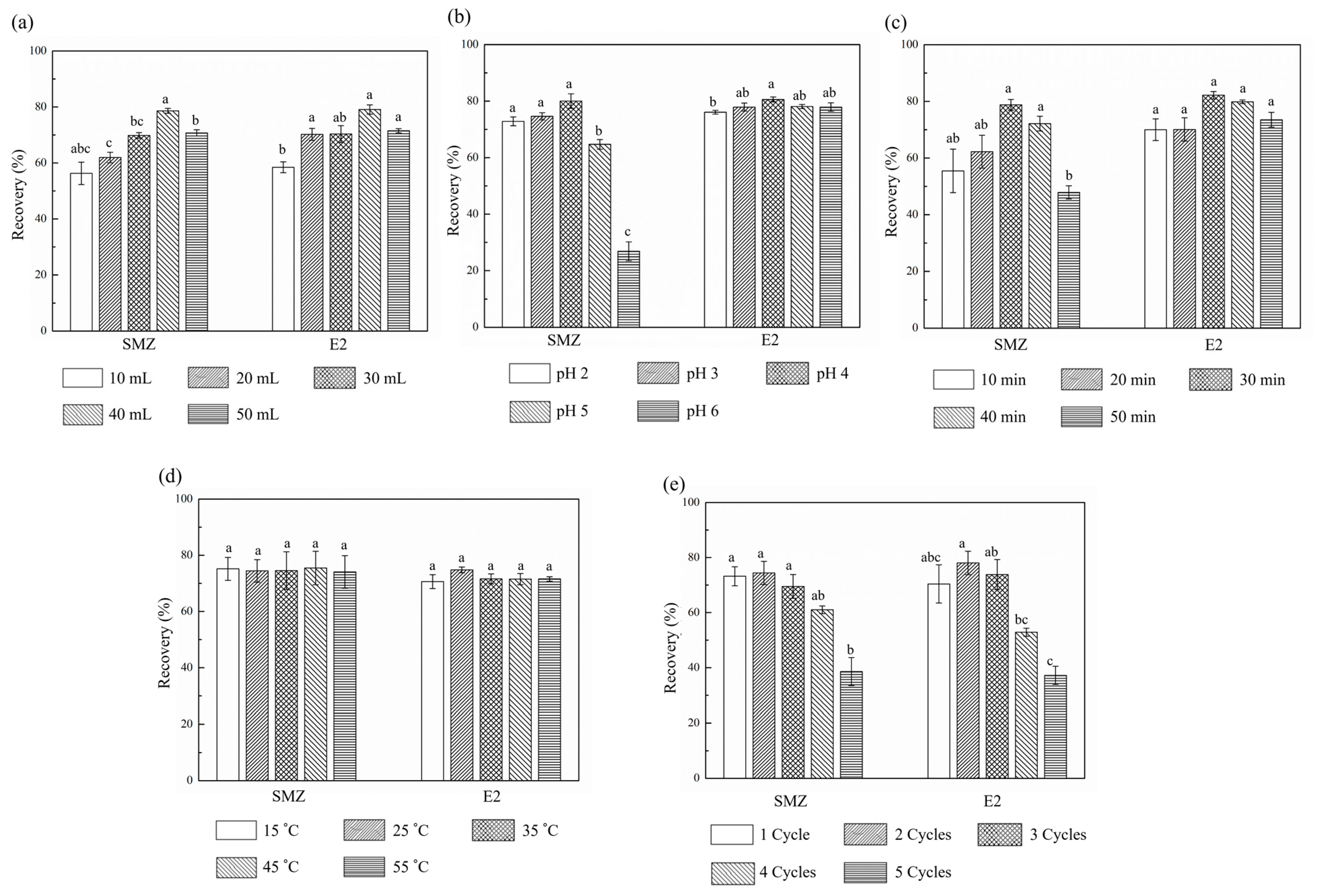
2.2. Single Factor Tests of Ultrasonic Extraction Pretreatment
2.2.1. Volumes of Extraction Solution
2.2.2. pH Values of Extraction Solution
2.2.3. Ultrasonication Time
2.2.4. Ultrasonication Temperature
2.2.5. Total Cycles of Extraction
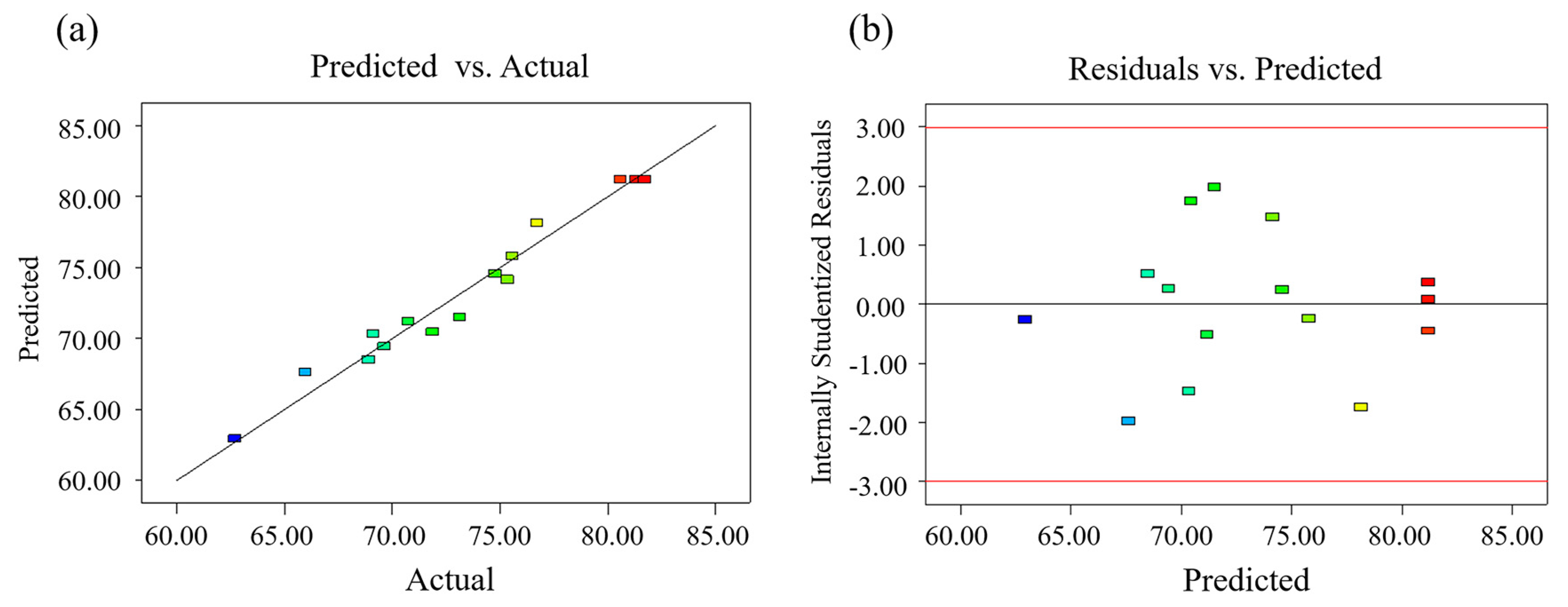
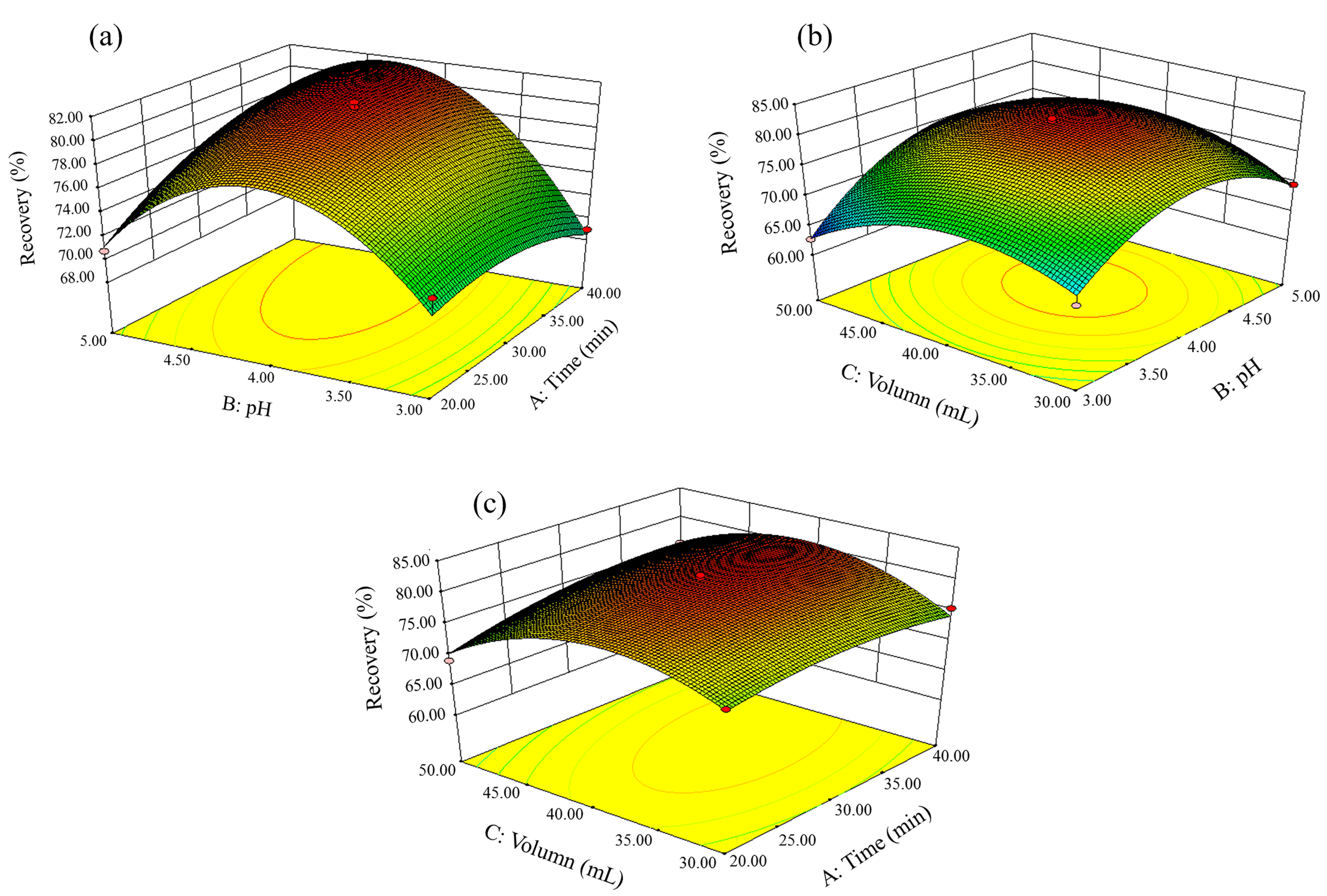
2.3. RSM Optimization
2.4. Method Validation for Real Samples
2.5. Comparison with Other Existing Methods
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Processing
3.3. Detection Conditions with HPLC
3.4. Extraction Method
3.5. Single-Factor Experiments
3.6. Response Surface Optimization (RSM)
3.7. Method Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, P.P.; Mao, D.Q.; Luo, Y.; Wang, L.M.; Xu, B.J.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Moore, P.R.; Evenson, A. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [PubMed]
- Knapp, C.W.; Dolfing, J.; Ehlert, P.A.I.; Graham, D.W. Evidence of Increasing Antibiotic Resistance Gene Abundances in Archived Soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz1, P.; Białk-Bielińska1, A.; Dołżonek, J.; Kumirska, J.; Caban, M.; Stepnowski, P. A new approach for the extraction of tetracyclines from soil matrices: Application of the microwave-extraction technique. Anal. Bioanal. Chem. 2018, 410, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Tlili, I.; Caria, G.; Ouddane, B.; Ghorbel-Abid, I.; Ternane, R.; Trabelsi-Ayadi, M.; Net, S. Simultaneous detection of antibiotics and other drug residues in the dissolved and particulate phases of water by an off-line SPE combined with on-line SPE-LC-MS/MS: Method development and application. Sci. Total Environ. 2016, 563–564, 424–433. [Google Scholar] [CrossRef]
