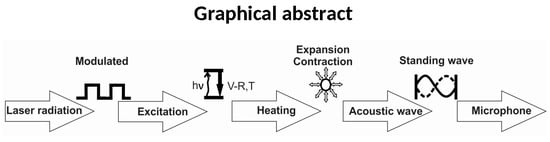
Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review
Abstract
:1. Introduction
2. Laser Photoacoustic Spectroscopy
2.1. Theoretical Background
- (1)
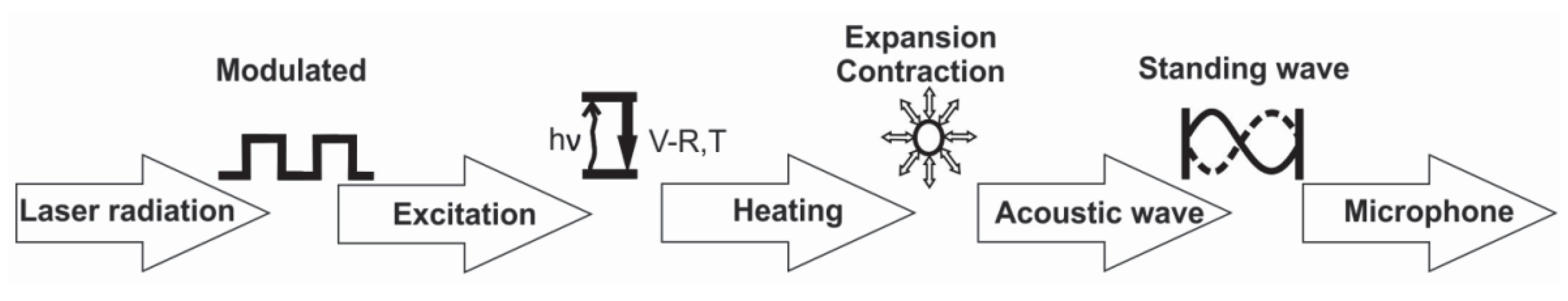
- Modulation of the laser radiation (either in amplitude or frequency) at a wavelength that overlaps with a spectral feature of the target species;
- (2)
- Excitation o of the target molecule by absorption of the incident laser radiation;
- (3)
- Energy exchange processes: the energy which is absorbed is almost completely converted to the kinetic energy of the gas molecules, the kinetic energy and then converted into periodic local heating at the modulation frequency;
- (4)
- Expansion and contraction of the gas in a closed volume that give rise to pressure variation which is an acoustic wave;
- (5)
- Detection of the resulting acoustic waves with microphones.
2.2. Laser Sources
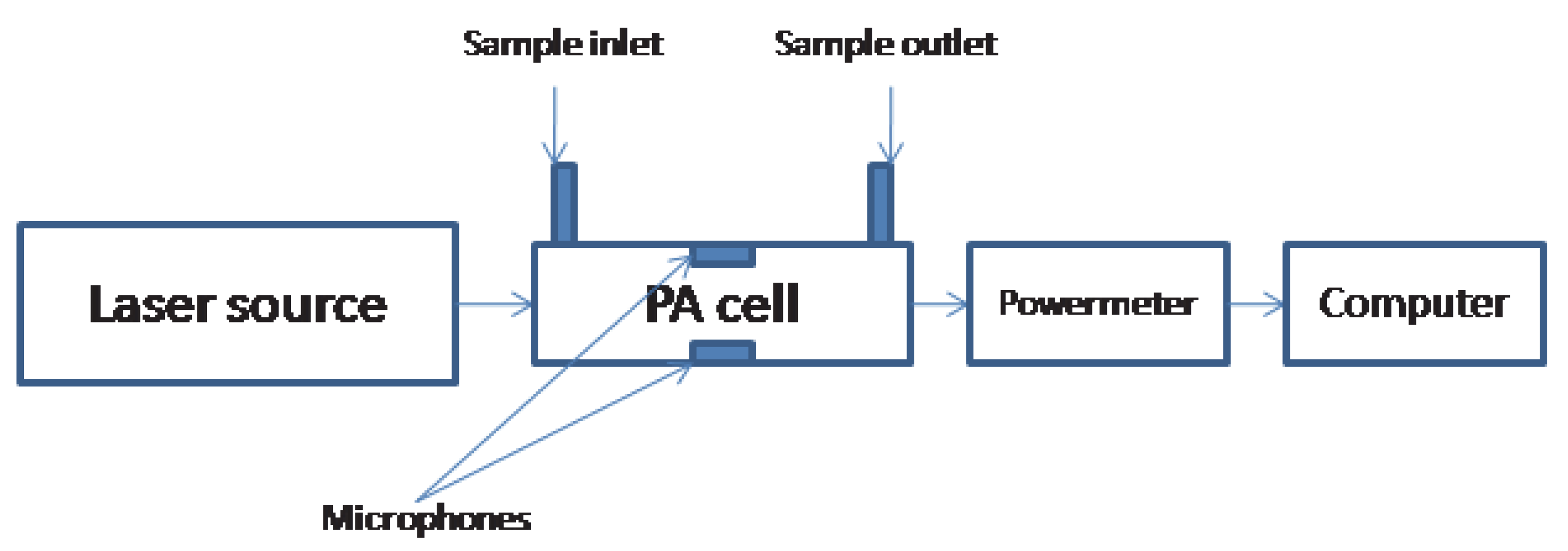
2.3. Photoacoustic Cell
2.4. Noises and Limiting Factors
- (a)
- Electrical noise, represent any random fluctuation (electronic or acoustic) which does not have a fixed phase relation with the modulation of the laser intensity.
- (b)
- Coherent acoustic background noise, meaning a signal caused by the modulation process, but not attributable to the presence of the light beam in the PA cell.
