Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
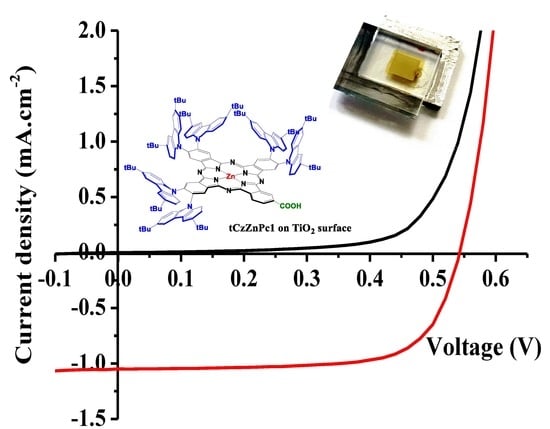
2.2. Photovoltaic Performance
3. Materials and Methods
3.1. General Methods and Characterization Techniques
3.2. Computational Details
3.3. Synthesis
3.4. Photovoltaics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urbani, M.; Ragoussi, M.-E.; Nazeeruddin, M.K.; Torres, T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Ragoussi, M.-E.; Torres, T. New generation solar cells: Concepts, trends and perspectives. Chem. Commun. 2015, 51, 3957–3972. [Google Scholar] [CrossRef] [Green Version]
- Ooyama, Y.; Harima, Y. Photophysical and electrochemical properties, and molecular structures of organic dyes for dye-sensitized solar cells. ChemPhysChem 2012, 13, 4032–4080. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Wu, H.; Cao, Y.; Zhang, J.; Xu, N.; Chen, S.; Decoppet, J.-D.; Zakeeruddin, S.M.; Grätzel, M. Stable and efficient organic dye-sensitized solar cell based on ionic liquid electrolyte. Joule 2018, 2, 2145–2153. [Google Scholar] [CrossRef] [Green Version]
- Feldt, S.M.; Wang, G.; Boschloo, G.; Hagfeldt, A. Effects of driving forces for recombination and regeneration on the photovoltaic performance of dye-sensitized solar cells using cobalt polypyridine redox couples. J. Phys. Chem. C 2011, 115, 21500–21507. [Google Scholar] [CrossRef]
- Harrath, K.; Hussain Talib, S.; Boughdiri, S. Theoretical design of metal-phthalocyanine dye-sensitized solar cells with improved efficiency. J. Mol. Model. 2018, 24, 279. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjörnsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Aumaitre, C.; Rodriguez-Seco, C.; Jover, J.; Bardagot, O.; Caffy, F.; Kervella, Y.; López, N.; Palomares, E.; Demadrille, R. Visible and near-infrared organic photosensitizers comprising isoindigo derivatives as chromophores: Synthesis, optoelectronic properties and factors limiting their efficiency in dye solar cells. J. Mater. Chem. A 2018, 6, 10074–10084. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Chen, J.; Xu, S.; Zhu, L.; Zhou, Y.; Xiang, Y.; Xia, D. Self-assembled core–shell nanospheres and dendritic nanostructure of novel tetra-(3-phenyprop-2-allyloxy) phthalocyanine in different solvents. RSC Adv. 2015, 5, 43489–43495. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.Y.; Giribabu, L.; Lyness, C.; Snaith, H.J.; Vijaykumar, C.; Chandrasekharam, M.; Lakshmikantam, M.; Yum, J.-H.; Kalyanasundaram, K.; Grätzel, M.; et al. Efficient sensitization of nanocrystalline TiO2 films by a near-IR-absorbing unsymmetrical zinc phthalocyanine. Angew. Chem. Int. Ed. 2007, 46, 373–376. [Google Scholar] [CrossRef]
- Cid, J.-J.; Yum, J.-H.; Jang, S.-R.; Nazeeruddin, M.K.; Martínez-Ferrero, E.; Palomares, E.; Ko, J.; Grätzel, M.; Torres, T. Molecular cosensitization for efficient panchromatic dye-sensitized solar cells. Angew. Chem. Int. Ed. 2007, 46, 8358–8362. [Google Scholar] [CrossRef]
- Giribabu, L.; Vijay Kumar, C.; Gopal Reddy, V.; Yella Reddy, P.; Srinivasa Rao, C.; Jang, S.-R.; Yum, J.-H.; Nazeeruddin, M.K.; Grätzel, M. Unsymmetrical alkoxy zinc phthalocyanine for sensitization of nanocrystalline TiO2 films. Solar Energy Mater. Solar Cells 2007, 91, 1611–1617. [Google Scholar] [CrossRef]
- Giribabu, L.; Kumar, C.V.; Reddy, P.Y.; Yum, J.-H.; Grätzel, M.; Nazeeruddin, M.K. Unsymmetrical extended π-conjugated zinc phthalocyanine for sensitization of nanocrystalline TiO2 films. J. Chem. Sci. 2009, 121, 75. [Google Scholar] [CrossRef]
- Nagata, M.; Kimura, M.; Taya, M. Design of Dye-Sensitized Solar Cells with New Light-Harvesting Dyes; SPIE: Bellingham, WA, USA, 2008; Volume 6927. [Google Scholar]
- Ragoussi, M.-E.; Cid, J.-J.; Yum, J.-H.; de la Torre, G.; Di Censo, D.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Carboxyethynyl anchoring ligands: a means to improving the efficiency of phthalocyanine-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 4375–4378. [Google Scholar] [CrossRef]
- Ragoussi, M.-E.; Yum, J.-H.; Chandiran, A.K.; Ince, M.; de la Torre, G.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Sterically hindered phthalocyanines for dye-sensitized solar cells: influence of the distance between the aromatic core and the anchoring group. ChemPhysChem 2014, 15, 1033–1036. [Google Scholar] [CrossRef]
