Pre-Clinical Investigation of Keratose as an Excipient of Drug Coated Balloons
Abstract
1. Introduction
2. Results
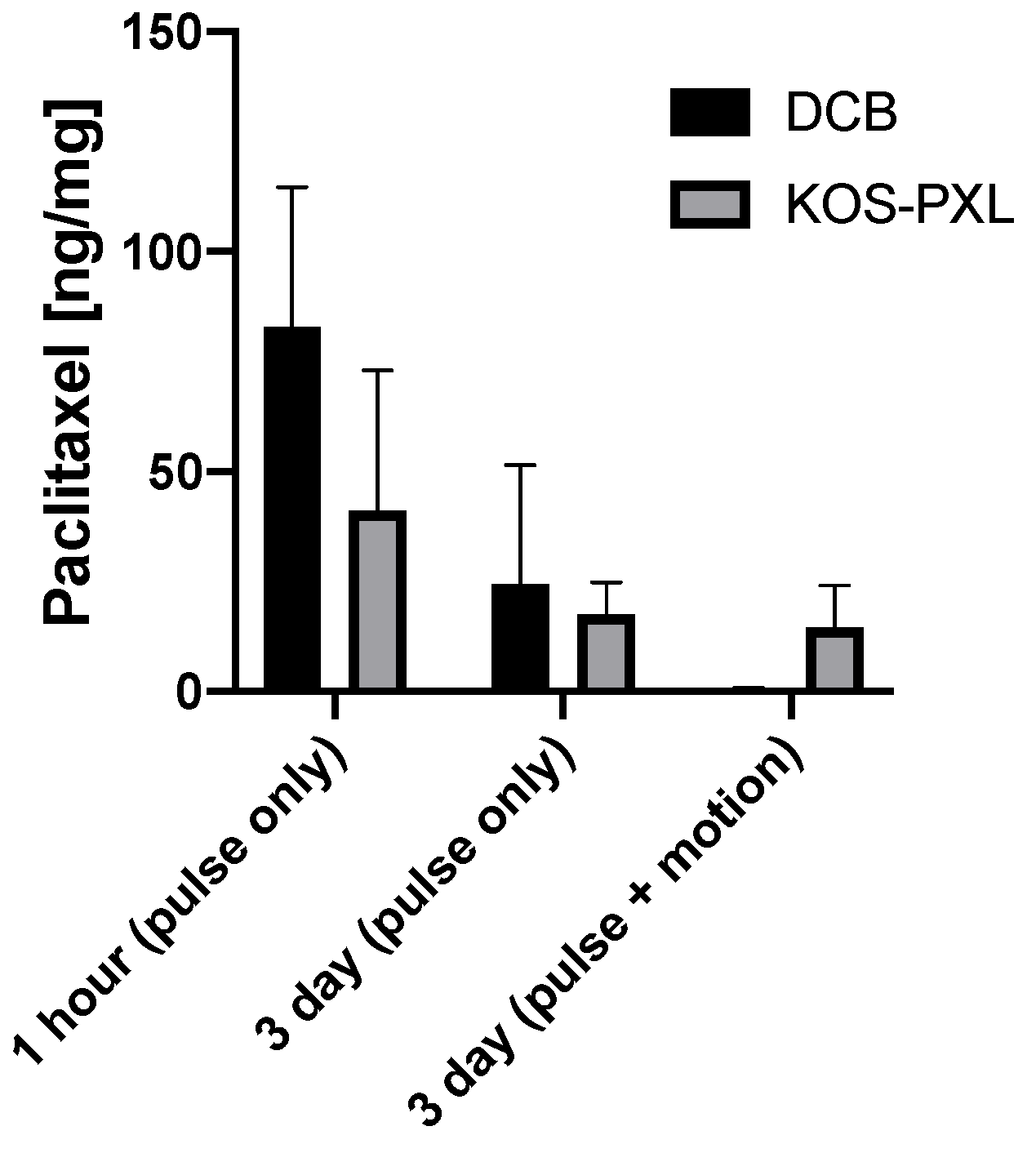
2.1. Ex Vivo Results
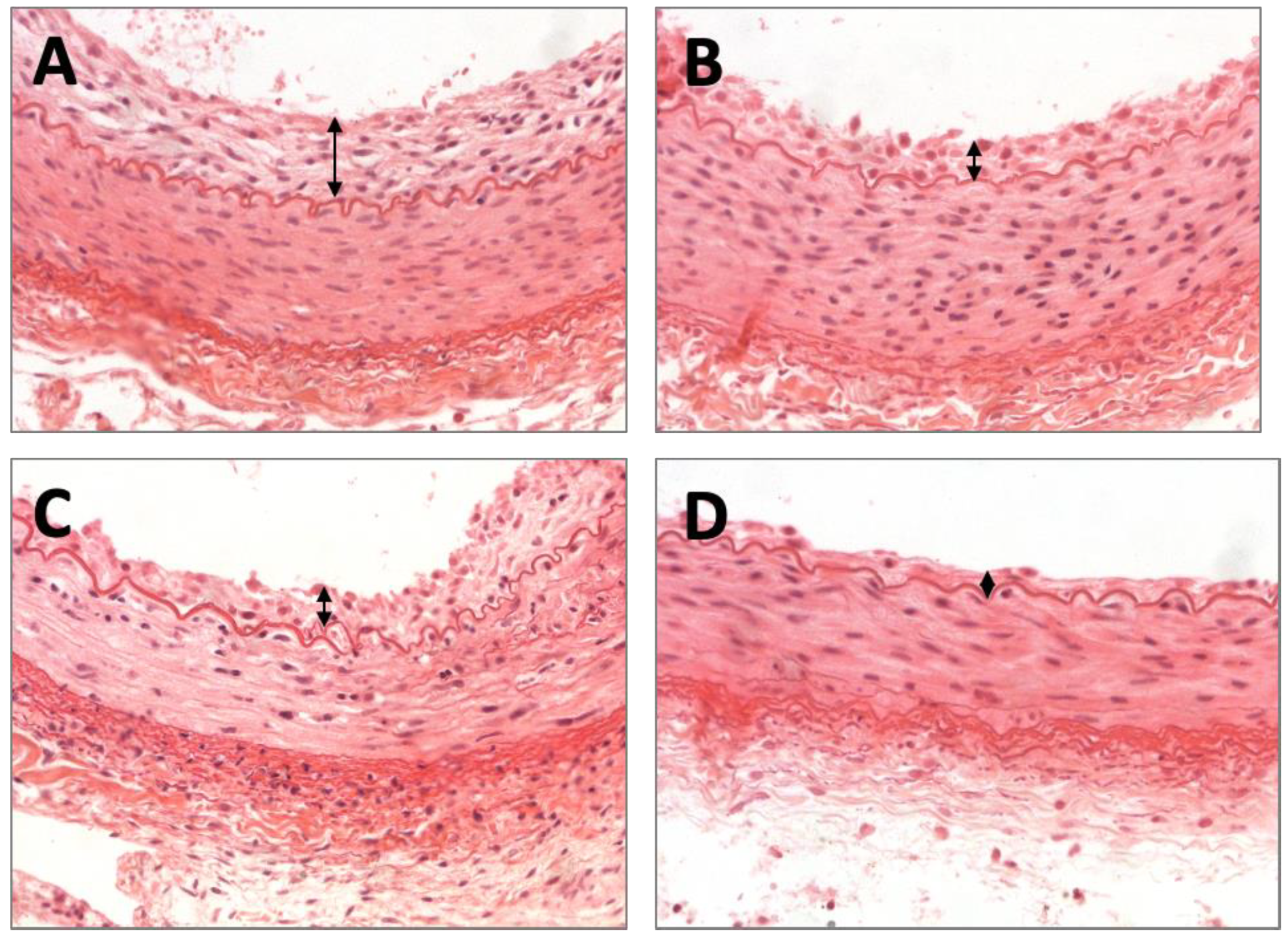
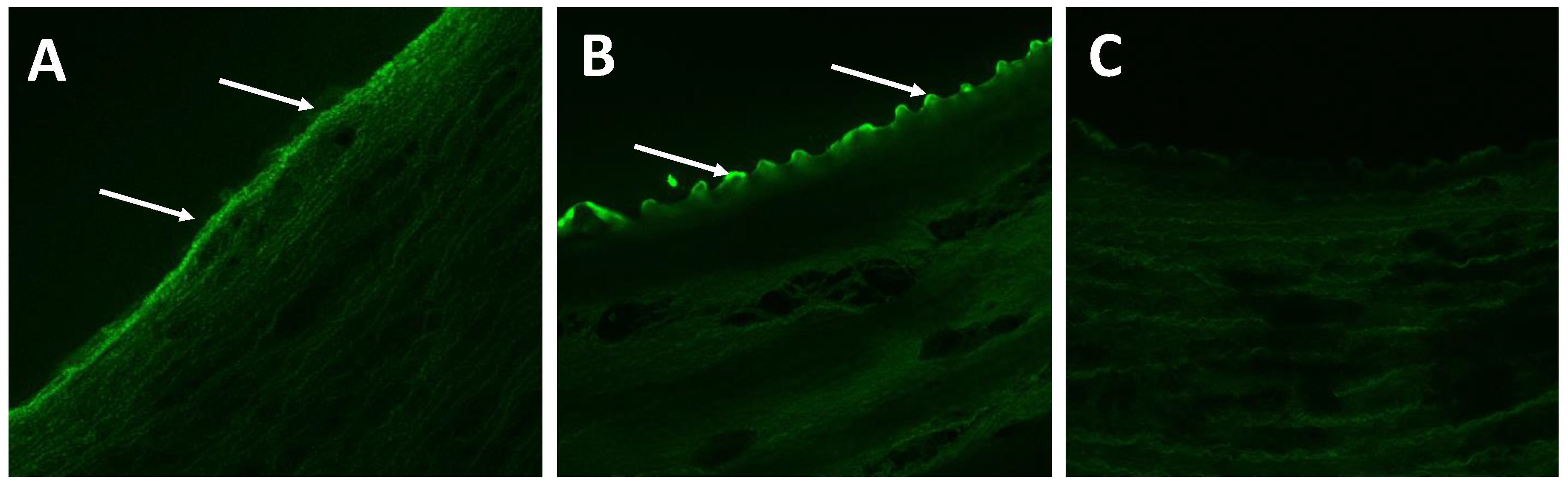
2.2. Histomorphometric Results
3. Discussion
4. Materials and Methods
4.1. Keratose-Coated Balloons
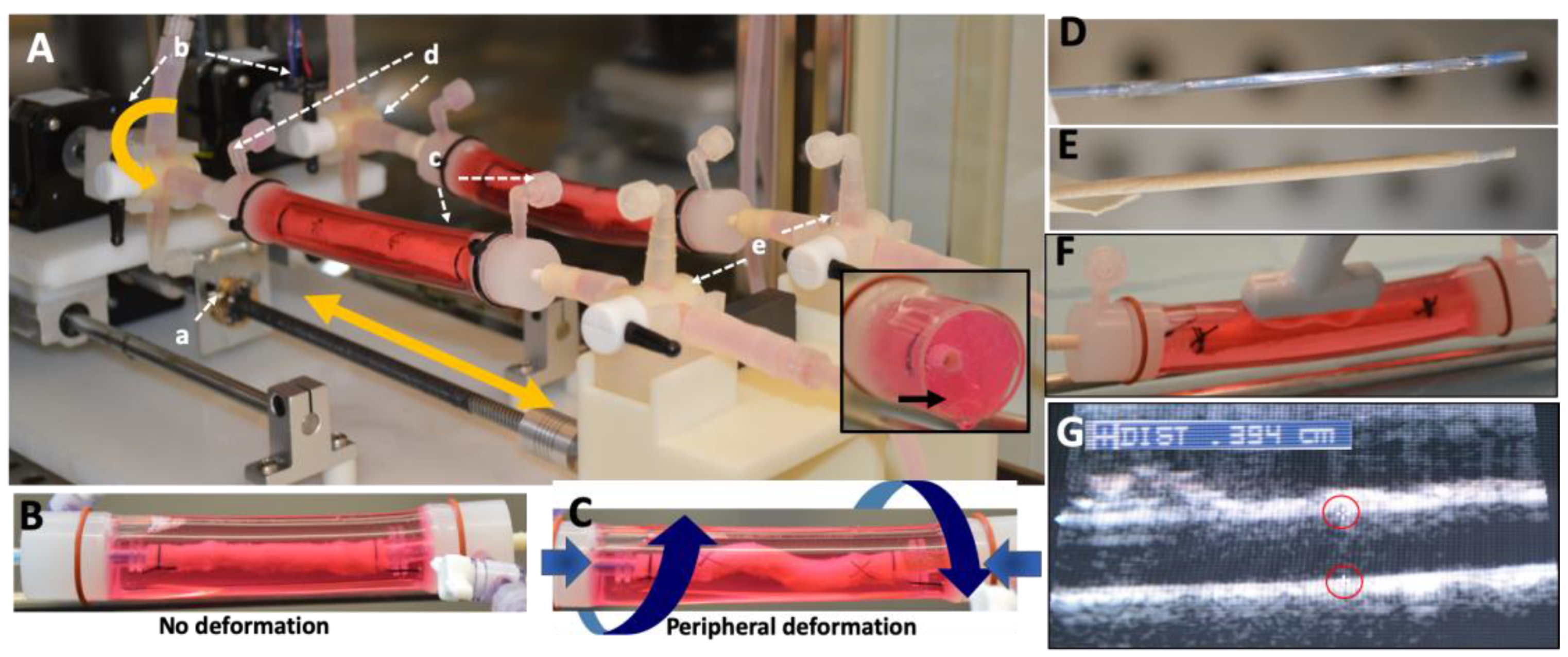
4.2. Peripheral-Simulating Bioreactor
4.3. Ex Vivo DCB Testing and Arterial Time Drug Concentration
4.4. Rabbit Injury Model
4.5. Arterial Sections
4.6. Histomorphometric Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scheinert, D.; Duda, S.; Zeller, T.; Krankenberg, H.; Ricke, J.; Bosiers, M.; Tepe, G.; Naisbitt, S.; Rosenfield, K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: First-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc. Interv. 2014, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Schnorr, B.; Albrecht, T.; Brechtel, K.; Claussen, C.D.; Scheller, B.; Speck, U.; Zeller, T. Angioplasty of Femoral-Popliteal Arteries With Drug-Coated Balloons: 5-Year Follow-Up of the THUNDER Trial. JACC Cardiovasc. Interv. 2015, 8, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Werk, M.; Albrecht, T.; Meyer, D.R.; Ahmed, M.N.; Behne, A.; Dietz, U.; Eschenbach, G.; Hartmann, H.; Lange, C.; Schnorr, B.; et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: Evidence from the randomized PACIFIER trial. Circ. Cardiovasc. Interv. 2012, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Werk, M.; Langner, S.; Reinkensmeier, B.; Boettcher, H.F.; Tepe, G.; Dietz, U.; Hosten, N.; Hamm, B.; Speck, U.; Ricke, J. Inhibition of restenosis in femoropopliteal arteries: Paclitaxel-coated versus uncoated balloon: Femoral paclitaxel randomized pilot trial. Circulation 2008, 118, 1358–1365. [Google Scholar] [CrossRef]
- Tepe, G.; Laird, J.; Schneider, P.; Brodmann, M.; Krishnan, P.; Micari, A.; Metzger, C.; Scheinert, D.; Zeller, T.; Cohen, D.J.; et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015, 131, 495–502. [Google Scholar] [CrossRef]