- (c)
- Coherent photoacoustic background signal. This signal, which is always present in the PA detector, is caused by the laser beam, it is due to laser beam heating of the windows and of the absorbates at their surfaces, and heating of the PA resonator walls by the reflected or scattered light owing to imperfections of the focusing lens, windows and inner walls of the PA resonator. This signal is in phase with, and at the same frequency as, the laser intensity modulation and therefore, it is not filtered out by the lock-in amplifier connected to the microphone. Thus, a background signal proportional to the laser power becomes the main factor that limits sensitivity.
3. Applications of PAS in Human Health
3.1. Human Breath Biomarkers
3.1.1. Ammonia
3.1.2. Nitric Oxide
3.1.3. Carbon Dioxide
3.1.4. Acetone
3.1.5. Etylene
3.2. LPAS for Breath Analysis: Clinical Implementation
3.2.1. Oxidative Stress
3.2.2. LPAS in Subjects with Autism
3.2.3. LPAS in Subjects with Schizophrenia
3.2.4. LPAS in Smokers
3.2.5. LPAS in Radiotherapy
3.2.6. LPAS in Lung Cancer
3.2.7. LPAS in Kidney Failure
3.2.8. LPAS in Diabetes
3.2.9. LPAS in Surgical Smoke Gases Detection
3.2.10. LPAS in the Study of Dietary Effects on VOCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Francesco, F.D.; Fuoco, R.; Trivella, M.G.; Ceccarini, A. Breath analysis: Trends in techniques and clinical applications. Microchem. J. 2005, 79, 405–410. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5, S540. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Giulio, C.D.; Pokorski, M. Pathologies currently identified by exhaled biomarkers. Respir. Physiol. Neurobiol. 2013, 187, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M. Breath tests in medicine. Sci. Am. 1992, 267, 74–79. [Google Scholar] [CrossRef]
- Risby, T.H. Volatile organic compounds as markers in normal and diseased states. In Disease Markers in Exhaled Breath; Marczin, N., Yacoub, M.H., Eds.; IOS Press: Amsterdam, The Netherlands, 2002; pp. 113–122. [Google Scholar]
- Amann, A.; Poupart, G.; Telser, S.; Ledochowski, M.; Schmid, A.; Mechtcheriakov, S. Applications of breath gas analysis in medicine. Int. J. Mass Spectrom. 2004, 239, 227–233. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranish, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M. Method for the collection and assay of volatile organic compounds in breath. Anal. Biochem. 1997, 247, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Gleeson, K.; Hughes, J.M.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, P.W. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Popov, T.A. Human exhaled breath analysis. Ann. Allergy Asthma Immunol. 2011, 106, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Stiegel, M.A.; Risby, T.H. Clinical Breath Analysis: Discriminating Between Human Endogenous Compounds and Exogenous (Environmental) Chemical Confounders. J. Breath Res. 2013, 7, 017107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agapiou, A.; Amann, A.; Mochalski, P.; Statheropoulos, M.; Thomas, C.L.P. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. Trends Anal. Chem. 2015, 66, 158–175. [Google Scholar] [CrossRef]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hacki, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef] [Green Version]
- Mochalski, P.; King, J.; Klieber, M.; Unterkofler, K.; Hinterhuber, H.; Baumann, M.; Amann, A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst 2013, 138, 2134–2145. [Google Scholar] [CrossRef] [Green Version]
- Mochalski, P.; King, J.; Haas., M.; Unterkofler, K.; Amann, A.; Mayer, G. Blood and breath profiles of volatile organic compounds in patients with end-stage renal disease. BMC Nephrol. 2014, 15, 43. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of exhaled breath for disease detection. Ann. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef]
- Del Rio, R.F.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile biomarkers in breath associated with liver cirrhosis—Comparisons of pre-and post-liver transplant breath samples. Ebiomedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Dryahina, K.; Smith, D.; Bortlik, M.; Machkova, N.; Lukas, M.; Spanel, P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. J. Breath Res. 2018, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mundada, M.; Patel, U.; Zhang, X. Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. PLoS ONE 2013, 8, e75274. [Google Scholar] [CrossRef] [PubMed]
- Dymerski, T.; Gebicki, J.; Wi’sniewska, P.; ’Sliwi’nska, M.; Wardencki, W.; Namie’snik, J. Application of the electronic nose technique to differentiation between model mixtures with COPD markers. Sensors 2013, 13, 5008–5027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Magureanu, A.M.; Petrus, M.; Popa, C. Laser photoacoustic spectroscopy: Principles, instrumentation, and characterization. J. Optoelectron. Adv. Mater. 2007, 9, 3655–3701. [Google Scholar]
- Harren, F.J.M.; Cotti, G.; Oomens, J.; te Lintel Hekkert, S. Environment: Trace Gas Monitoring. In Encyclopedia of Analytical Chemistry; Sigrist, M.W., Meyers, R.A., Eds.; Wiley: Chichester, UK, 2000; Volume 3, pp. 2203–2226. [Google Scholar]