- Garcíagalán, M.J.; Díazcruz, M.S.; Barceló, D. Occurrence of sulfonamide residues along the Ebro River basin: Removal in wastewater treatment plants and environmental impact assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef]
- Hou, J.; Pan, B.; Niu, X.K.; Chen, J.Z.; Xing, B.S. Sulfamethoxazole sorption by sediment fractions in comparison to pyrene and bisphenol A. Environ. Pollut. 2010, 158, 2826–2832. [Google Scholar] [CrossRef]
- Orso, D.; Floriano, L.; Ribeiro, L.C.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R. Simultaneous Determination of Multiclass Pesticides and Antibiotics in Honey Samples Based on Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Method 2016, 9, 1638–1653. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Vajda, A.M.; Barber, L.B.; Gray, J.L.; Lopez, E.M.; Woodling, J.D.; Norris, D.O. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ. Sci. Technol. 2008, 42, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Casey, F.X.M.; Hakk, H.; Larsen, G.L. Persistence and fate of 17β-estradiol and testosterone in agricultural soils. Chemosphere 2007, 67, 886–895. [Google Scholar] [CrossRef]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cabrera, M.L.; Radcliffe, D.E.; Zhang, H.; Huang, Q.G. Laccase mediated transformation of 17β-estradiol in soil. Environ. Pollut. 2015, 197, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Sun, W.L.; Pan, W.Y.; Xu, N. Adsorption of sulfamethoxazole and 17β-estradiol by carbon nanotubes/CoFe2O4 composites. Chem. Eng. J. 2015, 27, 417–429. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Sarri, N. Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef]
- Huang, Y.J.; Cheng, M.M.; Li, W.H.; Wu, L.H.; Chen, Y.S.; Luo, Y.M.; Christie, P.; Zhang, H.B. Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Methods 2013, 5, 3721–3731. [Google Scholar] [CrossRef]