- Mahoney, E.M.; Wang, K.; Keo, H.H.; Duval, S.; Smolderen, K.G.; Cohen, D.J.; Steg, G.; Bhatt, D.L.; Hirsch, A.T. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 642–651. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; Komori, K.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef]
- Tsetis, D.; Belli, A.M. Guidelines for stenting in infrainguinal arterial disease. Cardiovasc. Interv. Radiol. 2004, 27, 198–203. [Google Scholar] [CrossRef]
- Schillinger, M.; Sabeti, S.; Dick, P.; Amighi, J.; Mlekusch, W.; Schlager, O.; Loewe, C.; Cejna, M.; Lammer, J.; Minar, E. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007, 115, 2745–2749. [Google Scholar] [CrossRef]
- Tosaka, A.; Soga, Y.; Iida, O.; Ishihara, T.; Hirano, K.; Suzuki, K.; Yokoi, H.; Nanto, S.; Nobuyoshi, M. Classification and clinical impact of restenosis after femoropopliteal stenting. J. Am. Coll. Cardiol. 2012, 59, 16–23. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Scheinert, D.; Scheinert, S.; Sax, J.; Piorkowski, C.; Braunlich, S.; Ulrich, M.; Biamino, G.; Schmidt, A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 2005, 45, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Micheli, A.; Picchi, A.; Coppolaro, A.; Bandinelli, L.; Severi, S.; Limbruno, U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomized clinical trial. Heart 2010, 96, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Speck, U.; Romeike, B.; Schmitt, A.; Sovak, M.; Böhm, M.; Stoll, H.P. Contrast media as carriers for local drug delivery: Successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 2003, 24, 1462–1467. [Google Scholar] [CrossRef]
- Hehrlein, C.; Richardt, G.; Wiemer, M.; Schneider, H.; Naber, C.; Hoffmann, E.; Dietz, U. Description of Pantera Lux paclitaxel-releasing balloon and preliminary quantitative coronary angiography (QCA) results at six months in patients with coronary in-stent restenosis. EuroIntervention 2011, 7, K119–K1124. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.; Meyer, D.R.; Lux, B.; Ruecker, F.; Martorana, M.; Duda, S. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: Outcomes from the ILLUMENATE first-in-human study. Catheter. Cardiovasc. Interv. 2015, 86, 278–286. [Google Scholar] [CrossRef]