- Kerschhofer, A.; Breitegger, P.; Bergmann, A. Laser Driver and Analysis Circuitry Development for Quartz-Enhanced Photoacoustic Spectroscopy of NO2 for IoT Purpose. Proceedings 2018, 2, 1062. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Španěl, P.; Smith, D.; Hanna, G.B. Selected Ion Flow Tube Mass Spectrometry Analysis of Exhaled Breath for Volatile Organic Compound Profiling of Esophago-Gastric. Cancer Anal. Chem. 2013, 85, 6121–6128. [Google Scholar] [CrossRef]
- Milligan, D.P.; Wilson, P.F.; Mautner, M.N.; Freeman, C.G.; McEwan, M.J.; Clough, T.J.; Sherlock, R.R. Atmospheric Pollutants and Trace Gases. J. Environ. Qual. 2002, 31, 515. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Koss, A.R.; Warneke, C.; Coggon, M.; Sekimoto, K.; de Gouw, J.A. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem. Rev. 2017, 117, 13187–13229. [Google Scholar] [CrossRef]
- Amelynck, C.; Schoon, N.; Dhooghe, F. SIFT Ion Chemistry Studies Underpinning the Measurement of Volatile Organic Compound Emissions by Vegetation. Curr. Anal. Chem. 2013, 9, 540–549. [Google Scholar] [CrossRef]
- Davis, B.M.; Senthilmohan, S.T.; Wilson, P.F.; McEwan, M.J. Major volatile compounds in head-space above olive oil analysed by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Filipiak, W.; Amann, A. Determining concentration patterns of volatile compounds in exhaled breath by PTR-MS. J. Breath Res. 2009, 3, 027002. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Vaidya, J.; Kulkarni, Y.A.; Gaud, R.S. Systematic approaches for biodiagnostics using exhaled air. J. Controll. Release 2017, 268, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Spanel, P.; Herbig, J.; Beauchamp, J. Mass spectrometry for real-time quantitative breath analysis. J. Breath Res. 2014, 8, 027101. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Kesy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. BMC Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef]
- Lord, H.; Yu, Y.F.; Segal, A.; Pawliszyn, J. Breath analysis and monitoring by membrane extraction with sorbent interface. Anal. Chem. 2002, 74, 5650–5657. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Kerr, E.L.; Atwood, J. The laser illuminated absorptivity spectrophone: A method for measurement of weak absorptivity in gases at laser wavelength. Appl. Opt. 1968, 7, 915–921. [Google Scholar] [CrossRef]
- Kreuzer, L.B. Ultralow gas concentration infrared absorption spectroscopy. J. Appl. Phys. 1971, 42, 2934–2943. [Google Scholar] [CrossRef]
- Patel, C.K.N.; Tam, A.C. Pulsed optoacoustic spectroscopy of condensed matter. Rev. Mod. Phys. 1981, 53, 517–554. [Google Scholar] [CrossRef]
- West, G.A. Photoacoustic spectroscopy. Rev. Sci. Instrum. 1983, 54, 797–817. [Google Scholar] [CrossRef]
- Hess, P. Resonant photoacoustic spectroscopy. In Topics in Current Chemistry; Boschke, F.L., Ed.; Springer: Berlin, Germny, 1983; Volume 11, pp. 1–32. [Google Scholar]
- Tam, A.C. Applications of photoacoustic sensing techniques. Rev. Mod. Phys. 1986, 58, 381–431. [Google Scholar] [CrossRef]
- Sigrist, M.W. Laser generation of acoustic waves in liquids and gases. J. Appl. Phys. 1986, 60, R83–R121. [Google Scholar] [CrossRef]
- Meyer, P.L.; Sigrist, M.W. Atmospheric pollution monitoring using CO2-laser photoacoustic spectroscopy and other techniques. Rev. Sci. Instrum. 1990, 61, 1779–1807. [Google Scholar] [CrossRef]
- Harren, F.J.M.; Reuss, J. Spectroscopy, photoacoustic. In Encyclopedia of Applied Physics; Trigg, G.L., Ed.; VCH Publishers: New York, NY, USA, 1997; Volume 19, pp. 413–435. [Google Scholar]
- Miklos, A.; Hess, P.; Bozoki, Z. Application of acoustic resonators in photoacoustic trace gas analysis and metrology. Rev. Sci. Instrum. 2001, 72, 1937–1955. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, M.W. Trace gas monitoring by laser photoacoustic spectroscopy and related techniques. Rev. Sci. Instrum. 2003, 74, 486–490. [Google Scholar] [CrossRef]
- Schmid, T. Photoacoustic spectroscopy for process analysis. Anal. Bioanal. Chem. 2006, 384, 1071–1086. [Google Scholar] [CrossRef]
- Henderson, B.; Khodabakhsh, A.; Metsälä, M.; Ventrillard, I.; Schmidt, F.M.; Romanini, D.; Ritchie, G.A.D.; te Lintel Hekkert, S.; Briot, R.; Risby, T.; et al. Laser spectroscopy for breath analysis: Towards clinical implementation. Appl. Phys. B 2018, 124, 161. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Sahay, P. Breath analysis using laser spectroscopic techniques: Breath biomarkers, spectral fingerprints, and detection limits. Sensor 2009, 9, 8230–8262. [Google Scholar] [CrossRef]
- Nieam, L.; Chen, X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem. Soc. Rev. 2014, 43, 7132–7170. [Google Scholar]
- Liu, Y.; Nie, L.; Chen, X. Photoacoustic Molecular Imaging: From Multiscale Biomedical Applications Towards Early-Stage Theranostics. Trends Biotechnol. 2016, 34, 420–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 20, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.G. Upon the production of sound by radiant energy. Phil. Mag. J. Sci. 1881, 11, 510–528. [Google Scholar] [CrossRef]
- Tyndall, J. Action of an intermittent beam of radiant heat upon gaseous matter. Proc. R. Soc. 1881, 31, 307–317. [Google Scholar]
- Röntgen, W.C. Über Töne, welche durch intermittierende Bestralung eines Gases entstehen. Ann. Phys. Chem. 1881, 1, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Preece, W.H. On the conversion of radiant energy into sonorous vibrations. Proc. R. Soc. 1881, 31, 506–520. [Google Scholar]
