Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermophysical Properties on Choline ILs
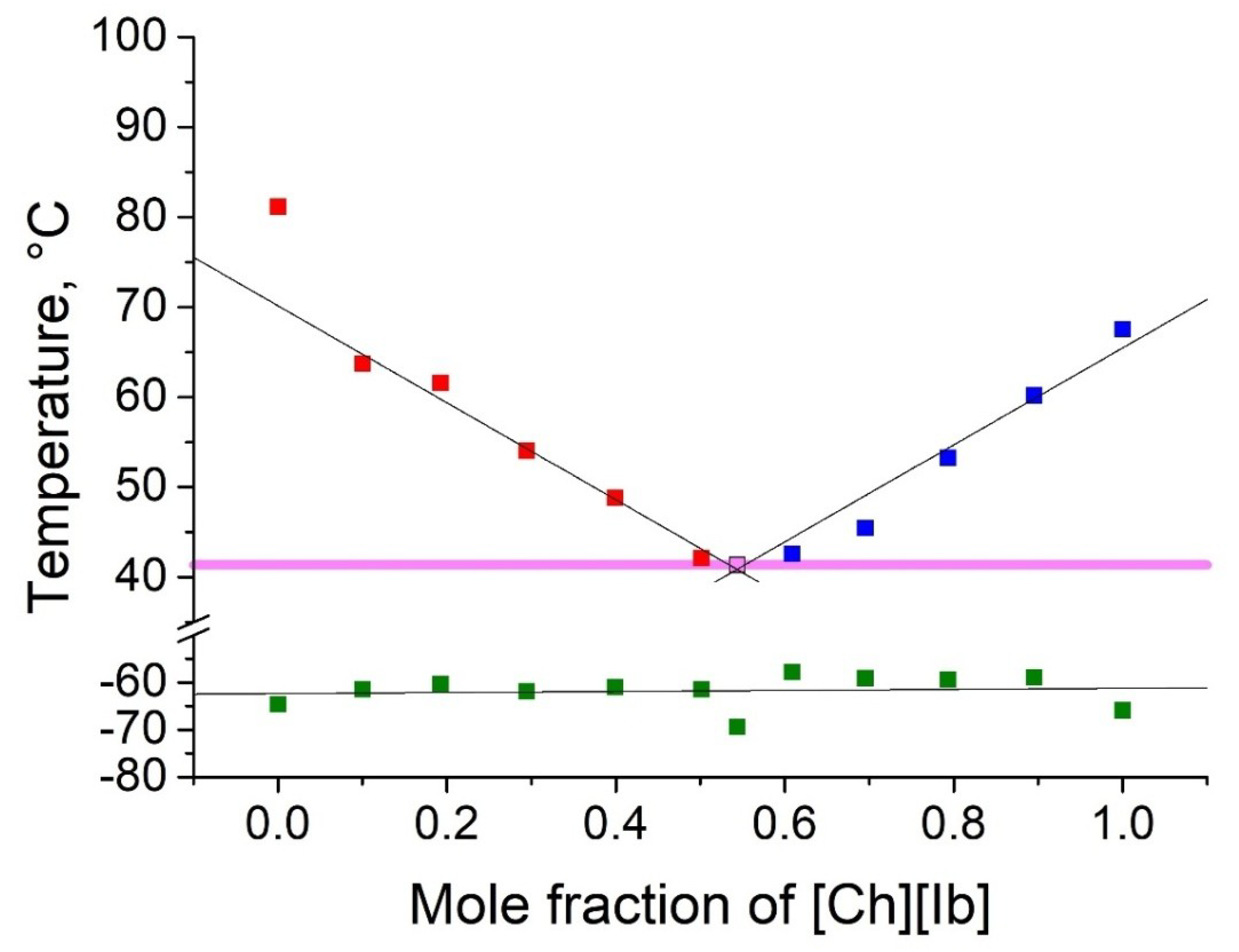
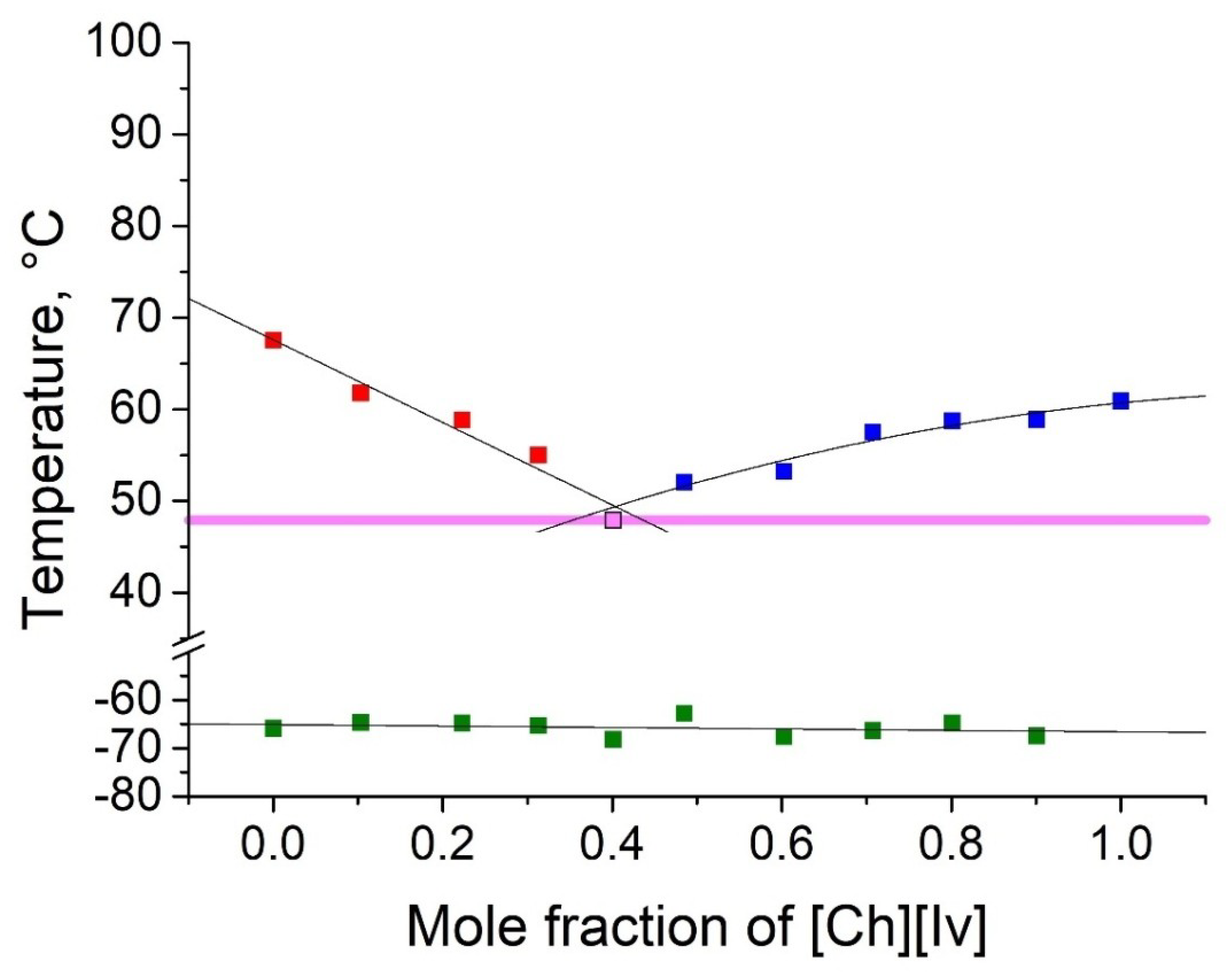
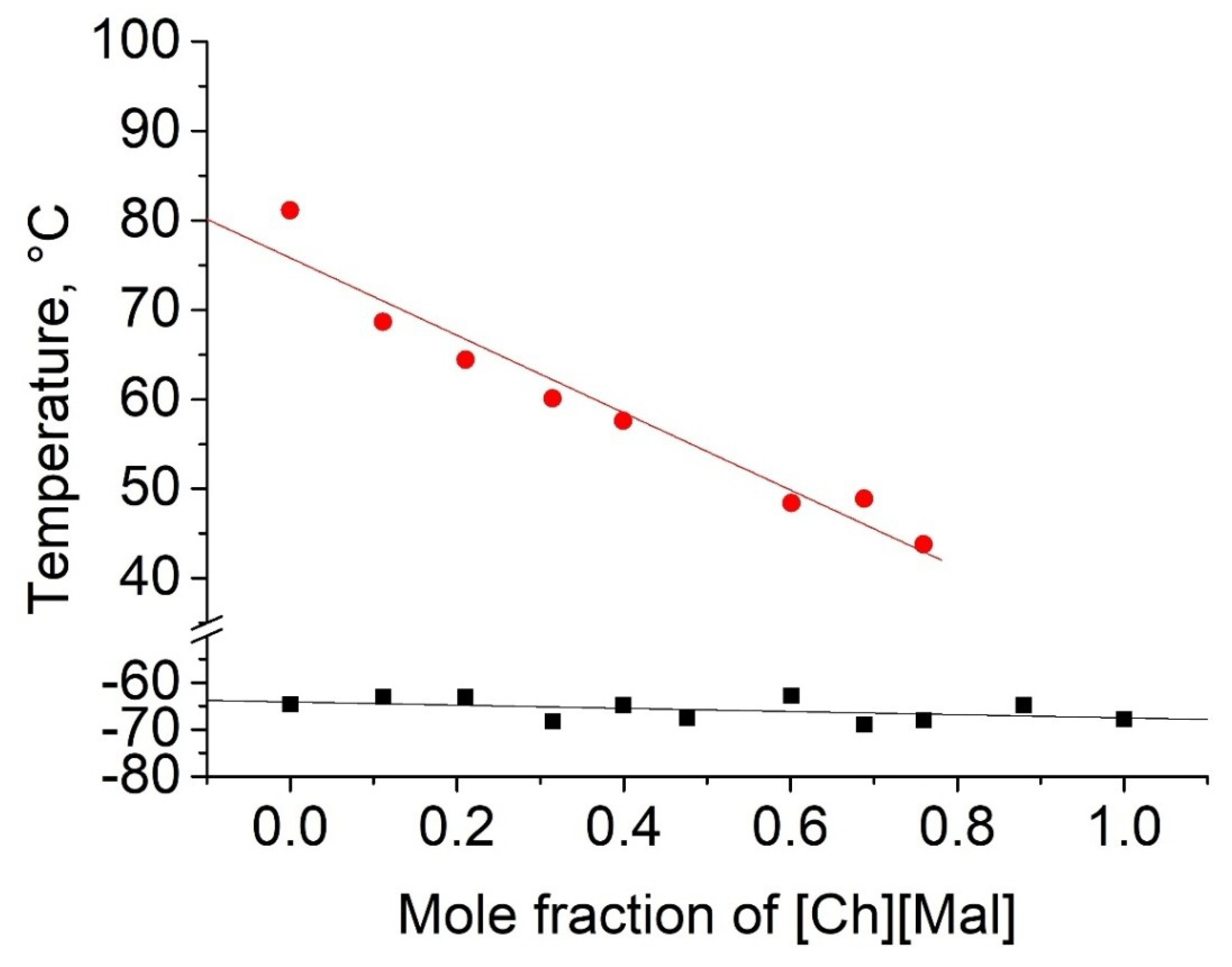
2.2. Thermophyscial Behaviour of the IL Mixtures
3. Materials and Methods
3.1. Materials
3.2. DSC Sample Preparation
3.3. DSC Measurements
3.4. TGA Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, J.; Chen, G.; Li, N. Ionic Liquid Solutions as a Green Tool for the Extraction and Isolation of Natural Products. Molecules 2018, 23, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacFarlane, D.R.; Chong, A.L.; Forsyth, M.; Kar, M.; Vijayaraghavan, R.; Somers, A.; Pringle, J.M. New dimensions in salt–solvent mixtures: A 4th evolution of ionic liquids. Faraday Discuss. 2018, 206, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, S.; Xu, H.; Dai, G. Preparations, properties, and formation mechanism of novel cellulose hydrogel membrane based on ionic liquid. J. Appl. Polym. Sci. 2018, 135, 45488. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef] [Green Version]
- Natour, S.; Levi-Zada, A.; Abu-Reziq, R. Magnetic Polyurea Nano-Capsules Synthesized via Interfacial Polymerization in Inverse Nano-Emulsion. Molecules 2019, 24, 2663. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wu, X.-S.; Wen, G.-Y.; Qiao, Y. Imidazolium Ionic Liquid Functionalized Carbon Nanotubes for Improved Interfacial Charge Transfer and Simultaneous Determination of Dihydroxybenzene Isomers. Molecules 2016, 21, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Hou, Q.; Li, W.; Bai, C.; Bai, X.; Ju, M. Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid. Molecules 2019, 24, 594. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.B.; Umpierre, A.P.; Santos, V.O., Jr.; Soares, V.C.D.; Dupont, J.; Rubim, J.C.; Suarez, P.A.Z. The use of Differential Scanning Calorimetry (DSC) to characterize phase diagrams of ionic mixtures of 1-n-butyl-3-methylimidazolium chloride and niobium chloride or zinc chloride. Thermochim. Acta 2010, 502, 20–23. [Google Scholar] [CrossRef]
- Kick, M.; Keil, P.; König, A. Solid–liquid phase diagram of the two Ionic Liquids EMIMCl and BMIMCl. Fluid Phase Equilib. 2013, 338, 172–178. [Google Scholar] [CrossRef]
- Stolarska, O.; Rodríguez, H.; Smiglak, M. Eutectic mixtures of pyrrolidinium-based ionic liquids. Fluid Phase Equilib. 2016, 408, 1–9. [Google Scholar] [CrossRef]
- Stolarska, O.; Soto, A.; Rodríguez, H.; Smiglak, M. Thermal behaviour of mixtures of 1-alkylpyridinium halides with and without a common ion. J. Mol. Liq. 2018, 268, 781–790. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Klein, R.; Müller, E.; Kraus, B.; Brunner, G.; Estrine, B.; Touraud, D.; Heilmann, J.; Kellermeier, M.; Kunz, W. Biodegradability and cytotoxicity of choline soaps on human cell lines: Effects of chain length and the cation. RSC Advances 2013, 3, 23347–23354. [Google Scholar] [CrossRef]
- Rengstl, D.; Kraus, B.; Van Vorst, M.; Elliott, G.D.; Kunz, W. Effect of choline carboxylate ionic liquids on biological membranes. Colloids Surf. B: Biointerfaces 2014, 123, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.-D.; Liu, Q.-P.; Smith, T.J.; Li, N.; Zong, M.-H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, L.; Wen, P.; Ye, X.; Dong, R.; Sun, W.; Fan, M.; Yang, D.; Zhou, F.; Liu, W. The ecotoxicity and tribological properties of choline amino acid ionic liquid lubricants. Tribol. Int. 2018, 121, 435–441. [Google Scholar] [CrossRef]