- Bevacqua, C.E.; Rice, C.P.; Torrents, A.; Ramirez, M. Steroid hormones in biosolids and poultry litter: A comparison of potential environmental inputs. Sci. Total Environ. 2011, 409, 2120–2126. [Google Scholar] [CrossRef]
- Blackwell, P.A.; Lützhøft, H.H.; Ma, H.P.; Halling-Sørensen, B.; Boxall, A.B.A.; Kay, P. Ultrasonic extraction of veterinary antibiotics from soils and pig slurry with SPE clean-up and LC-UV and fluorescence detection. Talanta 2004, 64, 1058–1064. [Google Scholar] [CrossRef]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef]
- Aust, M.O.; Godlinski, F.; Travis, G.R.; Hao, X.; Mcallister, T.A.; Leinweber, P. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environ. Pollut. 2008, 156, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Elena, M.C.; Carmen, G.B.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Messi, P.; Guerrieri, E.; Bondi, M. Antibiotic resistance and antibacterial activity in heterotrophic bacteria of mineral water origin. Sci. Total Environ. 2005, 346, 213–219. [Google Scholar] [CrossRef]
- Anderson, C.R.; Rupp, H.S.; Wu, W.H. Complexities in tetracycline analysis-chemistry, matrix extraction, cleanup, and liquid chromatography. J. Chromatogr. A 2005, 1075, 23–32. [Google Scholar] [CrossRef]
- Zheng, W.L.; Zhang, L.F.; Zhang, K.Y.; Wang, X.Y.; Xue, F.Q. Determination of Tetracyclines and Their Epimers in Agricultural Soil Fertilized with Swine Manure by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Integr. Agr. 2012, 11, 1189–1198. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, C.J.; Zhang, W.J.; Liu, Y.W.; Li, Z.J.; Hu, H.Y.; Xue, J.M.; Davis, M. A simple and economic method for simultaneous determination of 11 antibiotics in manure by solid-phase extraction and high-performance liquid chromatography. J. Soil Sediment. 2016, 16, 2242–2251. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Ahlborn, J.; Hamscher, G. Simultaneous determination of 14 sulfonamides and tetracyclines in biogas plants by liquid-liquid-extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2513–2524. [Google Scholar] [CrossRef]