- Hubert, M.H. Laser Optoacustic Detector Measurement of Signatures of a Selection of Environmental Contaminant; Rep. No. 83-715−1; Ultra Lasertech Inc.: Mississauga, ON, Canada, 1983; pp. 1–107. [Google Scholar]
- Kosterev, A.A.; Tittel, F.K. Chemical Sensors Based on Quantum Cascade Lasers. IEEE J. Quant. Electron. 2002, 38, 582–591. [Google Scholar] [CrossRef]
- Kosterev, A.; Wysocki, G.; Bakhirkin, Y.; So, S.; Lewicki, R.; Fraser, M.; Tittel, F.; Curl, R.F. Application of Quantum Cascade Lasers to Trace Gas Analysis. Appl. Phys. B 2008, 90, 165–176. [Google Scholar] [CrossRef]
- Myers, L.E.; Bosenberg, W.R.; Pierce, J.W. Periodically Poled Lithium Niobate and Quasi-Phase-Matched Optical Parametric Oscillators. IEEE J. Quant. Electron. 1997, 33, 1663–1672. [Google Scholar] [CrossRef]
- Bruhns, H.; Wolff, M.; Saalberg, Y.; Spohr, K.M. Quantitative Evaluation of Broadband Photoacoustic Spectroscopy in the Infrared with an Optical Parametric Oscillator. Sensors 2018, 18, 3971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harren, F.J.M.; Mandon, J.; Cristescu, S.M. Photoacoustic Spectroscopy in Trace Gas Monitoring, Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Repond, P.; Sigrist, M.W. Photoacoustic spectroscopy on trace gases with continuously tunable CO2 laser. Appl. Opt. 1996, 35, 4065–4085. [Google Scholar] [CrossRef] [PubMed]
- Bohren, A.; Sigrist, M.W. Optical Parametric OscillatorBased Difference Frequency Laser Source for Photoacoustic Trace Gas Spectroscopy in the 3 mm mid-IR Range. Infrared Phys. Technol. 1997, 38, 423–435. [Google Scholar] [CrossRef]
- Patel, C.K.N.; Burkhardt, E.G.; Lambert, C.A. Spectroscopic Measurements of Stratospheric Nitric Oxide and Water Vapor. Science 1974, 184, 1173–1176. [Google Scholar] [CrossRef]
- Vansteenkiste, T.H.; Faxvog, F.R.; Roessler, D.M. Photoacoustic Measurement of Carbon Monoxide Using a Semiconductor Diode Lase. Appl. Spectrosc. 1981, 35, 194–196. [Google Scholar] [CrossRef]
- Bozo’ki, Z.; Sneider, J.; Szabo’, G.; Miklo’s, A.; Sere’nyi, M.; Nagy, G.; Feh’er, M. Intracavity Photoacoustic Gas Detection With an External Cavity Diode Laser. Appl. Phys. B 1996, 63, 399–401. [Google Scholar] [CrossRef]
- Avetisov, V.; Bjoroey, O.; Wang, J.; Geiser, P.; Paulsen, K.G. Hydrogen Sensor Based on Tunable Diode Laser Absorption Spectroscopy. Sensors 2019, 19, 5313. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.P.; Lahmann, W. Optoacoustic Detection of Sulfur Dioxide below the Parts per Billion Level. Appl. Phys. Lett. 1978, 32, 289–291. [Google Scholar] [CrossRef]
- Nägele, M.; Sigrist, M.W. Mobile laser spectrometer with novel resonant multipass photoacoustic cell for trace-gas sensing. Appl. Phys. B 2000, 70, 895–901. [Google Scholar] [CrossRef]
- Fung, K.H.; Lin, H.-B. Trace gas detection by laser intracavity photothermal spectroscopy. Appl. Opt. 1986, 25, 749–752. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Cong, Z.; Wang, Z.; Wang, F. Dual Path Lock-In System for Elimination of Residual Amplitude Modulation and SNR Enhancement in Photoacoustic Spectroscopy. Sensors 2018, 18, 4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Ji, Y.; Li, Y.; Li, T. Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy. Sensors 2018, 18, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratu, A.M.; Popa, C.; Matei, C.; Banita, S.; Dutu, D.C.A.; Dumitras, D.C. Removal of interfering gases in breath biomarker measurements. J. Optoelectron. Adv. Mater. 2011, 13, 1045–1050. [Google Scholar]
- Wang, J.Y.; Zhang, W.; Li, L.; Yu, Q. Breath ammonia detection based on tunable fiber laser photoacoustic spectroscopy. Appl. Phys. B 2011, 103, 263–269. [Google Scholar] [CrossRef]
- Risby, T.H.; Pleil, J.D. Breath analysis—Past, present and future: A special issue in honour of Michael Phillips’ 70th birthday. J. Breath Res. 2013, 7, 010201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, I.; Hunt, J.; Barnes, P.J. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 6, 523–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochalski, P.; Wiesenhofer, H.; Allers, M.; Zimmermann, S.; Güntner, A.T.; Pineau, N.J.; Lederer, W.; Agapiou, A.; Mayhew, C.A.; Ruzsanyi, V. Monitoring of selected skin- and breath-borne volatile organic compounds emitted from the human body using gas chromatography ion mobility spectrometry (GC-IMS). J. Chromatogr. B 2018, 1076, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaugg, M.T.; Gomez, D.G.; Barrios-Collado, C.; Vidal-de-Miguel, G.; Kohler, M.; Zenobi, R.; Sinues, P.M.L. Expanding metabolite coverage of real-time breath analysis by coupling a universal secondary electrospray ionization source and high resolution mass spectrometry-a pilot study on tobacco smokers. J. Breath Res. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.L.; Pownraj, P.; Abdulla, S.; Pullithandathil, B. Technologies for clinical diagnosis using expired human breath analysis. Diagnnostics 2015, 5, 27–60. [Google Scholar] [CrossRef]
- Spagnolo, V.; Lewicki, R.; Dong, L.; Tittel, F.K. Quantum-cascade-laser-based optoacoustic detection for breath sensor applications. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 30—31 May 2011. [Google Scholar] [CrossRef]
- Stönner, C.; Edtbauer, A.; Williams, J. Real-world volatile organic compound emission rates from seated adults and children for use in indoor air studies. Indoor Air 2018, 28, 164–172. [Google Scholar] [CrossRef]