- Cremers, B.; Clever, Y.; Schaffner, S.; Speck, U.; Böhm, M.; Scheller, B. Treatment of coronary in-stent restenosis with a novel paclitaxel urea coated balloon. Minerva Cardioangiol. 2010, 58, 583–588. [Google Scholar]
- Lockwood, N. BioInterface. In Drug Delivery to the Vessel Wall: Coated Balloons and the Role of the Excipient; Surfaces in Biomaterials Foundation, BioInterface Workshop and Symposium: Scottsdale, AZ, USA, 2015. [Google Scholar]
- Seidlitz, A.; Kotzan, N.; Nagel, S.; Reske, T.; Grabow, N.; Harder, C.; Petersen, S.; Sternberg, K.; Weitschies, W. In vitro determination of drug transfer from drug-coated balloons. PLoS ONE 2013, 8, e83992. [Google Scholar] [CrossRef]
- Kempin, W.; Kaule, S.; Reske, T.; Grabow, N.; Petersen, S.; Nagel, S.; Schmitz, K.P.; Weitschies, W.; Seidlitz, A. In vitro evaluation of paclitaxel coatings for delivery via drug-coated balloons. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2015, 96, 322–328. [Google Scholar] [CrossRef]
- Buszman, P.P.; Tellez, A.; Afari, M.; Cheng, Y.; Conditt, G.B.; McGregor, J.C.; Milewski, K.; Stenoien, M.; Kaluza, G.L.; Granada, J.F. Stent healing response following delivery of paclitaxel via durable polymeric matrix versus iopromide-based balloon coating in the familial hypercholesterolaemic swine model of coronary injury. EuroIntervention 2013, 9, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Spectranetics, Stellarex™ DCB with EnduraCoat Technology. Available online: https://www.usa.philips.com (accessed on 10 February 2020).
- Venkatasubbu, G.D.; Ramasamy, S.; Avadhani, G.S.; Ramakrishnan, V.; Kumar, J. Surface modification and paclitaxel drug delivery of folic acid modified polyethylene glycol functionalized hydroxyapatite nanoparticles. Powder Technol. 2012, 235, 437–442. [Google Scholar] [CrossRef]