- Tylova, T.; Olsovska, J.; Novak, P.; Flieger, M. High-throughput analysis of tetracycline antibiotics and their epimers in liquid hog manure using Ultra Performance Liquid Chromatography with UV detection. Chemosphere 2010, 78, 353–359. [Google Scholar] [CrossRef]
- Salvia, M.V.; Experton, J.; Geandel, C.; Cren-Olivé, C.; Vulliet, E. Fate of pharmaceutical compounds and steroid hormones in soil: Study of transfer and degradation in soil columns. Environ. Sci. Pollut. Res. 2014, 21, 10525–10535. [Google Scholar] [CrossRef]
- Meziane, S. Optimization of oil extraction from olive pomace using response surface methodology. Food Sci. Technol. Int. 2013, 19, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.S.; Gu, S.B.; Jin, Z.Y.; Hao, M.Q.; Yin, Z.Z.; Wang, J.H. Optimization of Ultrasonic-Assisted simultaneous extraction of three active compounds from the fruits of Forsythia suspensa and comparison with conventional extraction methods. Molecules 2018, 23, 2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.Y.; Tang, G.S.; Cai, E.B.; Liu, S.L.; Yang, H.; Zhang, L.X.; Wang, S.J. Optimization of Ultrasonic Extraction of Phenolic Compounds from Epimedium brevicornum Maxim Using Response Surface Methodology and Evaluation of Its Antioxidant Activities In Vitro. J. Anal. Methods Chem. 2014, 11, 1–7. [Google Scholar]
- Yuan, H.P.; Zhu, N.W.; Song, L.J. Conditioning of sewage sludge with electrolysis: Effectiveness and optimizing study to improve dewaterability. Bioresour. Technol. 2010, 101, 4285–4290. [Google Scholar] [CrossRef]