- Souvik, D.; Saurabh, P.; Mitra, M. Significance of Exhaled Breath Test in Clinical Diagnosis: A Special Focus on the Detection of Diabetes Mellitus. J. Med. Biol. Eng. 2016, 36, 605–624. [Google Scholar]
- Španěl, P.; Smith, D. What is the real utility of breath ammonia concentration measurements in medicine and physiology? J. Breath Res. 2018, 12, 027102. [Google Scholar] [CrossRef] [PubMed]
- Spacek, L.A.; Mudalel, M.; Tittel, F.; Risby, T.H.; Solga, S.F. Clinical utility of breath ammonia for evaluation of ammonia physiology in healthy and cirrhotic adults. J. Breath Res. 2015, 9, 047109. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, T.; Killard, A.J. Breath Ammonia Analysis: Clinical Application and Measurement. Crit. Rev. Anal. Chem. 2011, 41, 21–35. [Google Scholar] [CrossRef]
- Solga, S.F.; Mudalel, M.L.; Spacek, L.A.; Risby, T.H. Fast and Accurate Exhaled Breath Ammonia Measurement. J. Vis. Exp. 2014, 88, e51658. [Google Scholar] [CrossRef] [Green Version]
- Lewicki, R.; Kosterev, A.A.; Bakhirkin, Y.A.; Thomazy, D.M.; Doty, J.; Dong, L.; Tittel, F.K. Real Time Ammonia Detection in Exhaled Human Breath with a Quantum Cascade Laser Based Sensor. In Proceedings of the Conference on Lasers and Electro-Optics and Conference on Quantum electronics and Laser Science Conference, Baltimore, MD, USA, 2–4 June 2009; pp. 1–2. [Google Scholar]
- Grob, N.M.; Aytekin, M.; Dweik, R.A. Biomarkers in exhaled breath condensate: A review of collection, processing and analysis. J. Breath Res. 2008, 2, 037004. [Google Scholar] [CrossRef] [Green Version]
- Hatziagorou, E.; Tsanakas, J. Assessment of airway inflammation with exhaled NO measurement. Hippokratia 2007, 11, 51–62. [Google Scholar]
- Dong, L.; Spagnolo, V.; Lewicki, R.; Tittel, F.K. Ppb-level detection of nitric oxide using an external cavity quantum cascade laser based QEPAS sensor. Opt. Express 2011, 19, 24037–24045. [Google Scholar] [CrossRef]
- Gondal, M.A.; Khalil, A.A.; Al-Suliman, N. High sensitive detection of nitric oxide using laser induced photoacoustic spectroscopy at 213 nm. Appl. Opt. 2012, 51, 5724–5734. [Google Scholar] [CrossRef]
- Kearney, D.J.; Hubbard, T.; Putnam, D. Breath ammonia measurement in helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 2523–2530. [Google Scholar] [CrossRef]
- Hibbard, T.; Killard, A.J. Breath ammonia levels in a normal human population study as determined by photoacoustic laser spectroscopy. J. Breath Res. 2011, 5, 037101. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Nikan, E.; Khodadadi, A.A.; Mortazavi, Y. Highly sensitive carbon nanotubes–SnO2 nanocomposite sensor for acetone detection in diabetes mellitus breath. Sens. Actuators B Chem. 2014, 205, 261–267. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giubileo, G. Laser based assessment of lipid peroxidation in humans. Proc. Spie 1998, 3405, 642–653. [Google Scholar]
- Lazo-de-la-Vega-Monroy, M.L.; Fernández-Mejía, C. Oxidative Stress in Diabetes Mellitus and the Role Of Vitamins with Antioxidant Actions, Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants. Morales-González, J.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Freidovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? N.Y. Acad. Sci. 1999, 893, 913. [Google Scholar] [CrossRef] [PubMed]
- Yun-Zhong, F.; Sheng, Y.; Wu, W.G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar]
- Yavuzer, S.; Yavuzer, H.; Cengiz, M.; Erman, H.; Demirdag, F.; Doventas, A.; Balci, H.; Erdincler, D.S.; Uzun, H. The role of protein oxidation and DNA damage in elderly hypertension. Aging Clin. Exp. Res. 2015, 28, 625–632. [Google Scholar] [CrossRef]
- Fatani, A.J.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Protective effect of lutein supplementation on oxidative stress and inflammatory progression in cerebral cortex of streptozotocin-induced diabetes in rats. Neurochem. J. 2016, 10, 69–76. [Google Scholar] [CrossRef]
- De Bandeira, S.M.; Guedes, G.; da Fonseca, L.J.S.; Pires, A.S.; Gelain, D.P.; Moreira, J.C.F.; Rabelo, L.A.; Vasconcelos, S.M.L.; Goulart, M.O.F. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: Increase in lipid peroxidation and SOD activity. Oxid. Med. Cell. Longev. 2012, 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Dix, T.A.; Aikens, J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem. Res. Toxicol. 1993, 6, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, S.M.; Kiss, R.; Hekkert, S.T.; Dalby, M.; Harren, F.J.M.; Risby, T.H.; Marczin, N.; Harefield, B.S.I. Real-time monitoring of endogenous lipid peroxidation by exhaled ethylene in patients undergoing cardiac surgery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L509–L515. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faras, H.; Al Ateeqi, N.; Tidmarsh, L. Autism spectrum disorders. Ann. Saudi Med. 2010, 30, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; McGee, J.P. Educating Children with Autism; National Research Council, National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Estes, A.; Munson, J.; Rogers, S.J.; Greenson, J.; Winter, J.; Dawson, G. Long-Term Outcomes of Early Intervention in 6-Year-Old Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Reichow, B.; Hume, K.; Barton, E.E.; Boyd, B.A. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst. Rev 2018, 5, CD009260. [Google Scholar] [CrossRef]
