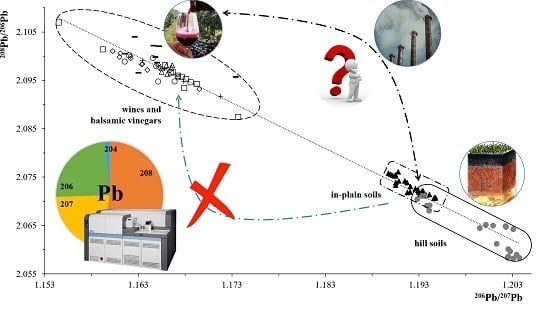
Use of Lead Isotopic Ratios as Geographical Tracer for Lambrusco PDO Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Soil and Wine Samples
2.3. Sample Pre-treatment
2.4. Pb Concentration Measurement
2.5. Pb Separation by SPE
2.6. Pb Isotope Ratios Determination
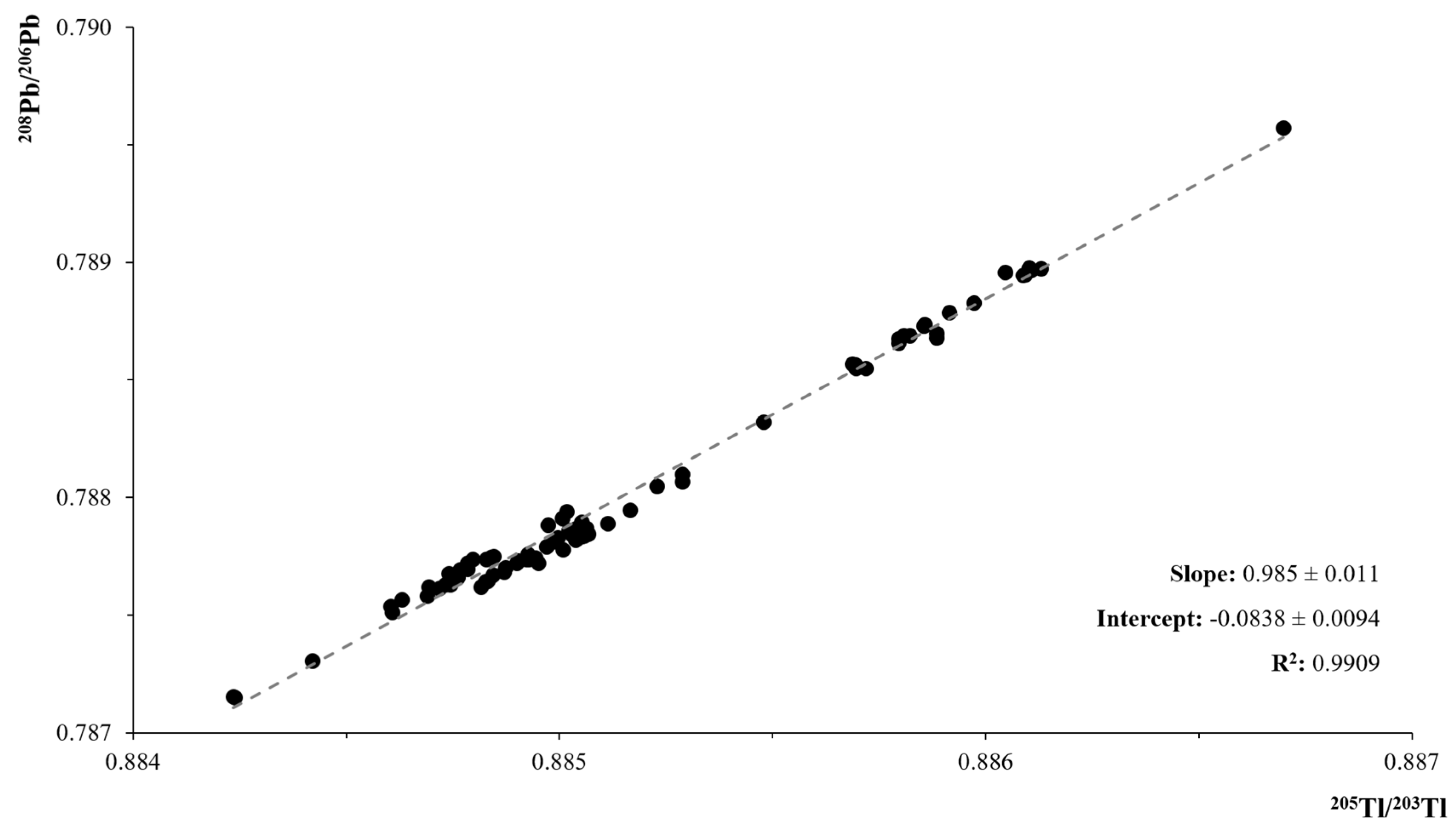
2.7. Mass Bias Correction Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Instrumental Mass Dependent Fractionation Correction
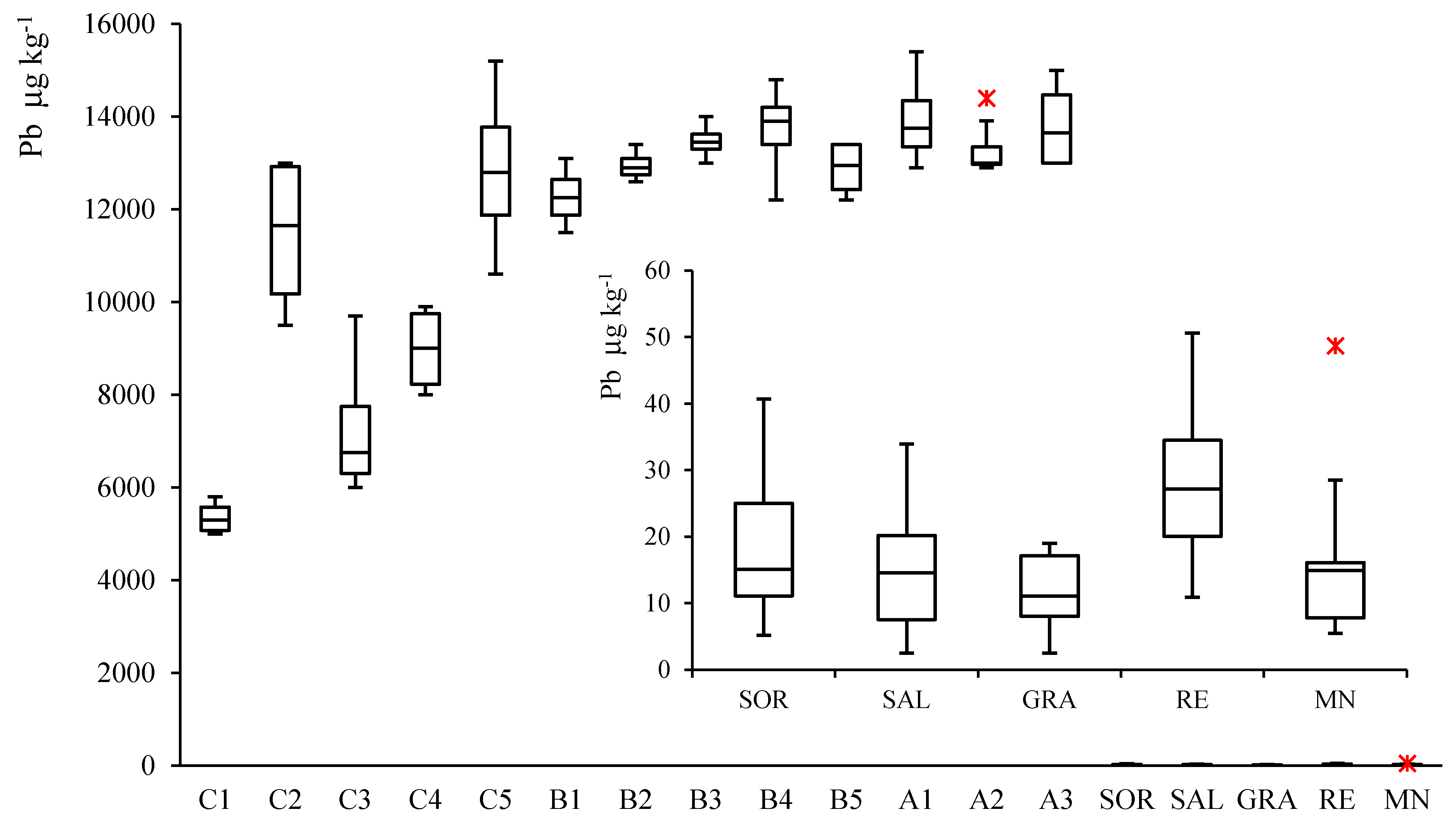
3.2. Pb Concentration in Soil and Wine Samples
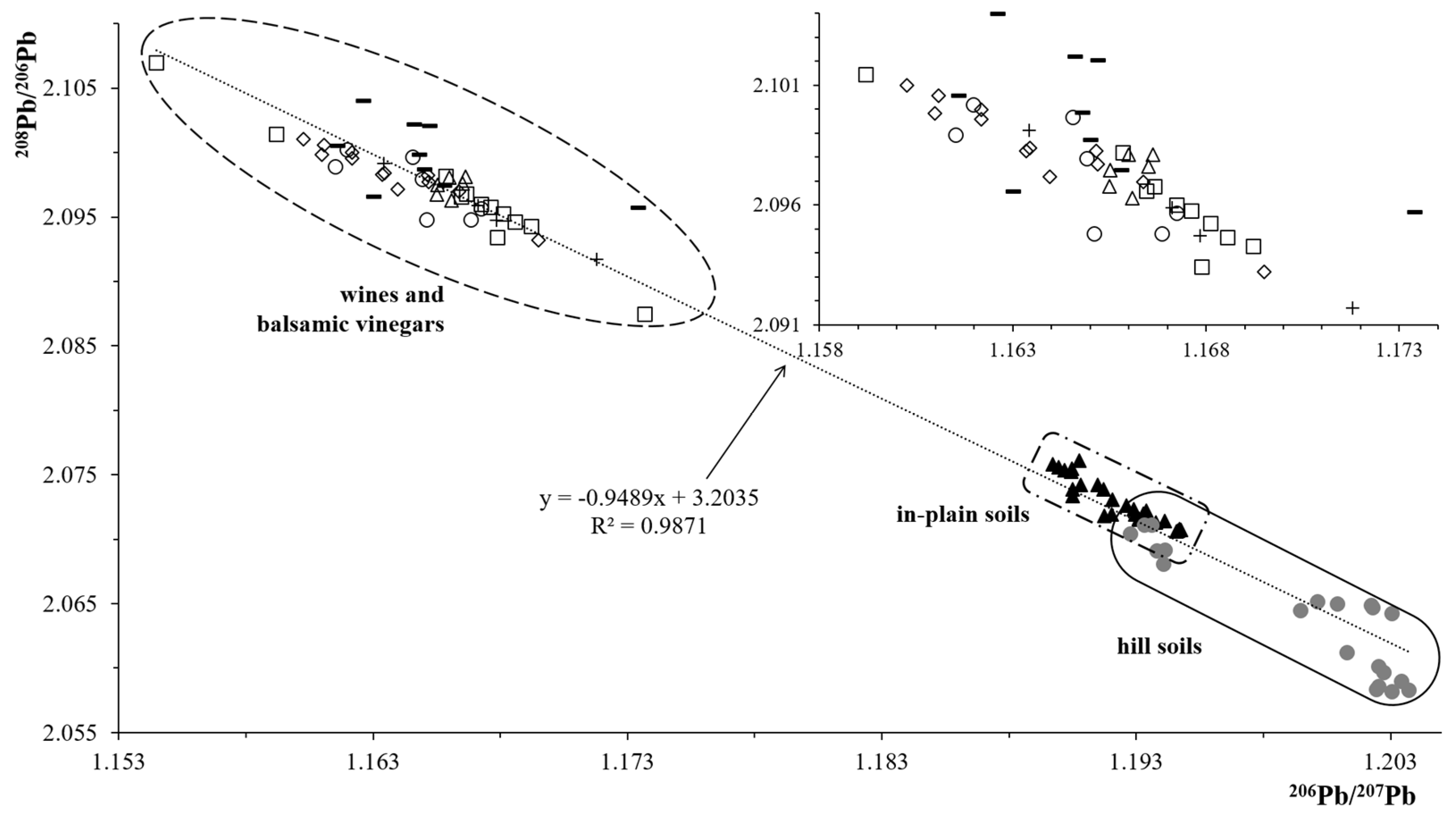
3.3. Determination of Isotopic Ratios: Soils and Wines
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CRM | Certificate Reference Material |
| DL | Detection Limit |
| ICP/QMS | Inductively Coupled Plasma Quadrupole Mass Spectrometry |
| IR | Isotope Ratio |
| ISO | International Organization for Standardization |
| MC-ICP/MS | Multi Collector Inductively Coupled Plasma Mass Spectrometry |
| NIST | National Institute of Standard and Technology |
| OIV | International Organization of Vine and Wine |
| PDO | Protected Designation of Origin |
| PFA | Perfluoroalkoxy |
| PGI | Protected Geographical Identification |
| SPE | Solid Phase Extraction |
| SRM | Standard Reference Material |
| TDS | Total Dissolved Solids |
| TIMS | Thermal Ionization Mass Spectrometry |
| TSG | Traditional Specialty Guaranteed |
Appendix A. Methodological procedure for the instrumental mass bias correction in MC-ICP/MS isotope ratio determinations
Appendix A.1. Mass Bias Correction with Cfactor (Method a)
Appendix A.2. Mass Bias Internal Correction with 205Tl/203Tl Isotope Ratio (Methods b and c)
References
- Arfini, F.; Belletti, G.; Marescotti, A. Prodotti Tipici e Denominazioni Geografiche: Strumenti di Tutela e Valorizzazione; Edizioni Tellus: Roma, Italy, 28 September 2010. (In Italy) [Google Scholar]
- Council Regulation 510/2006, 20 March 2006, on the protection of Geographical Indications and Designations of Origin for Agricultural Products and Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2006/510/oj (accessed on 22 January 2020).