- Ji, L.L.; Wan, Y.Q.; Zheng, S.R.; Zhu, D.Q. Adsorption of Tetracycline and Sulfamethoxazole on Crop Residue-Derived Ashes: Implication for the Relative Importance of Black Carbon to Soil Sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef]
- Shafrir, M.; Avisar, D. Development method for extracting and analyzing antibiotic and hormone residues from treated wastewater sludge and composted biosolids. Water Air Soil Pollut. 2012, 223, 2571–2587. [Google Scholar] [CrossRef]
- Malintan, N.T.; Mohd, M.A. Determination of sulfonamides in selected Malaysian swine wastewater by high-performance liquid chromatography. J. Chromatogr. A 2006, 1127, 154–160. [Google Scholar] [CrossRef]
- Shareef, A.; Page, D.; Vanderzalm, J.; Williams, M.; Kookana, R. Biodegradation of Simazine and Diuron Herbicides under Aerobic and Anoxic Conditions Relevant to Managed Aquifer Recharge of Storm Water. Clean-Soil Air Water 2014, 42, 745–752. [Google Scholar] [CrossRef]
- Zhou, J.H.; Xue, X.F.; Li, Y.; Zhang, J.Z.; Chen, F.; Wu, L.M.; Chen, L.Z.; Zhao, J. Multiresidue determination of tetracycline antibiotics in propolis by using HPLC-UV detection with ultrasonic-assisted extraction and two-step solid phase extraction. Food Chem. 2009, 115, 1074–1080. [Google Scholar] [CrossRef]
- Amini, H.; Ahmadiani, A. Rapid and simultaneous determination of sulfamethoxazole and trimethoprim in human plasma by high-performance liquid chromatography. J. Pharm. Biomed. 2007, 43, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.B.; Keefe, S.H.; Leblanc, D.R.; Bradley, P.M.; Chapelle, F.H.; Meyer, M.T.; Rubio, F. Fate of Sulfamethoxazole, 4-Nonylphenol, and 17β-Estradiol in Groundwater Contaminated by Wastewater Treatment Plant Effluent. Environ. Sci. Technol. 2009, 43, 4843–4850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrić, F.; Šegan, S.; Tešić, Ž.; Milojković-Opsenica, D. Chromatographic methods in determination of the soil–water partition coefficient. J. Liq. Chromatog. 2016, 39, 249–256. [Google Scholar]