- Schendel, D.E.; Diguiseppi, C.; Croen., L.A.; Fallin, M.D.; Reed, P.L.; Schieve, L.A.; Wiggins, L.D.; Daniels, J.; Grether, J.; Levy, S.E.; et al. The Study to Explore Early Development (SEED): A multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. J. Autism Dev. Disord. 2012, 42, 2121–2140. [Google Scholar]
- Hammock, E.; Veenstra-VanderWeele, J.; Yan, Z.; Kerr, T.M.; Morris, M.; Anderson, G.M.; Carter, C.S.; Cook, E.H.; Jacob, S. Examining autism spectrum disorders by biomarkers: Example from the oxytocin and serotonin systems. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, B.; Sarkans, U.; Schumann, G.; Persico, A.M. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef]
- Anderson, S.R.; Avery, D.L.; DiPietro, E.K.; Edwards, G.L.; Christian, W.P. Intensive home-based early intervention with autistic children. Educ. Treat. Child. 1987, 10, 352–366. [Google Scholar]
- Cook, E.H.; Leventhal, B.L. The serotonin system in autis. Curr. Opin. Pediatr. 1996, 8, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; de Bildt, A.; van Lang, N.D.; den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929. [Google Scholar] [CrossRef]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 11, e479. [Google Scholar] [CrossRef] [PubMed]
- Ewen, J.B.; Sweeney, J.A.; Potter, W.Z. Conceptual, Regulatory and Strategic Imperatives in the Early Days of EEG-Based Biomarker Validation for Neurodevelopmental Disabilities. Front. Integr. Neurosci. 2019, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Loth, E.; Spooren, W.; Ham, L.M.; Isaac, M.B.; Auriche-Benichou, C.; Banaschewski, T.; Baron-Cohen, S.; Broich, K.; Bölte, S.; Bourgeron, T.; et al. Identification and validation of biomarkers for autism spectrum disorders. Nat. Rev. Drug Discov. 2016, 15, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osredkar, J.; Gosar, D.; Maˇcek, J.; Kumer, K.; Fabjan, T.; Finderle, P.; Šterpin, S.; Zupan, M.; Jekovec Vrhovšek, M. Urinary Markers of Oxidative Stress in Children with Autism Spectrum Disorder (ASD). Antioxidants 2019, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impairedmethylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Gonzalez Fraguela, M.E.; Diaz Hung, M.-L.; Vera, H.; Maragoto, C.; Noris, E.; Blanco, L.; Galvizu, R.; Robinson, M. Oxidative stress markers in children with autism spectrum disorders. Br. J. Med. Med. Res. 2013, 3, 307–317. [Google Scholar] [CrossRef]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative stress and erythrocyte membrane alterations in children with autism: Correlation with clinical features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risby, T.H.; Sehnert, S.S. Clinical application of breath biomarkers of oxidative stress status. Free Radic. Biol. Med. 1999, 27, 1182–1192. [Google Scholar] [CrossRef]
- Bratu, A.M.; Petrus, M.; Popa, C. Laser-based spectrometer for optical trace gas detection in young adults with autism. Microchem. J. 2018, 138, 203–208. [Google Scholar] [CrossRef]
- National Institute of Mental Health. Schizophrenia. 2016. Available online: https://nimh.nih.gov/health/topics/.schizophrenia/index.shtm. (accessed on 11 February 2020).
- Phillips, M.; Sabas, M.; Greenberg, J. Increased pentane and carbon disulfide in the breath of patients with schizophrenia. Clin. Pathol. 1993, 46, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Erickson, G.A.; Sabas, M.; Smith, J.P.; Greenberg, J. Volatile organic compounds in the breath of patients with schizophrenia. Clin. Pathol. 1995, 48, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; van Berkel, J.J.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef]
- Puri, B.K.; Counsell, S.J.; Ross, B.M.; Hamilton, G.; Bustos, M.G.; Treasaden, I.H. Evidence from in vivo 31-phosphorus magnetic resonance spectroscopy phosphodiesters that exhaled ethane is a biomarker of cerebral n-3 polyunsaturated fatty acid peroxidation in humans. BMC Psychiatry 2008, 8 (Suppl. S1), S2. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.; Petrus, M.; Bratu, A.M. Ammonia and ethylene biomarkers in the respiration of the people with schizophrenia using photoacoustic spectroscopy. J. Biomed. Opt. 2015, 20, 057006. [Google Scholar] [CrossRef]
- Tobacco: Leading Cause of Death, Illness and Impoverishment. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 4 March 2020).
- Bukreeva, E.B.; Bulanova, A.A.; Kistenev, Y.V.; Nikiforova, O.Y. Photoacoustic spectroscopy evaluation of the impact of smoking on the composition of exhaled air in patients with bronchopulmonary diseases. Ter Arkh. 2017, 89, 34–37. [Google Scholar] [CrossRef]
- Giubileo, G.; Puiu, A.P.; Dumitras, D.C. Detection of ethylene in smokers breath by laser photoacoustic spectroscopy. Proc. Spie Int. Soc. Opt. Eng. 2004, 5486, 280–286. [Google Scholar] [CrossRef]
- Popa, C.; Banita, S.; Patachia, M.; Matei, C.; Bratu, A.M.; Petrus, M.; Dumitras, D.C. CO2 laser-photoacoustic study of exhaled breath produced by electronic vs. traditional cigarettes. Rom. Rep. Phys. 2015, 67, 946–953. [Google Scholar]
- Department of Health. Achieving a world-class radiotherapy service across the UK. A report for Cancer Research UK. 2009. Available online: https://www.cancerresearchuk.org/sites/default/files/policy-achieving-a-world-class-radiotherapy-service-across-the-uk.pdf (accessed on 20 February 2020).