- Alcalde, R.; Gutiérrez, A.; Atilhan, M.; Aparicio, S. An experimental and theoretical investigation of the physicochemical properties on choline chloride – Lactic acid based natural deep eutectic solvent (NADES). J. Mol. Liq. 2019, 290, 110916. [Google Scholar] [CrossRef]
- Harifi-Mood, A.R.; Buchner, R. Density, viscosity, and conductivity of choline chloride + ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. J. Mol. Liq. 2017, 225, 689–695. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Yin, J.; Li, S.; Jia, Y.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine – Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Alcalde, R.; Atilhan, M.; Aparicio, S. On the properties of (choline chloride + lactic acid) deep eutectic solvent with methanol mixtures. J. Mol. Liq. 2018, 272, 815–820. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Synthesis of citric acid monohydrate-choline chloride based deep eutectic solvents (DES) and characterization of their physicochemical properties. J. Mol. Liq. 2019, 288, 111081. [Google Scholar] [CrossRef]
- Chemat, F.; Anjum, H.; Shariff, A.M.; Kumar, P.; Murugesan, T. Thermal and physical properties of (Choline chloride+urea+l-arginine) deep eutectic solvents. J. Mol. Liq. 2016, 218, 301–308. [Google Scholar] [CrossRef]
- Kuddushi, M.; Nangala, G.S.; Rajput, S.; Ijardar, S.P.; Malek, N.I. Understanding the peculiar effect of water on the physicochemical properties of choline chloride based deep eutectic solvents theoretically and experimentally. J. Mol. Liq. 2019, 278, 607–615. [Google Scholar] [CrossRef]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis – an alternative to ionic liquids? Green Chem. 2012, 14, 2969. [Google Scholar] [CrossRef] [Green Version]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Patidar, P.; Bahadur, A.; Prasad, K.; Tiwari, S.; Aswal, V.K.; Bahadur, P. Synthesis, self-assembly and micellization characteristics of choline alkanoate ionic liquids in association with a star block copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 691–698. [Google Scholar] [CrossRef]