- Li, L.Q.; Sun, M.X.; Zhou, H.; Zhou, Y.; Chen, P.; Min, H.; Shen, G.Q. Response Surface Optimization of a Rapid Ultrasound-Assisted Extraction Method for Simultaneous Determination of Tetracycline Antibiotics in Manure. J. Anal. Methods Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, L.C.; Li, Q.P.; Jin, W.F.; Chen, W.Y.; Han, J.; Zhang, Y.Y. Simultaneous optimization for Ultrasound-Assisted extraction and antioxidant activity of flavonoids from Sophora flavescens using response surface methodology. Molecules 2019, 24, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.W.; Wang, Y.X.; Cao, H.Y.; Cui, S.Y.; Su, Y.H. A novel extraction method for analysing available sulfamethoxazole in soil. Chem. Ecol. 2019, 35, 284–299. [Google Scholar] [CrossRef]
- Chen, J.; Lichwa, J.; Snehota, M.; Mohanty, S.; Ray, C. Determination of hormones and non-ionic surfactant degradation products in small-volume aqueous samples from soil columns using LC-ESI-MS-MS and GC-MS. Chromatographia 2006, 64, 413–418. [Google Scholar] [CrossRef]
- EPA. Method 8000B, Determinative Chromatographic Separations; U.S. Environmental Protection Agency, Ed.; U.S. Government Printing Office: Washington, DC, USA, 2018.
- Xu, B.J.; Mao, D.Q.; Luo, Y.; Xu, L. Sulfamethoxazole biodegradation and biotransformation in the water-sediment system of a natural river. Bioresour. Technol. 2011, 102, 7069–7076. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.Q. Box-Behnken response surface design for the optimization of electrochemical detection of cadmium by Square Wave Anodic Stripping Voltammetry on bismuth film/glassy carbon electrode. Sens. Actuators B-Chem. 2016, 11, 67–73. [Google Scholar] [CrossRef]
