A Pilot Study on the Effect of Anti-Thrombopoietin Antibody on Platelet Count in Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Enrolled Patients and Anthropometry
4.2. Biochemical Assays
4.3. Detection of Anti-THPO Antibodies
4.4. Transient Elastography
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wolber, E.-M.; Jelkmann, W. Thrombopoietin: The novel hepatic hormone. News Physiol. Sci. 2002, 17, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K.; Broudy, V.C.; Lin, N.; Jorgensen, M.J.; McCarty, J.; Fox, N.; Zucker-Franklin, D.; Lofton-Day, C. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc. Natl. Acad. Sci. USA 1995, 92, 3234–3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushansky, K. The molecular mechanisms that control thrombopoiesis. J. Clin. Investig. 2005, 115, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Murone, M.; Carpenter, D.A.; de Sauvage, F.J. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells 1998, 16, 1–6. [Google Scholar] [CrossRef]
- Abina, M.A.; Tulliez, M.; Duffour, M.T.; Debili, N.; Lacout, C.; Villeval, J.L.; Wendling, F.; Vainchenker, W.; Haddada, H. Thrombopoietin (TPO) knockout phenotype induced by cross-reactive antibodies against TPO following injection of mice with recombinant adenovirus encoding human TPO. J. Immunol. 1998, 160, 4481–4489. [Google Scholar]
- Vadhan-Raj, S.; Verschraegen, C.F.; Bueso-Ramos, C.; Broxmeyer, H.E.; Kudelkà, A.P.; Freedman, R.S.; Edwards, C.L.; Gershenson, D.; Jones, D.; Ashby, M.; et al. Recombinant human thrombopoietin attenuates carboplatin-induced severe thrombocytopenia and the need for platelet transfusions in patients with gynecologic cancer. Ann. Intern. Med. 2000, 132, 364–368. [Google Scholar] [CrossRef]
- Nomura, S.; Dan, K.; Hotta, T.; Fujimura, K.; Ikeda, Y. Effects of pegylated recombinant human megakaryocyte growth and development factor in patients with idiopathic thrombocytopenic purpura. Blood 2002, 100, 728–730. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, H.; Miyawaki, S.; Kuwaki, T.; Hagiwara, T.; Kato, T.; Miyazaki, H. Autoantibodies neutralizing thrombopoietin in a patient with amegakaryocytic thrombocytopenic purpura. Blood 2000, 95, 2187–2188. [Google Scholar]
- Aledort, L.M.; Hayward, C.P.M.; Chen, M.-G.; Nichol, J.L.; Bussel, J. ITP Study Group Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am. J. Hematol. 2004, 76, 205–213. [Google Scholar] [CrossRef]
- Ziakas, P.D.; Routsias, J.G.; Giannouli, S.; Tasidou, A.; Tzioufas, A.G.; Voulgarelis, M. Suspects in the tale of lupus-associated thrombocytopenia. Clin. Exp. Immunol. 2006, 145, 71–80. [Google Scholar] [CrossRef]