- Mori, S.; Nagata, M.; Nakahata, Y.; Yasuta, K.; Goto, R.; Kimura, M.; Taya, M. Enhancement of incident photon-to-current conversion efficiency for phthalocyanine-sensitized solar cells by 3D molecular structuralization. J. Am. Chem. Soc. 2010, 132, 4054–4055. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Murakami, T.N.; Masaki, N.; Furube, A.; Kimura, M.; Mori, S. Dye aggregation effect on interfacial electron-transfer dynamics in zinc phthalocyanine-sensitized solar cells. J. Phys. Chem. C 2014, 118, 17205–17212. [Google Scholar] [CrossRef]
- Ikeuchi, T.; Nomoto, H.; Masaki, N.; Griffith, M.J.; Mori, S.; Kimura, M. Molecular engineering of zinc phthalocyanine sensitizers for efficient dye-sensitized solar cells. Chem. Commun. 2014, 50, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, L.; Singh, V.K.; Kumar, C.V.; Soujanya, Y.; Reddy, P.Y.; Kantam, M.L. Triphenylamine–phthalocyanine based sensitizer for sensitization of nanocrystalline TiO2 films. Solar Energy 2011, 85, 1204–1212. [Google Scholar] [CrossRef]
- Ince, M.; Cardinali, F.; Yum, J.-H.; Martínez-Díaz, M.V.; Nazeeruddin, M.K.; Grätzel, M.; Torres, T. Convergent synthesis of near-infrared absorbing, “Push–Pull”, Bisthiophene-substituted, Zinc(II) phthalocyanines and their application in dye-sensitized solar cells. Chem. A Eur. J. 2012, 18, 6343–6348. [Google Scholar] [CrossRef] [PubMed]
- Milan, R.; Selopal, G.S.; Cavazzini, M.; Orlandi, S.; Boaretto, R.; Caramori, S.; Concina, I.; Pozzi, G. Dye-sensitized solar cells based on a push-pull zinc phthalocyanine bearing diphenylamine donor groups: Computational predictions face experimental reality. Sci. Rep. 2017, 7, 15675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M.; Suzuki, H.; Tohata, Y.; Ikeuchi, T.; Yamamoto, S.; Kobayashi, N. Carbazole-fused Zinc(II)−phthalocyanine sensitizers. Asian J. Org. Chem. 2017, 6, 544–550. [Google Scholar] [CrossRef]
- Majeed, S.A.; Ghazal, B.; Nevonen, D.E.; Goff, P.C.; Blank, D.A.; Nemykin, V.N.; Makhseed, S. Evaluation of the intramolecular charge-transfer properties in solvatochromic and electrochromic Zinc Octa(carbazolyl)phthalocyanines. Inorg. Chem. 2017, 56, 11640–11653. [Google Scholar] [CrossRef]
- Majeed, S.A.; Ghazal, B.; Nevonen, D.E.; Nemykin, V.N.; Makhseed, S. Spectroscopic and TDDFT studies on the charge-transfer properties of metallated Octa(carbazolyl)phthalocyanines. Dye. Pigment. 2019, 170, 107593. [Google Scholar] [CrossRef]
- Monda, F.; Madsen, R. Zinc oxide-catalyzed dehydrogenation of primary alcohols into carboxylic acids. Chem. A Eur. J. 2018, 24, 17832–17837. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Plenum: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Kieboom, A.P.G. Purification of Laboratory Chemicals, 3rd ed.; Perrin, D.D., Armarego, W.L.F., Eds.; Pergamon Press: Oxford, UK; Wiley-Vch Verlag: Weinheim, Germany, 1988; Volume 107, p. 685. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Enes, R.F.; Cid, J.-J.; Hausmann, A.; Trukhina, O.; Gouloumis, A.; Vázquez, P.; Cavaleiro, J.A.S.; Tomé, A.C.; Guldi, D.M.; Torres, T. Synthesis and photophysical properties of fullerene–Phthalocyanine–Porphyrin triads and pentads. Chem. A Eur. J. 2012, 18, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Dye | Cells | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| tCzZnPc1 | Opaque | 1.05 (0.98) | 0.54 (0.54) | 70.8 (69.8) | 0.40 (0.37) |
| tCzZnPc1 | Transparent | 0.91 (0.77) | 0.54 (0.51) | 71.7 (73.3) | 0.35 (0.29) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazal, B.; Azizi, K.; Ewies, E.F.; Youssef, A.S.A.; Mwalukuku, V.M.; Demadrille, R.; Torres, T.; Makhseed, S. Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells. Molecules 2020, 25, 1692. https://doi.org/10.3390/molecules25071692
Ghazal B, Azizi K, Ewies EF, Youssef ASA, Mwalukuku VM, Demadrille R, Torres T, Makhseed S. Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells. Molecules. 2020; 25(7):1692. https://doi.org/10.3390/molecules25071692
Chicago/Turabian StyleGhazal, Basma, Kobra Azizi, Ewies F. Ewies, Ahmed S. A. Youssef, Valid Mwatati Mwalukuku, Renaud Demadrille, Tomás Torres, and Saad Makhseed. 2020. "Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells" Molecules 25, no. 7: 1692. https://doi.org/10.3390/molecules25071692
APA StyleGhazal, B., Azizi, K., Ewies, E. F., Youssef, A. S. A., Mwalukuku, V. M., Demadrille, R., Torres, T., & Makhseed, S. (2020). Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells. Molecules, 25(7), 1692. https://doi.org/10.3390/molecules25071692