- Lagunas-Allué, L.; Sanz-Asensio, J.; Martínez-Soria, M.T. Response surface optimization for determination of pesticide residues in grapes using MSPD and GC-MS: Assessment of global uncertainty. Anal. Bioanal. Chem. 2010, 398, 1509–1523. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
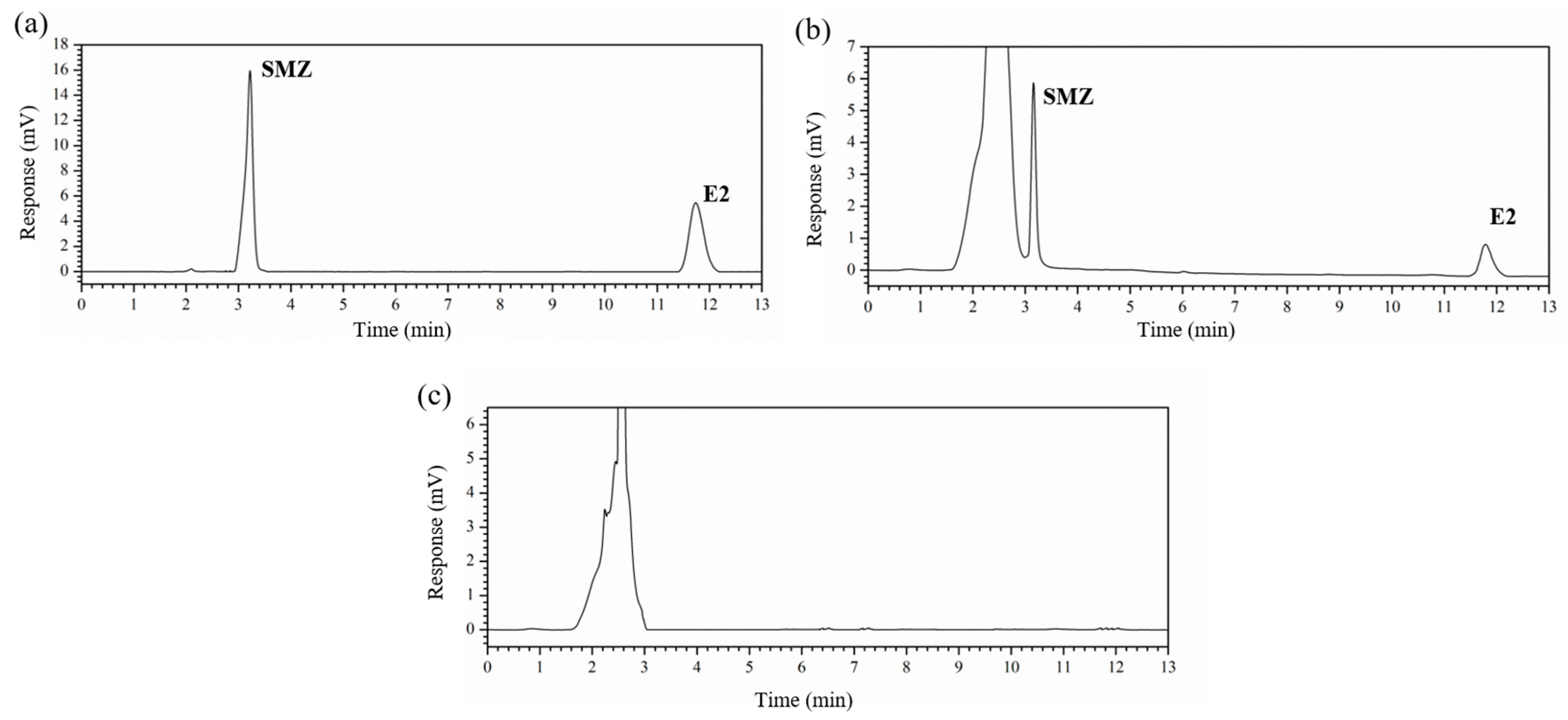
| Compound | Linear Regression Equation | R2 | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|
| SMZ | Y = 33.696X + 2115.651 | 0.99998 | 0.3 | 1 |
| E2 | Y = 4.367X + 60.425 | 0.99991 | 10 | 30 |
| X1 | X2 | X3 | Y | |
|---|---|---|---|---|
| Ultrasonication Time (min) | pH Values | Volume (mL) | Recovery (%) | |
| 1 | 20.00 (−1) | 4.00 (0) | 50.00 (+1) | 69.12 |
| 2 | 30.00 (0) | 4.00 (0) | 40.00 (0) | 81.70 |
| 3 | 30.00 (0) | 3.00 (−1) | 30.00 (−1) | 65.97 |
| 4 | 30.00 (0) | 5.00 (+1) | 30.00 (−1) | 69.64 |
| 5 | 30.00 (0) | 5.00 (+1) | 50.00 (+1) | 73.13 |
| 6 | 40.00 (+1) | 3.00 (−1) | 40.00 (0) | 68.91 |
| 7 | 20.00 (−1) | 4.00 (0) | 30.00 (−1) | 74.78 |
| 8 | 20.00 (−1) | 5.00 (+1) | 40.00 (0) | 70.75 |
| 9 | 40.00 (+1) | 5.00 (+1) | 40.00 (0) | 76.72 |
| 10 | 40.00 (+1) | 4.00 (0) | 50.00 (+1) | 75.57 |
| 11 | 30.00 (0) | 4.00 (0) | 40.00 (0) | 80.59 |
| 12 | 40.00 (+1) | 4.00 (0) | 30.00 (−1) | 75.36 |
| 13 | 30.00 (0) | 3.00 (−1) | 50.00 (+1) | 62.71 |
| 14 | 20.00 (−1) | 3.00 (−1) | 40.00 (0) | 71.88 |
| 15 | 30.00 (0) | 4.00 (0) | 40.00 (0) | 81.30 |
| Source | Sum of Squares | Df a | Mean Square | F-value b | P value c |
|---|---|---|---|---|---|
| Model | 421.87 | 9 | 46.87 | 17.29 | 0.0029 |
| X1, time (min) | 12.58 | 1 | 12.58 | 4.64 | 0.0839 |
| X2, pH | 53.92 | 1 | 53.92 | 19.89 | 0.0066 |
| X3, volume (mL) | 3.41 | 1 | 3.41 | 1.26 | 0.3133 |
| X1X2 | 19.98 | 1 | 19.98 | 7.37 | 0.0421 |
| X1X3 | 8.61 | 1 | 8.61 | 3.18 | 0.1348 |
| X2X3 | 11.39 | 1 | 11.39 | 4.20 | 0.0957 |
| X12 | 9.97 | 1 | 9.97 | 3.68 | 0.1133 |
| X22 | 207.05 | 1 | 207.05 | 76.35 | 0.0003 |