- Sastry, N.V.; Singh, D.K.; Trivedi, P.A. Studies on Micellar Behavior of PEO-PBO or PEO-PBO-PEO Copolymers, or Surface Active Amphiphilic Ionic Liquids in Aqueous Media and Exploration of the Micellar Solutions for Solubilization of Dexamethasone and its Delayed Release. J. Surfactants Deterg. 2018, 21, 65–79. [Google Scholar] [CrossRef]
- Mourão, T.; Tomé, L.C.; Florindo, C.; Rebelo, L.P.N.; Marrucho, I.M. Understanding the Role of Cholinium Carboxylate Ionic Liquids in PEG-Based Aqueous Biphasic Systems. ACS Sustain. Chem. Eng. 2014, 2, 2426–2434. [Google Scholar] [CrossRef]
- Kunz, W.; Maurer, E.; Klein, R.; Touraud, D.; Rengstl, D.; Harrar, A.; Dengler, S.; Zech, O. Low Toxic Ionic Liquids, Liquid Catanionics, and Ionic Liquid Microemulsions. J. Dispers. Sci. Technol. 2011, 32, 1694–1699. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Muhammad, N.; Hossain, M.I.; Man, Z.; El-Harbawi, M.; Bustam, M.A.; Noaman, Y.A.; Mohamed Alitheen, N.B.; Ng, M.K.; Hefter, G.; Yin, C.-Y. Synthesis and Physical Properties of Choline Carboxylate Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2191–2196. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids—toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Sun, J.; Forsyth, M.; MacFarlane, D.R. Room-Temperature Molten Salts Based on the Quaternary Ammonium Ion. J. Phys. Chem. B 1998, 102, 8858–8864. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Boisset, A.; Menne, S.; Jacquemin, J.; Balducci, A.; Anouti, M. Deep eutectic solvents based on N-methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 20054. [Google Scholar] [CrossRef] [Green Version]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Macedo, E.A. Thermal Analysis and Heat Capacities of 1-Alkyl-3-methylimidazolium Ionic Liquids with NTf2−, TFO−, and DCA− Anions. Ind. Eng. Chem. Res. 2013, 52, 2103–2110. [Google Scholar] [CrossRef]
- Meng, X.; Ballerat-Busserolles, K.; Husson, P.; Andanson, J.-M. Impact of water on the melting temperature of urea + choline chloride deep eutectic solvent. New J. Chem. 2016, 40, 4492–4499. [Google Scholar] [CrossRef] [Green Version]
- Annat, G.; Forsyth, M.; MacFarlane, D.R. Ionic Liquid Mixtures—Variations in Physical Properties and Their Origins in Molecular Structure. J. Phys. Chem. B 2012, 116, 8251–8258. [Google Scholar] [CrossRef] [PubMed]
- Maximo, G.J.; Santos, R.J.B.N.; Brandão, P.; Esperança, J.M.S.S.; Costa, M.C.; Meirelles, A.J.A.; Freire, M.G.; Coutinho, J.A.P. Generating Ionic Liquids from Ionic Solids: An Investigation of the Melting Behavior of Binary Mixtures of Ionic Liquids. Cryst. Growth Des. 2014, 14, 4270–4277. [Google Scholar] [CrossRef]
Sample Availability: Samples of the choline IL mixtures and pure choline ILs are available from the authors. |



| IL | Abbreviation | Anion Structure | Tg | Tm | w% | Td, 5%, N2 | Td, 5%, air |
|---|---|---|---|---|---|---|---|
| choline acetate | [Ch][Ac] | 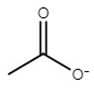 | −65 | 81 | 2.7 | 178 | 176 |
| choline isobutyrate | [Ch][Ib] |  | −66 | 68 | 0.1 | 178 | 172 |
| choline isovalerate | [Ch][Iv] |  | NA* | 61 | 0.0 | 176 | 176 |
| choline 2-methylbutyrate | [Ch][2mb] |  | −61 | 90 | 0.3 | 175 | 174 |
| choline malonate | [Ch][Mal] |  | −67 | ND** | 0.2 | 165 | 129 |
| choline lactate | [Ch][Lac] | 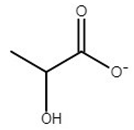 | −62 | ND** | 0.8 | 207 | 206 |