- Füreder, W.; Firbas, U.; Nichol, J.L.; Pistillo, J.; Winkler, S.; Hiesberger, H.; Sperr, W.R.; Smolen, J.; Schett, G. Serum thrombopoietin levels and anti-thrombopoietin antibodies in systemic lupus erythematosus. Lupus 2002, 11, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Xia, Y.; Bertino, A.; Glaspy, J.; Roberts, M.; Kuter, D.J. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 2001, 98, 3241–3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xu, M.; Qin, P.; Zhang, H.-y.; Yuan, C.-l.; Zhao, H.-g.; Cui, Z.-g.; Meng, Y.-s.; Wang, L.; Zhou, F.; et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood 2015, 125, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.K.; Pack, S.P.; Oh, J.-G.; Kang, N.K.; Chang, M.H.; Chung, Y.H.; Kim, S.-J.; Lee, J.W.; Heo, T.-H. Anti-erythropoietin and anti-thrombopoietin antibodies induced after administration of recombinant human erythropoietin. Int. Immunopharmacol. 2011, 11, 2237–2241. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.E. Diabetes mellitus: A hypercoagulable state. J. Diabetes Complications 2001, 15, 44–54. [Google Scholar] [CrossRef]
- Jindal, S.; Gupta, S.; Gupta, R.; Kakkar, A.; Singh, H.V.; Gupta, K.; Singh, S. Platelet indices in diabetes mellitus: Indicators of diabetic microvascular complications. Hematology 2011, 16, 86–89. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Radaelli, M.G.; Martucci, F.; Perra, S.; Accornero, S.; Castoldi, G.; Lattuada, G.; Manzoni, G.; Perseghin, G. NAFLD/NASH in patients with type 2 diabetes and related treatment options. J. Endocrinol. Investig. 2018, 41, 509–521. [Google Scholar] [CrossRef]
- Dubey, I.; Singh Gaur, B.; Singh, R. A study to find correlation of platelet indices with HbA1c in diabetic patients with absence/presence of vascular complications. Int. J. Res. Med. Sci. 2017, 5, 1042–1047. [Google Scholar] [CrossRef] [Green Version]
- Pujani, M.; Gahlawat, H.; Agarwal, C.; Chauhan, V.; Singh, K.; Lukhmana, S. Platelet parameters: Can they serve as biomarkers of glycemic control or development of complications in evaluation of type 2 diabetes mellitus? Iraq J. Hematol. 2018, 7, 72. [Google Scholar]
- Daly, M.E. Determinants of platelet count in humans. Haematologica 2011, 96, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Yang, J.; Lu, X.; Okada, T.; Kondo, T.; Ruan, C.; Wu, Y.; Xin, X. Effects of biological variations on platelet count in healthy subjects in China. Thromb. Haemost. 2004, 91, 367–372. [Google Scholar] [PubMed]
- Ferroni, P.; Basili, S.; Falco, A.; Davi, G. Platelet activation in type 2 diabetes mellitus. J. Thromb. Haemost. 2004, 2, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Alçelik, A.; Tekce, B.K.; Şavlı, H.; Uyeturk, U.; Kurt, M.; Tekelioglu, V.; Yüce, Y. Mean platelet volume and red cell distribution width in hepatosteatosis. Natl. J. Med. Res. 2013, 3, 264–266. [Google Scholar]
- Kim, J.H.; Bae, H.Y.; Kim, S.Y. Clinical marker of platelet hyperreactivity in diabetes mellitus. Diabetes Metab. J. 2013, 37, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, M.; Fujii, H.; Sumida, Y.; Hyogo, H.; Itoh, Y.; Ono, M.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. 2011, 46, 1300–1306. [Google Scholar] [CrossRef]
