Targeted UHPLC–HRMS (Orbitrap) Polyphenolic and Capsaicinoid Profiling for the Chemometric Characterization and Classification of Paprika with Protected Designation of Origin (PDO) Attributes
Abstract
1. Introduction
2. Results and Discussion
2.1. Targeted UHPLC–HRMS Polyphenolic Profiling
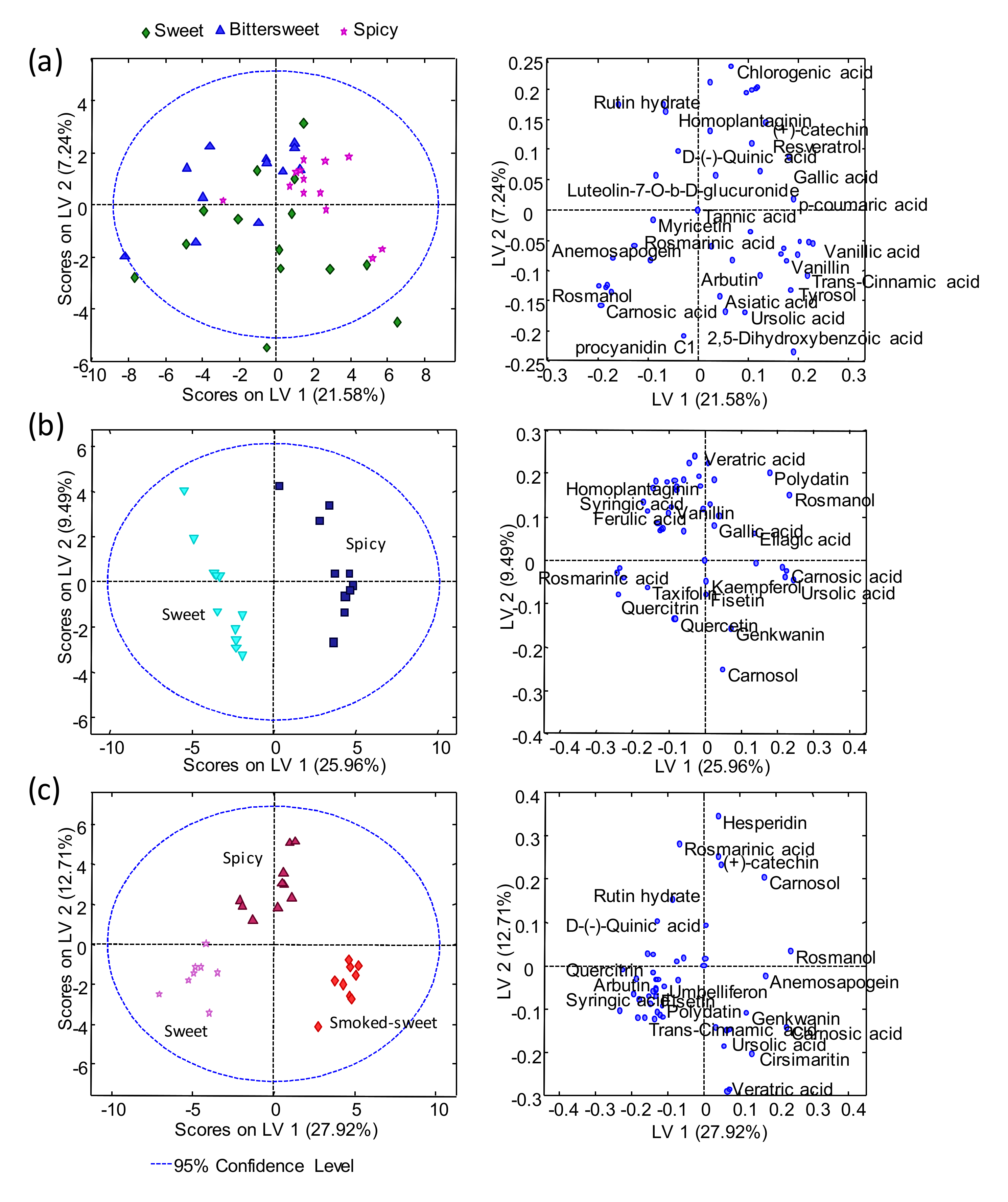
2.2. Sample Exploration by PCA
2.3. Sample Classification by PLS-DA
2.4. Supervised PLS-DA Method Validation
2.5. Targeted UHPLC-HRMS Polyphenolic and Capsaicinoid Profiling
3. Materials and Methods
3.1. Chemicals and Standard Solutions
3.2. Instrumentation
3.3. Samples and Sample Treatment
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- García, C.C.; Barfuss, M.H.J.; Sehr, E.M.; Barboza, G.E.; Samuel, M.R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.C.; Nahm, S.H.; Huh, J.H.; Yoo, H.S.; Yu, J.W.; Lee, M.H.; Kim, B.-D. An interspecific (Capsicum annuum x C. chinese) F2 linkage map in pepper using RFLP and AFLP markers. Theor. Appl. Genet. 2001, 102, 531–539. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Topuz, A.; Dinçer, C.; Özdemir, K.S.; Feng, H.; Kushad, M. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv., Jalapeno). Food Chem. 2011, 129, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Swiner, D.J.; Capone, P.C.; Badu-Tawiah, A.K. Thread spray mass spectrometry for direct analysis of capsaicinoids in pepper products. Anal. Chim. Acta 2018, 1023, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Gorgulu, A.; Akar, T.; Celik, S. Decolorization of Reactive Blue 49 contaminated solutions by Capsicum annuum seeds: Batch and continuous mode biosorption applications. Chem. Eng. J. 2011, 168, 125–133. [Google Scholar] [CrossRef]
- European Commission. Council Regulation (EC) No 510/2006 of 20 March 2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, 50, 12–25. [Google Scholar]
- European Commission. Agricultural and Rural Developmet, European Union Agricultural Product Quality Policy, Specific EU Quality Schemes E Guaranteeing Qaulity. Available online: https://ec.europa.eu/agriculture/quality_en (accessed on 2 February 2020).
- European Commission. Commission Regulation (EC) No 982/2007 of 21 August 2007 registering certain names in the Register of protected designations of origin and protected geographical indications (Pimentón de la Vera (PDO)—Karlovarský suchar (PGI)—Riso di Baraggia biellese e vercellese (PDO)). Off. J. Eur. Union 2007, L217, 22–23. [Google Scholar]
- European Commission. Commission Regulation (EC) No 464/2001 of 7 March 2001 supplementing the Annex to Regulation (EC) No 2400/96 on the entry of certain names in the ‘Register of protected designations of origin and protected geographical indications’ provided for in Council Regulation (EEC) No 2081/92 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2001, L66, 29–30. [Google Scholar]
- Zapata, M.; Bañon, S.; Cabrera, P. El pimiento para pimentón; Mundi Prensa Libros, S.A.: Madrid, Spain, 1992. [Google Scholar]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Mudrić, S.Ž.; Gašić, U.; Dramićanin, A.K.; Ćirić, I.; Milojković-Opsenica, D.; Popović-Đorđević, J.B.; Momirović, N.M.; Tešić, Ž. The polyphenolics and carbohydrates as indicators of botanical and geographical origin of Serbian autochthonous clones of red spice paprika. Food Chem. 2017, 217, 705–715. [Google Scholar]