| [Ch][Ac] | [Ch][Ib] | [Ch][Iv] | [Ch][2mb] | [Ch][Mal] | [Ch][Lac] | |
|---|---|---|---|---|---|---|
| [Ch][Ac] | ||||||
| [Ch][Ib] | eutectic, Tm, Tg | |||||
| [Ch][Iv] | eutectic, Tm, Tg | eutectic, Tm, Tg | ||||
| [Ch][2mb] | eutectic, Tm, Tg | eutectic, Tm, Tg | Tm, Tg | |||
| [Ch][Mal] | Tm, Tg | - | - | - | ||
| [Ch][Lac] | Tg | - | - | - | - |
| Mixture | Eutectic Composition | Melting Point Depression | ||||
|---|---|---|---|---|---|---|
| Comp. A | Comp. B | Tm, eu, °C | Comp. A | Comp. B | Tm,A, °C | Tm,B, °C |
| [Ch][Ac] | [Ch][Ib] | 41 | 0.46 | 0.54 | 40 | 26 |
| [Ch][Ac] | [Ch][2mb] | 45 | 0.42 | 0.58 | 36 | 45 |
| [Ch][Ac] | [Ch][Iv] | 39 | 0.43 | 0.57 | 42 | 22 |
| [Ch][Ib] | [Ch][2mb] | 54 | 0.51 | 0.49 | 13 | 36 |
| [Ch][Ib] | [Ch][Iv] | 48 | 0.60 | 0.40 | 20 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhi, F.; Gantman, M.; Nurk, G.; Schulz, P.S.; Wasserscheid, P.; Aabloo, A.; Põhako-Esko, K. Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures. Molecules 2020, 25, 1691. https://doi.org/10.3390/molecules25071691
Elhi F, Gantman M, Nurk G, Schulz PS, Wasserscheid P, Aabloo A, Põhako-Esko K. Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures. Molecules. 2020; 25(7):1691. https://doi.org/10.3390/molecules25071691
Chicago/Turabian StyleElhi, Fred, Mikhail Gantman, Gunnar Nurk, Peter S. Schulz, Peter Wasserscheid, Alvo Aabloo, and Kaija Põhako-Esko. 2020. "Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures" Molecules 25, no. 7: 1691. https://doi.org/10.3390/molecules25071691
APA StyleElhi, F., Gantman, M., Nurk, G., Schulz, P. S., Wasserscheid, P., Aabloo, A., & Põhako-Esko, K. (2020). Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures. Molecules, 25(7), 1691. https://doi.org/10.3390/molecules25071691