- GREEN PAPER on Agricultural Product Quality: Product Standards, Farming Requirements and Quality Schemes. 2008. Available online: http://ec.europa.eu/transparency/regdoc/rep/1/2008/EN/1-2008-641-EN-F1-1.Pdf (accessed on 22 January 2020).
- Durante, C.; Bertacchini, L.; Bontempo, L.; Camin, F.; Manzini, D.; Lambertini, P.; Marchetti, A.; Paolini, M. From soil to grape and wine: Variation of light and heavy elements isotope ratios. Food Chem. 2016, 210, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Baschieri, C.; Bertacchini, L.; Bertelli, D.; Cocchi, M.; Marchetti, A.; Manzini, D.; Papotti, G.; Sighinolfi, S. An analytical approach to Sr isotope ratio determination in Lambrusco wines for geographical traceability purposes. Food Chem. 2015, 173, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Bertacchini, L.; Durante, C. Heavy Isotopes. In Food Authentication: Management, Analysis and Regulation; Georgiou, C.A., Danezis, G.P., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 131–175. [Google Scholar]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, V. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar]
- Braschi, E.; Marchionni, S.; Priori, S.; Casalini, M.; Tommasini, S.; Natarelli, L.; Buccianti, A.; Buccelli, P.; Costantini, E.A.C.; Conticelli, S. Tracing the 87Sr/86Sr from rocks and soils to vine and wine: An experimental study on geologic and pedologic characterisation of vineyards using radiogenic isotope of heavy elements. STOTEN 2018, 628–629, 1317–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, C.; Bertacchini, L.; Cocchi, M.; Manzini, D.; Marchetti, A.; Rossi, M.C.; Sighinolfi, S.; Tassi, L. Development of 87Sr/86Sr maps as targeted strategy to support wine quality. Food Chem. 2018, 255, 139–146. [Google Scholar] [CrossRef] [PubMed]
- White, W.M. Chapter 8: Radiogenic Isotope Geochemistry. In Geochemistry; Wiley-Blackwell: Oxford, UK, 2013; pp. 313–360. [Google Scholar]