- Liauw, S.L.; Connell, P.P.; Weichselbaum, R.R. New paradigms and future challenges in Radiation Oncology: An Update of Biological Targets and Technology. Sci. Transl. Med. 2013, 20, 173sr2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, M.; Ito, H.; Matsui, H. Radiotherapy for cancer using X-ray fluorescence emitted from iodine. Sci. Rep. 2017, 7, 43667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.R.; Suhag, V.; Semwal, M.; Sharma, M.N. Radiotherapy: Basic Concepts and Recent Advances. Med. J. Armed Forces India 2010, 66, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Puiu, A.; Giubileo, G.; Bangrazi, C. Laser sensors for trace gases in human breath. Int. J. Environ. Anal. Chem. 2004, 85, 1001–1012. [Google Scholar] [CrossRef]
- Popa, C.; Verga, N.; Patachia, M.; Banita, S.; Matei, C.; Dumitras, D. Advantages of laser photoacoustic spectroscopy in radiotherapy characterization. Rom. Rep. Phys. 2014, 66, 120–126. [Google Scholar]
- Smith, R.A.; Manassaram-Baptiste, D.; Brooks, D.; Cokkinides, V.; Doroshenk, M.; Saslow, D.; Wender, R.C.; Brawley, O.W. Cancer screening in the United States, 2014: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2014, 64, 30–51. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Taivans, I.; Bukovskis, M.; Strazda, G.; Jurka, N. Breath testing as a method for detecting lung cancer. Expert Rev. Anticancer Ther. 2014, 14, 121–123. [Google Scholar] [CrossRef] [Green Version]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chrom. B. 2010, 878, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.C.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of lung cancer with volatile markers in the breath. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- Marcus, W.; Hinrich, G.G.; Matthias, D.; Hermann, H. Photoacoustic sensor for VOCs: First step towards a lung Cancer breath test. In Diagnostic Optical Spectroscopy in Biomedicine III, Proceedings of the European Conference on Biomedical Optics SPIE, Munich, Germany, 12–16 June 2005; Mycek, M.A., Ed.; OSA Publishing: Washington, DC, USA, 2005; Volume 5862, pp. 84–90. [Google Scholar]
- Kistenev, Y.V.; Borisov, A.V.; Nikolaev, V.V.; Vrazhnov, D.A.; Kuzmin, D.A. Laser photoacoustic spectroscopy applications in breathomics. J Biomed. Photonics Eng. 2019, 5, 010303. [Google Scholar] [CrossRef]
- Petrus, M.; Bratu, A.M.; Serbanescu, D. Laser spectroscopy method for non-invasive analysis of breath in pulmonary disease. Rom. Rep. Phys. 2015, 67, 1545–1557. [Google Scholar]
- European Dialysis and Transplant Nurses Association/European Renal Care Association (EDTNA/ERCA). Chronic Kidney Disease a Guide to Clinical Practice; Jenkins, K., Mahon, A., Eds.; European Dialysis and Transplant Nurses Association/European Renal Care Association (EDTNA/ERCA): Cham, Switzerland, 2008; ISBN 978-84-612-5925-0. [Google Scholar]
- Ashby, D.; Borman, N.; Burton, J.; Corbett, R.; Davenport, A.; Farrington, K.; Flowers, K.; Fotheringham, J.; Fox, R.N.A.; Franklin, G.; et al. Renal Association Clinical Practice Guideline on Haemodialysis. BMC Nephrol. 2019, 20, 379. [Google Scholar] [CrossRef]
- Davies, S.J.; Španěl, P.; Smith, D. Breath analysis of ammonia, volatile organic compounds and deuterated water vapor in chronic kidney disease and during dialysis. Bioanalysis 2014, 6, 843–857. [Google Scholar] [CrossRef]
- Španěl, P.; Davies, S.; Smith, D. Quantification of ammonia in human breath by the selected ion flow tube analytical method using H3O+ and O2+ precursor ions. Rapid Commun. Mass Spectrom. 1998, 12, 763–766. [Google Scholar] [CrossRef]
- Davies, S.; Španěl, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.; Dutu, D.C.A.; Cernat, R.; Matei, C.; Bratu, A.M.; Banita, S.; Dumitras, D.C. Ethylene and ammonia traces measurements from the patients’ breath with renal failure via LPAS method. Appl. Phys. B 2011, 105, 669–674. [Google Scholar] [CrossRef]
- Wang, J.W.; Xie, H.; Liang, L.R.; Zhang, W.; Peng, W.; Yu, Q.X. Tunable fiber laser based photoacoustic spectrometer for breath ammonia analysis during hemodialysis. Laser Phys. 2012, 22, 327–330. [Google Scholar] [CrossRef]
- Narasimhan, L.R.; Goodman, W.; Patel, K.N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tittel, F.K.; Lewicki, R.; Dong, L.; Liu, K.; Risby, T.H.; Solga, S.; Schwartz, T. Real time detection of exhaled human breath using quantum cascade laser based sensor technology. In Proceedings of the 3, Photons Plus Ultrasound: Imaging and Sensing SPIE 822, 82230E, San Francisco, CA, USA,, 23 February 2012. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016; ISBN 9789241565257. [Google Scholar]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int J Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar]
- Tachibana, K.; Okada, K.; Kobayashi, R.; Ishihara, Y. Development of a high-sensitivity and portable cell using Helmholtz resonance for noninvasive blood glucose-level measurement based on photoacoustic spectroscopy. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6477–6480. [Google Scholar] [CrossRef]