- Arase, N.; Arase, H. Cellular misfolded proteins rescued from degradation by MHC class II molecules are possible targets for autoimmune diseases. J. Biochem. 2015, 158, 367–372. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- ICH GCP|Good Clinical Practice—ICH GCP. Available online: https://ichgcp.net/ (accessed on 27 February 2020).
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar]
- European Association for the Study of Liver. EASL Clinical Practical Guidelines: Management of Alcoholic Liver Disease. J. Hepatol. 2012, 57, 399–420. [Google Scholar] [CrossRef]
- Kumar, R.; Rastogi, A.; Sharma, M.K.; Bhatia, V.; Tyagi, P.; Sharma, P.; Garg, H.; Chandan Kumar, K.N.; Bihari, C.; Sarin, S.K. Liver Stiffness Measurements in Patients with Different Stages of Nonalcoholic Fatty Liver Disease: Diagnostic Performance and Clinicopathological Correlation. Dig. Dis. Sci. 2013, 58, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A Novel VCTETM Guided Ultrasonic Attenuation Measurement for the Evaluation of Hepatic Steatosis: Preliminary Study and Validation in a Cohort of Patients with Chronic Liver Disease from Various Causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Nik Mustapha, N.R.; Wong, G.L.-H.; Wong, V.W.-S.; Mahadeva, S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United Eur. Gastroenterol. J. 2017, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- De Lédinghen, V.; Vergniol, J.; Foucher, J.; Merrouche, W.; le Bail, B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012, 32, 911–918. [Google Scholar] [CrossRef]

| Characteristics | Anti-THPO Antibody (−) | Anti-THPO Antibody (+) | p |
|---|---|---|---|
| Total number | 69 | 13 | - |
| Male | 30 (43.5) | 5 (38.5) | 0.74 |
| Age (years) | 64.1 ± 11.3 | 69.9 ± 10.2 | 0.09 |
| Duration of diabetes (years) | 13.9 ± 9.8 | 9.6 ± 7.7 | 0.15 |
| Body weight (kg) | 63.6 ± 13.7 | 57.3 ± 14.3 | 0.14 |
| Body mass index (kg/m2) | 24.3 ± 3.8 | 23.7 ± 4.1 | 0.56 |
| Smoking (none/past/current) | 40 (58.0)/23 (33.3)/6 (8.7) | 4 (30.8)/8 (61.5)/1 (7.7) | 0.15 |
| Consumption of alcohol (g/day) | 13.8 ± 4.7 | 8.8 ± 8.8 | 0.67 |
| Retinopathy (NDR/SDR/PDR) | 51 (73.9)/13 (18.8)/5 (7.3) | 10 (76.9)/1 (7.7)/2 (1.4) | 0.44 |
| Nephropathy (normo/micro/macroalbumiuria) | 45 (65.2)/19 (27.5)/5 (7.3) | 10 (76.9)/3 (23.1)/0 (0) | 0.74 |
| Neuropathy (no/yes) | 44 (63.8)/25 (36.2) | 6 (46.2)/7 (53.8) | 0.23 |
| Sulfonylurea | 26 (37.7) | 6 (46.2) | 0.57 |
| Dipeptidyl peptidase-4 inhibitor | 16 (23.2) | 5 (38.5) | 0.26 |
| Glucagon-like peptide-1 receptor agonist | 4 (5.8) | 0 (0) | 0.23 |
| Pioglitazone | 23 (33.3) | 6 (46.2) | 0.38 |
| Metformin | 32 (46.4) | 4 (30.8) | 0.29 |
| SGLT2 inhibitor | 2 (2.9) | 0 (0) | 0.40 |
| Insulin therapy | 22 (31.9) | 4 (30.8) | 0.94 |
| Systolic blood pressure (mmHg) | 130.1 ± 17.3 | 137.8 ± 22.3 | 0.16 |