- Komárek, M.; Ettler, V.; Chrastný, V.; Mihaljevič, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar]
- Dawson, J.J.; Tetzlaff, D.; Carey, A.M.; Raab, A.; Soulsby, C.; Killham, K.; Meharg, A.A. Characterizing Pb mobilization from upland soils to streams using 206Pb/207Pb isotopic ratios. Environ. Sci. Technol. 2009, 44, 243–249. [Google Scholar] [CrossRef]
- Yan, K.; Dong, Z.; Wijayawardena, M.A.A.; Liu, Y.; Naidu, R.; Semple, K. Measurement of soil lead bioavailability and influence of soil types and properties: A review. Chemosphere 2017, 184, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.M.R.; Vasconcelos, M.T.S.D. Lead Contamination in Portuguese Red Wines from the Douro Region: From the Vineyard to the Final Product. J. Agric. Food Chem. 2003, 51, 3012–3023. [Google Scholar] [CrossRef]
- Stockley, C.S.; Smith, L.H.; Tiller, T.L.K.G.; Gulson, B.L.; Osborn, C.D.; Lee, T.H. Lead in wine: A case study on two varieties at two wineries in South Australia. Aust. J. Grape Wine Res. 2003, 9, 47–55. [Google Scholar] [CrossRef]
- Durante, C.; Baschieri, C.; Bertacchini, L.; Cocchi, M.; Sighinolfi, S.; Silvestri, M.; Marchetti, A. Geographical traceability based on 87Sr/86Sr indicator: A first approach for PDO Lambrusco wines from Modena. Food Chem. 2013, 141, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Bertacchini, L.; Durante, C.; Marchetti, A.; Sighinolfi, S.; Silvestri, M.; Cocchi, M. Use of X-ray diffraction technique and chemometrics to aid soil sampling strategies in traceability studies. Talanta 2012, 98, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; Dietz, M.L.; Rhoads, S.; Felinto, C.; Gale, N.H.; Houghton, J. A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal. Chim. Acta 1994, 292, 263–273. [Google Scholar] [CrossRef]
- Weiss, D.J.; Kober, B.; Dolgopolova, A.; Gallagher, K.; Spiro, B.; Roux, G.L.; Mason, T.F.D.; Kylander, M.; Coles, B.J. Accurate and precise Pb isotope ratio measurements in environmental samples by MC-ICP-MS. Int. J. Mass Spectrom. 2004, 232, 205–215. [Google Scholar] [CrossRef]