- Tarr, B.D.; Yalkowsky, S.H. A new parenteral vehicle for the administration of some poorly water soluble anti-cancer drugs. J. Parenteral. Sci. Technol. 1987, 41, 31–33. [Google Scholar]
- Adams, J.D.; Flora, K.P.; Goldspiel, B.R.; Wilson, J.W.; Finley, R. Taxol®: A history of pharmaceutical development and current pharmaceutical concerns. J. Natl. Cancer Inst. Monogr. 1993, 15, 141–147. [Google Scholar]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Romero, M.E.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Comparison of Biologic Effect and Particulate Embolization after Femoral Artery Treatment with Three Drug-Coated Balloons in Healthy Swine Model. J. Vasc. Interv. Radiol. 2019, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Pacheco, E.; Yahagi, K.; Mori, H.; Ladich, E.; Virmani, R. Comparison of Particulate Embolization after Femoral Artery Treatment with IN.PACT Admiral versus Lutonix 035 Paclitaxel-Coated Balloons in Healthy Swine. J. Vasc. Interv. Radiol. 2016, 27, 1676–1685. [Google Scholar] [CrossRef]
- Tomblyn, S.; Pettit Kneller, E.L.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; Van Dyke, M.; Burnett, L.; Saul, J.M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef]
- Saul, J.M.; Ellenburg, M.D.; de Guzman, R.C.; Van Dyke, M. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J. Biomed. Mater. Res. Part A 2011, 98, 544–553. [Google Scholar] [CrossRef]
- Noishiki, Y.; Ito, H.; Hiyamoto, T.; Inagaki, H. Application of Denatured Wool Keratin Derivatives to an Antithrombogenic Biomaterial-Vascular Graft Coated with a Heparinized Keratin Derivative. Kobunshi Ronbunshu 1982, 39, 221–227. [Google Scholar] [CrossRef]
- Klein, A.J.; Chen, S.J.; Messenger, J.C.; Hansgen, A.R.; Plomondon, M.E.; Carroll, J.D.; Casserly, I.P. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter. Cardiovasc. Interv. 2009, 74, 787–798. [Google Scholar] [CrossRef]