- Domínguez-Martínez, I.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Proal-Nájera, J.B.; Gallardo-Velazquez, T. Determination of capsaicin, ascorbic acid, total phenolic compounds and antioxidant activity of Capsicum annuum L. var. serrano by mid infrared spectroscopy (Mid-FTIR) and chemometric analysis. J. Korean Soc. Appl. Boil. Chem. 2014, 57, 133–142. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Maraña, O.M.; Merás, I.D.; Galeano-Díaz, T.; De La Peña, A.M. Fluorescence properties of flavonoid compounds. Quantification in paprika samples using spectrofluorimetry coupled to second order chemometric tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.-H. Anti-inflammatory and antioxidant activities of red pepper (Capsicum annuum L.) stalk extracts: Comparison of pericarp and placenta extracts. J. Funct. Foods 2013, 5, 1724–1731. [Google Scholar] [CrossRef]
- Zaki, N.; Hakmaoui, A.; Ouatmane, A.; Fernández-Trujillo, J.P. Quality characteristics of Moroccan sweet paprika (Capsicum annuum L.) at different sampling times. Food Sci. Technol. 2013, 33, 577–585. [Google Scholar] [CrossRef]
- Kirschbaum-Titze, P.; Hiepler, C.; Mueller-Seitz, E.; Petz, M. Pungency in Paprika (Capsicum annuum). 1. Decrease of Capsaicinoid Content Following Cellular Disruption. J. Agric. Food Chem. 2002, 50, 1260–1263. [Google Scholar] [CrossRef]
- Musfiroh, I.D.A.; Mutakin, M.; Angelina, T.; Muchtaridi, M. Capsaicin level of various Capsicum fruits. Int. J. Pharm. Pharm. Sci. 2013, 5, 248–251. [Google Scholar]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum—production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef]
- Maraña, O.M.; De La Peña, A.M.; Galeano-Díaz, T. Isocratic LC–DAD–FLD method for the determination of flavonoids in paprika samples by using a rapid resolution column and post-column pH change. Talanta 2016, 152, 15–22. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.-C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Pardo-Mates, N.; Vera, A.; Barbosa, S.; Hidalgo-Serrano, M.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Characterization, classification and authentication of fruit-based extracts by means of HPLC-UV chromatographic fingerprints, polyphenolic profiles and chemometric methods. Food Chem. 2017, 221, 29–38. [Google Scholar] [CrossRef]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Núñez, O. Detection and Quantitation of Frauds in the Authentication of Cranberry-Based Extracts by UHPLC-HRMS (Orbitrap) Polyphenolic Profiling and Multivariate Calibration Methods. J. Agric. Food Chem. 2018, 66, 9353–9365. [Google Scholar] [CrossRef]
- Barbosa, S.; Campmajó, G.; Saurina, J.; Puignou, L.; Núñez, O. Determination of Phenolic Compounds in Paprika by Ultrahigh Performance Liquid Chromatography–Tandem Mass Spectrometry: Application to Product Designation of Origin Authentication by Chemometrics. J. Agric. Food Chem. 2019, 68, 591–602. [Google Scholar] [CrossRef]
- Serrano, N.; Cetó, X.; Núñez, O.; Aragó, M.; Gámez, A.; Ariño, C.; Díaz-Cruz, J. Characterization and classification of Spanish paprika (Capsicum annuum L.) by liquid chromatography coupled to electrochemical detection with screen-printed carbon-based nanomaterials electrodes. Talanta 2018, 189, 296–301. [Google Scholar] [CrossRef]