| X32 | 126.18 | 1 | 126.18 | 46.53 | 0.0010 |
| Residual | 13.56 | 5 | 2.71 | ||
| Lack of fit | 12.93 | 3 | 4.31 | 13.63 | 0.0691 |
| Pure error | 0.63 | 2 | 0.32 | ||
| R2 | 0.9689 | ||||
| Adj R2 | 0.9128 |
| Added (mg/kg) | Soil Samples a | SMZ Recovery (%) | E2 Recovery (%) | Average Recovery (%) | Integral Average Recovery (%) | RSD b (%) |
|---|---|---|---|---|---|---|
| 20 | S1 | 84.85 ± 0.81 | 81.26 ± 0.83 | 83.06 ± 0.68 | 81.78 ± 1.62 | 1.62 |
| S2 | 82.32 ± 2.63 | 76.66 ± 0.97 | 79.49 ± 0.85 | |||
| S3 | 87.51 ± 1.66 | 78.07 ± 2.90 | 82.79 ± 1.81 | |||
| 40 | S1 | 78.79 ± 2.96 | 86.06 ± 2.01 | 82.42 ± 4.81 | 82.02 ± 0.25 | 0.24 |
| S2 | 74.67 ± 1.29 | 89.32 ± 1.81 | 81.99 ± 1.51 | |||
| S3 | 76.54 ± 1.90 | 87.14 ± 0.46 | 81.84 ± 0.74 | |||
| 60 | S1 | 80.66 ± 2.35 | 88.27 ± 1.53 | 86.47 ± 0.57 | 85.05 ± 1.00 | 3.55 |
| S2 | 81.72 ± 1.74 | 86.86 ± 0.92 | 84.29 ± 0.86 | |||
| S3 | 82.44 ± 1.31 | 86.35 ± 1.55 | 84.40 ± 0.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Chen, Q.; Yan, L.; Rao, P.; Sun, P.; Wang, L.; Shen, G. Response Surface Optimization of an Extraction Method for the Simultaneous Detection of Sulfamethoxazole and 17β-Estradiol in Soil. Molecules 2020, 25, 1415. https://doi.org/10.3390/molecules25061415
Song R, Chen Q, Yan L, Rao P, Sun P, Wang L, Shen G. Response Surface Optimization of an Extraction Method for the Simultaneous Detection of Sulfamethoxazole and 17β-Estradiol in Soil. Molecules. 2020; 25(6):1415. https://doi.org/10.3390/molecules25061415
Chicago/Turabian StyleSong, Rui, Qincheng Chen, Lili Yan, Pinhua Rao, Peng Sun, Lumei Wang, and Guoqing Shen. 2020. "Response Surface Optimization of an Extraction Method for the Simultaneous Detection of Sulfamethoxazole and 17β-Estradiol in Soil" Molecules 25, no. 6: 1415. https://doi.org/10.3390/molecules25061415