- MacTaggart, J.N.; Phillips, N.Y.; Lomneth, C.S.; Pipinos, I.I.; Bowen, R.; Baxter, B.T.; Johanning, J.; Longo, G.M.; Desyatova, A.S.; Moulton, M.J.; et al. Three-dimensional bending, torsion and axial compression of the femoropopliteal artery during limb flexion. J. Biomech. 2014, 47, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Streicher, M.C.; Beck, R.J.; van den Bogert, A.J.; Tajaddini, A.; Davis, B.L. Simulation of lower limb axial arterial length change during locomotion. J. Biomech. 2012, 45, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Desyatova, A.; Poulson, W.; Deegan, P.; Lomneth, C.; Seas, A.; Maleckis, K.; MacTaggart, J.; Kamenskiy, A. Limb flexion-induced twist and associated intramural stresses in the human femoropopliteal artery. J. R. Soc. Interface 2017, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R., Jr.; Wyatt, H.R.; Thompson, H.; Peters, J.C.; Hill, J.O. Pedometer-measured physical activity and health behaviors in U.S. adults. Med. Sci. Sports Exerc. 2010, 42, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Yamauchi, K.; Hojo, H.; Yamamoto, Y.; Tanabe, T. Enhanced cell adhesion on RGDS-carrying keratin film. Mat. Sci. Eng. C Bio. S. 2003, 23, 467–472. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Edelman, E.R.; Carter, A.; Chronos, N.A.; Rogers, C.; Robinson, K.A.; Waksman, R.; Machan, L.; Weinberger, J.; Wilensky, R.L.; et al. Preclinical evaluation of drug-eluting stents for peripheral applications: Recommendations from an expert consensus group. Circulation 2004, 110, 2498–2505. [Google Scholar] [CrossRef]
- Milewski, K.; Tellez, A.; Aboodi, M.S.; Conditt, G.B.; Yi, G.H.; Thim, T.; Stenoien, M.; McGregor, J.C.; Gray, W.A.; Virmani, R.; et al. Paclitaxel-iopromide coated balloon followed by “bail-out” bare metal stent in porcine iliofemoral arteries: First report on biological effects in peripheral circulation. EuroIntervention 2011, 7, 362–368. [Google Scholar] [CrossRef]
- Albrecht, T.; Speck, U.; Baier, C.; Wolf, K.J.; Bohm, M.; Scheller, B. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Investig. Radiol. 2007, 42, 579–585. [Google Scholar] [CrossRef]