- Collerson, K.D.; Kamber, B.S.; Schoenberg, R. Applications of accurate, high-precision Pb isotope ratio measurement by multi-collector ICP-MS. Chem. Geol. 2002, 188, 65–83. [Google Scholar]
- Tanner, S.C. Space charge in ICP-MS: Calculation and implications. Spectrochim. Acta 1992, 47, 809–823. [Google Scholar] [CrossRef]
- Albarède, F.; Telouk, P.; Blichert-Toft, J.; Boyet, M.; Agranier, A.; Nelson, B. Precise and accurate isotopic measurements using multiple-collector ICPMS. Geochimica et Cosmochimica Acta 2004, 68, 2725–2744. [Google Scholar] [CrossRef]
- Yang, L. Accurate and precise determination of isotopic ratios by MC-ICP-MS: A review. Mass Spectrom. 2009, 28, 990–1011. [Google Scholar] [CrossRef]
- White, W.M.; Albarède, F.; Télouk, P. High-precision analysis of Pb isotope ratios by multi-collector ICP-MS. Chem. Geol. 2000, 167, 257–270. [Google Scholar]
- Todt, W.; Cliff, R.A.; Hanser, A.; Hofmann, A.W. Evaluation of a 202Pb–205Pb double spike for high-precision lead isotope analysis. In Earth Processes: Reading the Isotope Code; Geophys. Monogr. Ser.; Hart, S.R., Basu, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 95, pp. 429–437. [Google Scholar]
- Galer, S.J.G. Practical application of lead triple spiking for correction of instrumental mass discrimination. Mineral. Mag. 1998, 62, 491–492. [Google Scholar] [CrossRef]
- Thirlwall, M.F. Inter-laboratory and other errors in Pb isotope analyses investigated using a 207Pb–204Pb double spike. Chem. Geol. 2000, 163, 299–322. [Google Scholar]
- Hirata, T. Lead isotopic analyses of NIST Standard Reference Materials using multiple collector inductively coupled plasma mass spectrometry coupled with a modified external correction method for mass discrimination effect. Analyst 1996, 121, 1407–1411. [Google Scholar] [CrossRef]
- Rehkämper, M.; Halliday, A.N. Accuracy and long-term reproducibility of lead isotopic measurements by multiple-collector inductively coupled plasma mass spectrometry using an external method for correction of mass discrimination. Int. J. Mass Spectrom. 1998, 181, 123–133. [Google Scholar] [CrossRef]