- Puigventos, L.; Navarro, M.; Alechaga, E.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Determination of polyphenolic profiles by liquid chromatography-electrospray-tandem mass spectrometry for the authentication of fruit extracts. Anal. Bioanal. Chem. 2014, 407, 597–608. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Caravaca, G.-; García-Salas, P.; Segura-Carretero, A.; Gutierrez, A.F. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Navarro, M.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Characterization of Fruit Products by Capillary Zone Electrophoresis and Liquid Chromatography Using the Compositional Profiles of Polyphenols: Application to Authentication of Natural Extracts. J. Agric. Food Chem. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Gnayfeed, M.H.; Daood, H.G.; Biacs, P.A.; Alcaraz, C.F. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. J. Sci. Food Agric. 2001, 81, 1580–1585. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Ambrózy, Z.; Helyes, L. Determination of Polyphenols, Capsaicinoids, and Vitamin C in New Hybrids of Chili Peppers. J. Anal. Methods Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Juangsamoot, J.; Ruangviriyachai, C.; Techawongstien, S.; Chanthai, S. Determination of capsaicin and dihydrocapsaicin in some hot chilli varieties by RP-HPLC-PDA after magnetic stirring extraction and clean up with C 18 cartridge. Int. Food Res. J. 2012, 19, 1217–1226. [Google Scholar]
- Thompson, R.Q.; Phinney, K.W.; Welch, M.J.; White, E. Quantitative determination of capsaicinoids by liquid chromatography-electrospray mass spectrometry. Anal. Bioanal. Chem. 2005, 381, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, M.; Coutinho, J.P.; De Melo, A.M.T.; Godoy, H.T. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Res. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Han, J.-S.; Kozukue, E.; Lee, S.-J.; Kim, J.-A.; Lee, K.-R.; Levin, C.; Friedman, M. Analysis of Eight Capsaicinoids in Peppers and Pepper-Containing Foods by High-Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 9172–9181. [Google Scholar] [CrossRef]
- Ma, F.; Yang, Q.; Matthäus, B.; Li, P.; Zhang, Q.; Zhang, L. Simultaneous determination of capsaicin and dihydrocapsaicin for vegetable oil adulteration by immunoaffinity chromatography cleanup coupled with LC–MS/MS. J. Chromatogr. B 2016, 1021, 137–144. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Fujita, J.; Ono, M.; Oka, H.; Ito, Y.; Matsumoto, H.; Ozeki, N.; Itakura, Y.; Nakazawa, H. An HPLC method for the analysis of paprika color in food using capsanthin as an indicator. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 2347–2361. [Google Scholar] [CrossRef]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; Cordoba, M.D.G. Application of temperature-induced phase partition of proteins for the detection of smoked paprika adulteration by free zone capillary electrophoresis (FZCE). Food Chem. 2007, 105, 1219–1227. [Google Scholar] [CrossRef]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; Cordoba, M.D.G. Detection of Smoked Paprika “Pimentón de La Vera” Adulteration by Free Zone Capillary Electrophoresis (FZCE). J. Agric. Food Chem. 2006, 54, 4141–4147. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, Á.; Pons, F.D.P. Geographical characterization of Spanish PDO paprika by multivariate analysis of multielemental content. Talanta 2014, 128, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.; Esslinger, S.; Pfister, M.; Fauhl-Hassek, C.; Riedl, J. Non-targeted detection of paprika adulteration using mid-infrared spectroscopy and one-class classification—Is it data preprocessing that makes the performance? Food Chem. 2018, 257, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.G.; Raman, V.; Haider, S.; Ali, Z.; Chittiboyina, A.; Khan, I.A. Overview of Analytical Tools for the Identification of Adulterants in Commonly Traded Herbs and Spices. J. AOAC Int. 2019, 102, 376–385. [Google Scholar] [CrossRef]
- Cetó, X.; Serrano, N.; Aragó, M.; Gámez, A.; Esteban, M.; Díaz-Cruz, J.; Núñez, O. Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis. Sensors 2018, 18, 4479. [Google Scholar] [CrossRef]
- SOLO software, Eigenvecto Research. Available online: http://www.eigenvector.com/software/solo.htm (accessed on 10 February 2020).
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
Sample Availability: Samples of the polyphenolic and phenolic compounds (4-hydroxybenzoic acid, p-coumaric acid, sinapic acid, vanillic acid, homovanillic acid, homogentisic acid, chlorogenic acid, cryptochlorogenic acid, gallic acid, ferulic acid, gentisic acid, caffeic acid, syringic acid, rosmarinic acid, fisetin, taxifolin, rutin, quercetin, quercitrin, nepetin-7-glucoside, hesperidin, cirsimaritin, myricetin, luteolin-7-O-β-D-glucoronide, genkwanin, morin, kaempferol, quercetin, homoplantaginin, (+)-catechin, (-)-epicatechin, (-)-epigallocatechin gallate, carnosic acid, anemosapogenin, rosmanol, betulinic acid, asiatic acid, carnosol, 12-methoxycarnosic acid, procyanidin A2, procyanidin B2, procyanidin C1, polydatin, resveratrol, 3,4-dihydroxybenzaldehyde, syringaldehyde, vanillin, veratric acid, trans-cinnamic acid, tyrosol, arbutin, ethyl gallate, umbelliferon, and ellagic acid) and of capsaicinoids (capsaicin, iihydrocapsaicin, N-vanillylnonanamide, nordihydrocapsiate, and nordihydrocapsaicin) are available from the authors. |






| Target Name | +/− | Area | Formula | Expected m/z | Measured m/z | Delta m/z | Isotopic Pattern Score (%) |
|---|---|---|---|---|---|---|---|
| D-(-)-Quinic acid | − | 7.19 × 108 | C7H12O6 | 191.0561 | 191.0558 | −1.72 | 100 |
| Ethyl gallate | − | 5.99 × 106 | C9H10O5 | 197.0455 | 197.0452 | −1.39 | 100 |
| Polydatin | − | 4.15 × 107 | C20H22O8 | 389.1242 | 389.1254 | 3.02 | 89 |
| Syringic acid | − | 5.99 × 106 | C9H10O5 | 197.0455 | 197.0452 | −1.39 | 100 |
| Gallic acid | − | 2.35 × 107 | C7H6O5 | 169.0142 | 169.0137 | −2.85 | 95 |
| Arbutin | − | 1.58 × 108 | C12H16O7 | 271.0823 | 271.0817 | −2.23 | 100 |
| 3,4-Dihydroxybenzaldehyde | − | 7.75 × 107 | C7H6O3 | 137.0244 | 137.0239 | −3.68 | 80 |
| 4-Hydroxybenzoic acid | − | 7.75 × 107 | C7H6O3 | 137.0244 | 137.0239 | −3.68 | 80 |
| Chlorogenic acid | − | 3.91 × 107 | C16H18O9 | 353.0878 | 353.0870 | −2.16 | 100 |