- Atigh, M.K.; Turner, E.A.; Christians, U.; Yazdani, S.K. The use of an occlusion perfusion catheter to deliver paclitaxel to the arterial wall. Cardiovasc. Ther. 2017, 35, e12269. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Chronos, N.A.; Virmani, R. Preclinical restenosis models and drug-eluting stents: Still important, still much to learn. J. Am. Coll. Cardiol. 2004, 44, 1373–1385. [Google Scholar]
- Turner, E.; Erwin, M.; Atigh, M.; Christians, U.; Saul, J.M.; Yazdani, S.K. In vitro and in vivo Assessment of Keratose as a Novel Excipient of Paclitaxel Coated Balloons. Front. Pharmacol. 2018, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.A.; Atigh, M.K.; Erwin, M.M.; Christians, U.; Yazdani, S.K. Coating and Pharmacokinetic Evaluation of Air Spray Coated Drug Coated Balloons. Cardiovasc. Eng. Technol. 2018, 9, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.K.; Sheehy, A.; Nakano, M.; Nakazawa, G.; Vorpahl, M.; Otsuka, F.; Donn, R.S.; Perkins, L.E.; Simonton, C.A.; Kolodgie, F.D.; et al. Preclinical evaluation of second-generation everolimus- and zotarolimus-eluting coronary stents. J. Invasive Cardiol. 2013, 25, 383–390. [Google Scholar] [PubMed]
- Yazdani, S.K.; Pacheco, E.; Nakano, M.; Otsuka, F.; Naisbitt, S.; Kolodgie, F.D.; Ladich, E.; Rousselle, S.; Virmani, R. Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug-coated balloon in a swine femoral artery model. Catheter. Cardiovasc. Interv. 2014, 83, 132–140. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| KOS-PXL | Commercial DCB | p Value | |
|---|---|---|---|
| Time Points | ng/mg | ng/mg | |
| 1 h (pulse only) | 41.01 ± 32.11 | 82.88 ± 31.81 | 0.30 |
| 3 day (pulse only) | 17.56 ± 7.19 | 24.44 ± 27.03 | 0.96 |
| 3 day (pulse + vascular motion) | 14.89 ± 4.12 | 0.60 ± 0.26 | 0.018 |
| No Coating | KOS-only | PXL-only | KTO-PXL | p Value | |
|---|---|---|---|---|---|
| Morphometric Measurements | |||||
| EEL, mm2 | 1.87 ± 0.33 | 1.64 ± 0.63 | 1.98 ± 0.48 | 1.39 ± 0.39 | 0.37 |
| IEL, mm2 | 1.32 ± 0.24 | 1.15 ± 0.55 | 1.58 ± 0.24 | 0.99 ± 0.45 | 0.52 |