- Rehkamper, M.; Mezger, K. Investigation of matrix effects for Pb isotopes ratio measurements by multiple collector ICP-MS: Verification and application of optimized analytical protocols. J. Anal. Atom. Spectrom. 2000, 15, 1451–1460. [Google Scholar] [CrossRef]
- Reuer, M.K.; Boyle, E.A.; Grant, B.C. Lead isotope analysis of marine carbonates and seawater by multiple collector ICP-MS. Chem. Geol. 2003, 200, 137–153. [Google Scholar]
- Gallon, C.; Aggarwal, J.; Flegal, A.R. Comparison of Mass Discrimination Correction Methods and Sample Introduction Systems for the Determination of Lead Isotopic Composition Using a Multicollector Inductively Coupled Plasma Mass Spectrometer. Anal. Chem. 2008, 80, 8355–8363. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Health Criteria no. 165—Inorganic Lead. In International Programme on Chemical Safety; 1995; Available online: http://www.inchem.org/documents/ehc/ehc/ehc003.htm (accessed on 22 January 2020).
- International Code of Oenological Practices, published by the International Organization of Vine and Wine, OIV, Code Sheet—Edition 2019/01. ISBN 978-2-85038-002-0. Available online: http://www.oiv.int/public/medias/6558/code-2019-en.pdf (accessed on 22 January 2020).
- Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Chrastný, V. Lead isotopic signatures of wine and vineyard soils—tracers of lead origin. J. Geochem. Explor. 2006, 88, 130–133. [Google Scholar] [CrossRef]
- Reimann, C.; Flem, B.; Fabian, K.; Birke, M.; Ladenberger, A.; Négrel, P.; Demetriades, A.; Hoogewerff, J. Lead and lead isotopes in agricultural soils of Europe—The continental perspective. Appl. Geochem. 2012, 27, 532–542. [Google Scholar]
- Ndung’u, K.; Hibdon, S.; Véron, A.; Flegal, A.R. Lead isotopes reveal different sources of lead in balsamic and other vinegars. Sci. Total Environ. 2011, 409, 2754–2760. [Google Scholar] [CrossRef]
- Dean, J.R.; Ebdon, L.; Massey, R.C. Isotope ratio and isotope dilution analysis of lead in wine by inductively coupled plasmamass spectrometry. Food Addit. Contam. 1990, 7, 109–116. [Google Scholar]