| Diastolic blood pressure (mmHg) | 76.2 ± 11.8 | 81.0 ± 11.8 | 0.18 |
| LSM (kPa) | 4.63 ± 1.13 | 5.08 ± 1.29 | 0.19 |
| CAP (dB/m) | 262.1 ± 55.0 | 247.8 ± 55.9 | 0.39 |
| Fatty liver | 47 (68.1) | 7 (53.8) | 0.33 |
| FIB4 index | 1.51 ± 0.68 | 1.92 ± 0.58 | <0.05 |
| HbA1c (%) | 7.43 ± 1.04 | 7.44 ± 1.25 | 0.97 |
| Fasting plasma glucose (mg/dL) | 138.5 ± 35.5 | 144.6 ± 37.6 | 0.57 |
| Aspartate aminotransferase (IU/L) | 23.2 ± 9.4 | 25.2 ± 9.2 | 0.49 |
| Alanine aminotransferase (IU/L) | 23.9 ± 16.5 | 27.0 ± 16.7 | 0.53 |
| Gamma-glutamyl transferase (IU/L) | 32.8 ± 23.1 | 30.5 ± 17.3 | 0.73 |
| eGFR (mL/min/1.73 m2) | 76.6 ± 20.0 | 73.9 ± 19.1 | 0.66 |
| C-reactive protein (mg/dL) | 0.05 ± 0.03 | 0.14 ± 0.21 | 0.11 |
| Thrombopoietin (pg/mL) | 67.4 ± 63.0 | 91.1 ± 56.9 | 0.23 |
| White blood cell count (×109/L) | 7.07 ± 1.83 | 5.60 ± 1.36 | <0.01 |
| Red blood cell count (×1012/L) | 4.57 ± 0.50 | 4.73 ± 0.56 | 0.31 |
| Hemoglobin (g/dL) | 13.6 ± 1.5 | 14.1 ± 1.4 | 0.31 |
| MPV (fL) | 9.99 ± 0.91 | 9.68 ± 0.77 | 0.26 |
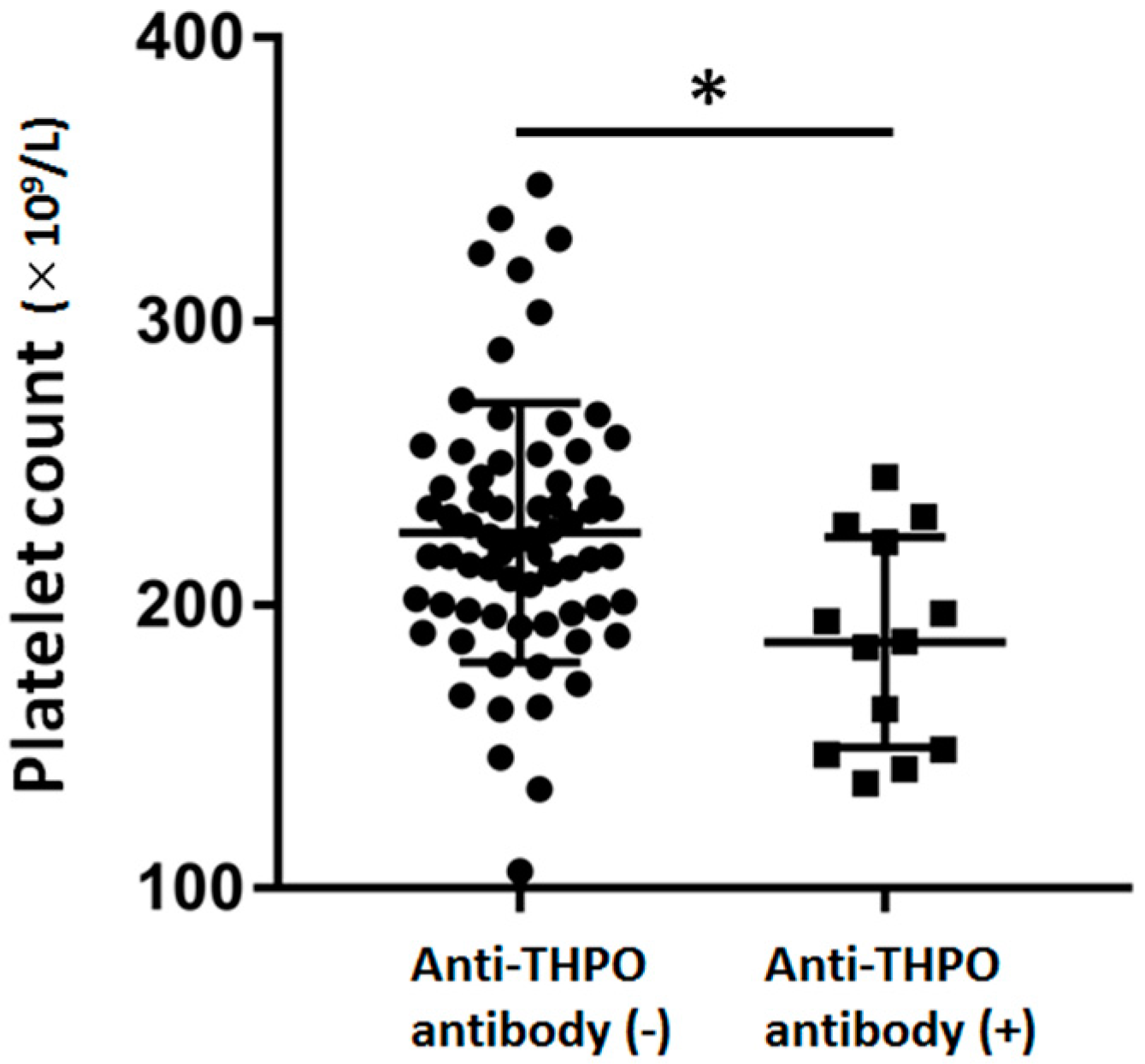
| Platelets (×109/L) | 228.7 ± 53.3 | 186.7 ± 37.1 | <0.01 |
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| r | p | β | p | |
| Age (years) | −0.35 | <0.01 | −0.27 | <0.01 |
| Sex (male) | 0.09 | 0.41 | - | - |
| Duration of diabetes (years) | −0.09 | 0.42 | - | - |
| HbA1c (%) | 0.32 | <0.01 | 0.25 | <0.05 |
| eGFR (mL/min/1.73 m2) | 0.21 | 0.06 | - | - |
| C-reactive protein (mg/dL) | 0.15 | 0.19 | - | - |
| LSM (kPa) | 0.15 | 0.17 | - | - |
| CAP (dB/m) | 0.25 | <0.05 | 0.12 | 0.23 |
| White blood cell count (×109/l) | 0.24 | <0.05 | 0.14 | 0.18 |
| Hemoglobin (g/dL) | 0.03 | 0.76 | - | - |
| MPV (fL) | −0.25 | <0.05 | −0.34 | <0.01 |
| Thrombopoietin (pg/mL) | 0.02 | 0.84 | - | - |
| anti-THPO antibody (yes) | −0.29 | <0.01 | −0.23 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, T.; Hamaguchi, M.; Osaka, T.; Hashimoto, Y.; Ushigome, E.; Asano, M.; Yamazaki, M.; Fukuda, E.; Yamaguchi, K.; Ogawa, K.; et al. A Pilot Study on the Effect of Anti-Thrombopoietin Antibody on Platelet Count in Patients with Type 2 Diabetes. Molecules 2020, 25, 1667. https://doi.org/10.3390/molecules25071667
Fukuda T, Hamaguchi M, Osaka T, Hashimoto Y, Ushigome E, Asano M, Yamazaki M, Fukuda E, Yamaguchi K, Ogawa K, et al. A Pilot Study on the Effect of Anti-Thrombopoietin Antibody on Platelet Count in Patients with Type 2 Diabetes. Molecules. 2020; 25(7):1667. https://doi.org/10.3390/molecules25071667
Chicago/Turabian StyleFukuda, Takuya, Masahide Hamaguchi, Takafumi Osaka, Yoshitaka Hashimoto, Emi Ushigome, Mai Asano, Masahiro Yamazaki, Eriko Fukuda, Kei Yamaguchi, Koji Ogawa, and et al. 2020. "A Pilot Study on the Effect of Anti-Thrombopoietin Antibody on Platelet Count in Patients with Type 2 Diabetes" Molecules 25, no. 7: 1667. https://doi.org/10.3390/molecules25071667