| p-coumaric acid | − | 4.52 × 107 | C9H8O3 | 163.0401 | 163.0395 | −3.74 | 87 |
| Caffeic acid | − | 7.57 × 107 | C9H8O4 | 179.0350 | 179.0346 | −2.28 | 100 |
| Homovanillic acid | − | 4.39 × 108 | C9H10O4 | 181.0506 | 181.0504 | −1.36 | 100 |
| Syringaldehyde | − | 4.39 × 108 | C9H10O4 | 181.0506 | 181.0504 | −1.36 | 100 |
| Veratric acid | − | 4.39 × 108 | C9H10O4 | 181.0506 | 181.0504 | −1.36 | 100 |
| Homogentisic acid | − | 3.73 × 107 | C8H8O4 | 167.0350 | 167.0345 | −2.87 | 94 |
| Vanillic acid | − | 3.73 × 107 | C8H8O4 | 167.0350 | 167.0345 | −2.99 | 94 |
| Trans-Cinnamic acid | − | 1.79 × 107 | C9H8O2 | 147.0452 | 147.0446 | −3.86 | 100 |
| Umbelliferon | − | 1.17 × 107 | C9H6O3 | 161.0244 | 161.0241 | −1.90 | 99 |
| Ferulic acid | − | 4.52 × 107 | C10H10O4 | 193.0506 | 193.0504 | −1.27 | 100 |
| Rutin | − | 4.86 × 107 | C27H30O16 | 609.1461 | 609.1454 | −1.17 | 100 |
| Taxifolin | − | 2.01 × 106 | C15H12O7 | 303.0510 | 303.0505 | −1.83 | 100 |
| (+)-catechin | − | 9.48 × 105 | C15H14O6 | 289.0718 | 289.0713 | −1.63 | 91 |
| Quercitrin hydrate | − | 1.19 × 109 | C21H20O11 | 447.0933 | 447.0924 | −2.07 | 100 |
| Homoplantaginin | − | 2.71 ×107 | C22H22O11 | 461.1089 | 461.1081 | −1.75 | 91 |
| Fisetin | − | 1.41 × 108 | C15H10O6 | 285.0405 | 285.0397 | −2.55 | 100 |
| Quercetin | − | 1.60 × 107 | C15H10O7 | 301.0354 | 301.0346 | −2.74 | 90 |
| Resveratrol | − | 3.49 × 106 | C14H12O3 | 227.0714 | 227.0705 | −3.92 | 89 |
| Rosmanol | − | 5.66 × 106 | C20H26O5 | 345.1707 | 345.1700 | −2.17 | 100 |
| Asiatic acid | − | 2.59 × 106 | C30H48O5 | 487.3429 | 487.3419 | −2.08 | 100 |
| Cirsimaritin | − | 1.95 × 106 | C17H14O6 | 313.0718 | 313.0710 | −2.51 | 91 |
| Carnosol | − | 5.15 × 106 | C20H26O4 | 329.1758 | 329.1750 | −2.37 | 86 |
| Carnosic acid | − | 2.41 × 106 | C20H28O4 | 331.1915 | 331.1909 | −1.85 | 100 |
| Ursolic acid | − | 6.52 × 105 | C30H46O3 | 453.3374 | 453.3362 | −2.55 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, S.; Saurina, J.; Puignou, L.; Núñez, O. Targeted UHPLC–HRMS (Orbitrap) Polyphenolic and Capsaicinoid Profiling for the Chemometric Characterization and Classification of Paprika with Protected Designation of Origin (PDO) Attributes. Molecules 2020, 25, 1623. https://doi.org/10.3390/molecules25071623
Barbosa S, Saurina J, Puignou L, Núñez O. Targeted UHPLC–HRMS (Orbitrap) Polyphenolic and Capsaicinoid Profiling for the Chemometric Characterization and Classification of Paprika with Protected Designation of Origin (PDO) Attributes. Molecules. 2020; 25(7):1623. https://doi.org/10.3390/molecules25071623
Chicago/Turabian StyleBarbosa, Sergio, Javier Saurina, Lluís Puignou, and Oscar Núñez. 2020. "Targeted UHPLC–HRMS (Orbitrap) Polyphenolic and Capsaicinoid Profiling for the Chemometric Characterization and Classification of Paprika with Protected Designation of Origin (PDO) Attributes" Molecules 25, no. 7: 1623. https://doi.org/10.3390/molecules25071623
APA StyleBarbosa, S., Saurina, J., Puignou, L., & Núñez, O. (2020). Targeted UHPLC–HRMS (Orbitrap) Polyphenolic and Capsaicinoid Profiling for the Chemometric Characterization and Classification of Paprika with Protected Designation of Origin (PDO) Attributes. Molecules, 25(7), 1623. https://doi.org/10.3390/molecules25071623