- Epova, E.N.; Bérail, S.; Séby, F.; Barre, J.P.G.; Vacchina, V.; Médina, B.; Sarthou, L.; Donard, O.F.X. Potential of lead elemental and isotopic signatures for authenticity and geographical origin of Bordeaux wines. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, A.; Ardini, F.; Becagli, S.; Traversi, R.; Udisti, R.; Cappelletti, D.; Grotti, M. Source assessment of atmospheric lead measured at Ny-Ålesund, Svalbard. Atmos. Environ. 2015, 113, 20–26. [Google Scholar]
- Chernyshev, I.V.; Chugaev, A.V.; Shatagin, K.N. High-precision Pb isotope analysis by multicollector-ICP-mass-spectrometry using 205Tl/203Tl normalization: Optimization and calibration of the method for the studies of Pb isotope variations. Geochem. Int. 2007, 45, 1065–1076. [Google Scholar] [CrossRef]
- Maréchal, C.N.; Télouk, P.; Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999, 15, 251–273. [Google Scholar] [CrossRef]
- Berni, A.; Baschieri, C.; Covelli, S.; Emili, A.; Marchetti, A.; Manzini, D.; Berto, D.; Rampazzo, F. DoE optimization of a mercury isotope ratio determination method for environmental studies. Talanta 2016, 152, 179–187. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: not available. |

| Step | Eluent | Volume (mL) | Function |
|---|---|---|---|
| 1 | H2O | 2 | Resin pre-washing |
| 2 | 8M HNO3 | 12 | Resin conditioning |
| 3 | Sample loading | 1.5–4.5 (soil) 20 (wine) | Sample loading |
| 4 | 8M HNO3 | 4 | Resin rinsing |
| 5 | H2O | 13 | Resin rinsing (Sr elution) |
| 6 | 6M HCl | 10 (soil) 3 (wine) | Pb elution |
| NIST SRM 981 # | |||
|---|---|---|---|
| I.R. | 208Pb/206Pb | 207Pb/206Pb | |
| Corr. Method | |||
| Certified values | 2.1681 ± 0.0008 | 0.91464 ± 0.00033 | |
| (b) fPb = fTl | 2.16665 ± 0.00023 | 0.914624 ± 0.000057 | |
| (c) fPb ≠ fTl | 2.16664 ± 0.00021 | 0.914645 ± 0.000021 | |
| Authors | Mass Spectrometer # | 208Pb/206Pb | 207Pb/206Pb | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb |
|---|---|---|---|---|---|---|