- Sim, J.Y.; Ahn, C.; Jeong, E.; Kim, B.K. In vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [Green Version]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntel, W. In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy. Anal. Chem. 2013, 85, 1013–1020. [Google Scholar] [CrossRef]
- Pai, P.P.; Sanki, P.K.; Banerjee, S.A. Photoacoustics based continuous non-invasive blood glucose monitoring system. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Turin, Italy, 7–9 May 2015; pp. 106–111. [Google Scholar] [CrossRef]
- Tyas, F.H.; Nikita, J.G.; Apriyanto, D.K.; Amin, M.N. The Performance of CO2 Laser Photoacoustic Spectrometer in Concentration Acetone Detection As Biomarker For Diabetes Mellitus Type 2. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1011, 012056. [Google Scholar] [CrossRef]
- Petrus, M.; Bratu, A.M.; Popa, C. Spectroscopic analysis of breath ethylene and oxidative stress relation with glycaemic status in type 2 diabetes. Opt. Quant. Electron. 2017, 49, 2. [Google Scholar] [CrossRef]
- Petrus, M.; Bratu, A.M.; Popa, C. The response of human body at oxidative stress in subjects with type 2 diabetes: Ammonia breath analysis by laser photoacoustic spectroscopy. Rev. Roum. Chim. 2016, 61, 89–95. [Google Scholar]
- Sigrist, M.W. Mid-infrared laser-spectroscopic sensing of chemical species. J. Adv. Res. 2015, 6, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Barrett, W.L.; Garber, S.M. Surgical smoke—A review of the literature. Is this just a lot of hot air? Surg. Endosc. 2003, 17, 979–987. [Google Scholar] [CrossRef]
- Romano, F.; Gustén, J.; De Antonellis, S.; Joppolo, C.M. Electrosurgical Smoke: Ultrafine Particle Measurements and Work Environment Quality in Different Operating Theatres. Int. J. Environ. Res. Public Health 2017, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Hu, X.; Yan, L.; Zhu, X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J. Cancer. 2019, 10, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.M.; Schramm, D.; Hahnloser, D.; Marinov, D.; Sigrist, M.W. Spectroscopic investigation of volatile compounds produced during thermal and radiofrequency bipolar cautery on porcine liver. Meas. Sci. Technol. 2008, 19, 5. [Google Scholar] [CrossRef]
- Petrus, M.; Bratu, A.M.; Patachia, M.; Dumitras, D.C. Spectroscopic analysis of surgical smoke produced in vitro by laser vaporization of animal tissues in aclosed gaseous environment. Rom. Rep. Phys. 2015, 67, 954–965. [Google Scholar]
- Hübner, M.; Sigrist, M.W.; Demartines, N.; Gianella, M.; Clavien, P.A.; Hahnloser, D. Gas emission during laparoscopic colorectal surgery using a bipolar vessel sealing device: A pilot study on four patients. Patient Saf. Surg. 2008, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Ajibola, O.A.; Smith, D.; Španěl, P.; Ferns, G.A.A. Effects of dietary nutrients on volatile breath metabolite. J. Nutr. Sci. 2013, 2, e34. [Google Scholar] [CrossRef] [Green Version]
- Hageman, J.H.J.; Nieuwenhuizen, A.G.; van Ruth, S.M.; Hageman, J.A.; Keijer, J. Application of Volatile Organic Compound Analysis in a Nutritional Intervention Study: Differential Responses during Five Hours Following Consumption of a High-and a Low-Fat Dairy Drink. Mol. Nutr. Food Res. 2019, 63, 1900189. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Lourenço, C.; Turner, C. Breath Analysis in Disease Diagnosis: Methodological Considerations and Applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef]
- Taucher, J.; Hansel, A.; Jordan, A.; Lindinger, W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996, 44, 3778–3782. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrus, M.; Bratu, A.M.; Popa, C. Spectroscopic study of dietary effects on volatile breath biomarkers. Rom. Rep. Phys. 2017, 69, 609. [Google Scholar]
| Breath Biomarker | Diseases | References |
|---|---|---|
| Ethane, Ethylene | Oxidative stress: Lipid peroxidation | [102] |
| Nitric oxide, Carbon monoxide, H2O2, isoprostanes, nitrite/nitrate | Lung disease: Asthma, COPD, lung cancer | [94,95] |
| Acetone | Metabolic disorders: Diabetes | [100,101] |
| H2, Carbon dioxide | Gastroenteric diseases: gastritis, ulcer, Helicobacter Pylori | [98,99] |
| Ammonia | Liver and/or kidneys disease | [54,89,90,91,92,93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitras, D.C.; Petrus, M.; Bratu, A.-M.; Popa, C. Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review. Molecules 2020, 25, 1728. https://doi.org/10.3390/molecules25071728
Dumitras DC, Petrus M, Bratu A-M, Popa C. Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review. Molecules. 2020; 25(7):1728. https://doi.org/10.3390/molecules25071728
Chicago/Turabian StyleDumitras, Dan C., Mioara Petrus, Ana-Maria Bratu, and Cristina Popa. 2020. "Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review" Molecules 25, no. 7: 1728. https://doi.org/10.3390/molecules25071728
APA StyleDumitras, D. C., Petrus, M., Bratu, A. -M., & Popa, C. (2020). Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review. Molecules, 25(7), 1728. https://doi.org/10.3390/molecules25071728




.jpg)


.jpg)