| Lumen, mm2 | 1.18 ± 0.24 | 1.00 ± 0.55 | 1.32 ± 0.30 | 0.92 ± 0.43 | 0.58 |
| Media, mm2 | 0.55 ± 0.10 | 0.50 ± 0.11 | 0.59 ± 0.18 | 0.40 ± 0.08 | 0.21 |
| Neointimal area, mm2 | 0.15 ± 0.06 | 0.14 ± 0.04 | 0.11 ± 0.05 | 0.06 ± 0.02 | 0.085 |
| Neointimal thickness, mm | 0.10 ± 0.011 | 0.069 ± 0.022 | 0.066 ± 0.018 | 0.053 ± 0.003 | 0.005 |
| Percent area stenosis, % | 10.88 ± 4.52 | 9.99 ± 3.78 | 7.92 ± 3.84 | 6.80 ± 2.74 | 0.45 |
| Histological Analysis | |||||
| Injury | 1.00 ± 0.41 | 0.75 ± 1.19 | 1.13 ± 1.32 | 0.50 ± 0.41 | 0.74 |
| EC Score | 0.25 ± 0.50 | 0.00 ± 0.00 | 0.25 ± 0.50 | 1.50 ± 0.58 | 0.013 |
| Inflammation | 0.25 ± 0.50 | 0.00 ± 0.00 | 0.50 ± 0.58 | 0.25 ± 0.50 | 0.86 |
| SMC Loss | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.50 | 1.00 ± 0.82 | 0.081 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, E.; Erwin, M.; Cawthon, C.V.; Schaff, C.; Fedor, N.; Rayl, T.; Wilson, O.; Christians, U.; Register, T.C.; Geary, R.L.; et al. Pre-Clinical Investigation of Keratose as an Excipient of Drug Coated Balloons. Molecules 2020, 25, 1596. https://doi.org/10.3390/molecules25071596
Goel E, Erwin M, Cawthon CV, Schaff C, Fedor N, Rayl T, Wilson O, Christians U, Register TC, Geary RL, et al. Pre-Clinical Investigation of Keratose as an Excipient of Drug Coated Balloons. Molecules. 2020; 25(7):1596. https://doi.org/10.3390/molecules25071596
Chicago/Turabian StyleGoel, Emily, Megan Erwin, Claire V. Cawthon, Carson Schaff, Nathaniel Fedor, Trevor Rayl, Onree Wilson, Uwe Christians, Thomas C. Register, Randolph L. Geary, and et al. 2020. "Pre-Clinical Investigation of Keratose as an Excipient of Drug Coated Balloons" Molecules 25, no. 7: 1596. https://doi.org/10.3390/molecules25071596
APA StyleGoel, E., Erwin, M., Cawthon, C. V., Schaff, C., Fedor, N., Rayl, T., Wilson, O., Christians, U., Register, T. C., Geary, R. L., Saul, J., & Yazdani, S. K. (2020). Pre-Clinical Investigation of Keratose as an Excipient of Drug Coated Balloons. Molecules, 25(7), 1596. https://doi.org/10.3390/molecules25071596