| Todt et al. [25] | TIMS | 2.16701 (43) | 0.91459 (13) | 16.9356 (23) | 15.4891 (30) | 36.7006 (112) |
| Galer [26] | TIMS | 2.16771 (10) | 0.914750 (35) | 16.9405 (15) | 15.4963 (16) | 36.7219 (44) |
| Thirlwall [27] | TIMS | 2.16770 (21) | 0.91469 (7) | 16.9409 (22) | 15.4956 (26) | 36.7228 (80) |
| Hirata [28] | MC-ICP/MS—Plasma 54 | 2.16636 (82) | 0.914623 (37) | 16.9311 (90) | 15.4856 | 36.6800 (210) |
| Rehkaemper, Halliday [29] | MC-ICP/MS—Plasma 54 | 2.16677 (14) | 0.91469 (5) | 16.9364 (55) | 15.4912 (51) | 36.7219 (44) |
| White et al. [24] | MC-ICP/MS—Plasma 54 | 2.1646 (8) | 0.91404 | 16.9467 (76) | 15.4899 (39) | 36.6825 (78) |
| Rehkaemper and Mezger [30] | MC-ICP/MS—IsoProbe | 2.16691 (29) | 0.91459 (13) | 16.9366 (29) | 15.4900 (17) | 36.7000 (23) |
| Reuer et al. [31] | MC-ICP/MS—IsoProbe | 2.16639 (304) | 0.91460 (18) | |||
| Weiss et al. [19] | MC-ICP/MS—IsoProbe | 2.16767 (63) | 0.914767 (120) | 16.9413 (39) | 15.4974 (51) | 36.7239 (115) |
| Gallon [32] | MC-ICP/MS—Neptune Spray chamber | 2.16606 (46) | 0.91441 (28) | 16.9315 (118) | 15.4824 (91) | 36.6746 (220) |
| Gallon [32] | MC-ICP/MS—Neptune Apex | 2.16607 (30) | 0.91451 (20) | 16.9308 (54) | 15.4835 (24) | 36.6733 (79) |
| This study | MC-ICP/MS—Neptune Spray chamber and Apex | 2.16664 (21) | 0.914645 (21) | 16.9310 (32) | 15.4858 (30) | 36.6834 (70) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancellotti, L.; Sighinolfi, S.; Marchetti, A.; Tassi, L. Use of Lead Isotopic Ratios as Geographical Tracer for Lambrusco PDO Wines. Molecules 2020, 25, 1641. https://doi.org/10.3390/molecules25071641
Lancellotti L, Sighinolfi S, Marchetti A, Tassi L. Use of Lead Isotopic Ratios as Geographical Tracer for Lambrusco PDO Wines. Molecules. 2020; 25(7):1641. https://doi.org/10.3390/molecules25071641
Chicago/Turabian StyleLancellotti, Lisa, Simona Sighinolfi, Andrea Marchetti, and Lorenzo Tassi. 2020. "Use of Lead Isotopic Ratios as Geographical Tracer for Lambrusco PDO Wines" Molecules 25, no. 7: 1641. https://doi.org/10.3390/molecules25071641