Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Univariate Experiments Design and Analysis
2.1.1. Effects of Solvent Type on UAE
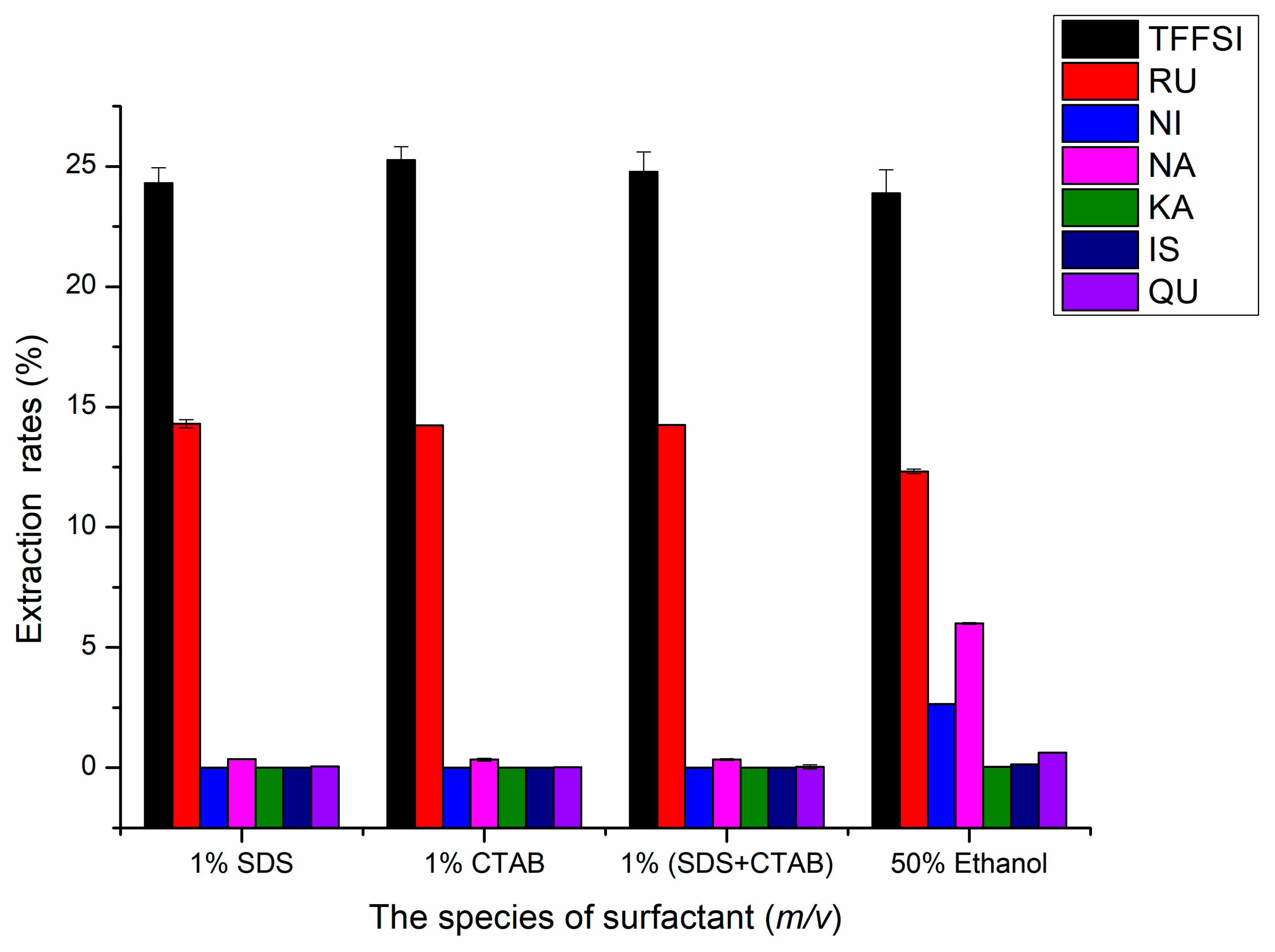
2.1.2. Effects of the Species of Surfactants on UAE
2.1.3. Effects of the Ethanol Concentration on UAE
2.1.4. Effects of Liquid-Solid Ratio on UAE
2.1.5. Effects of Ultrasound Extraction Time on UAE
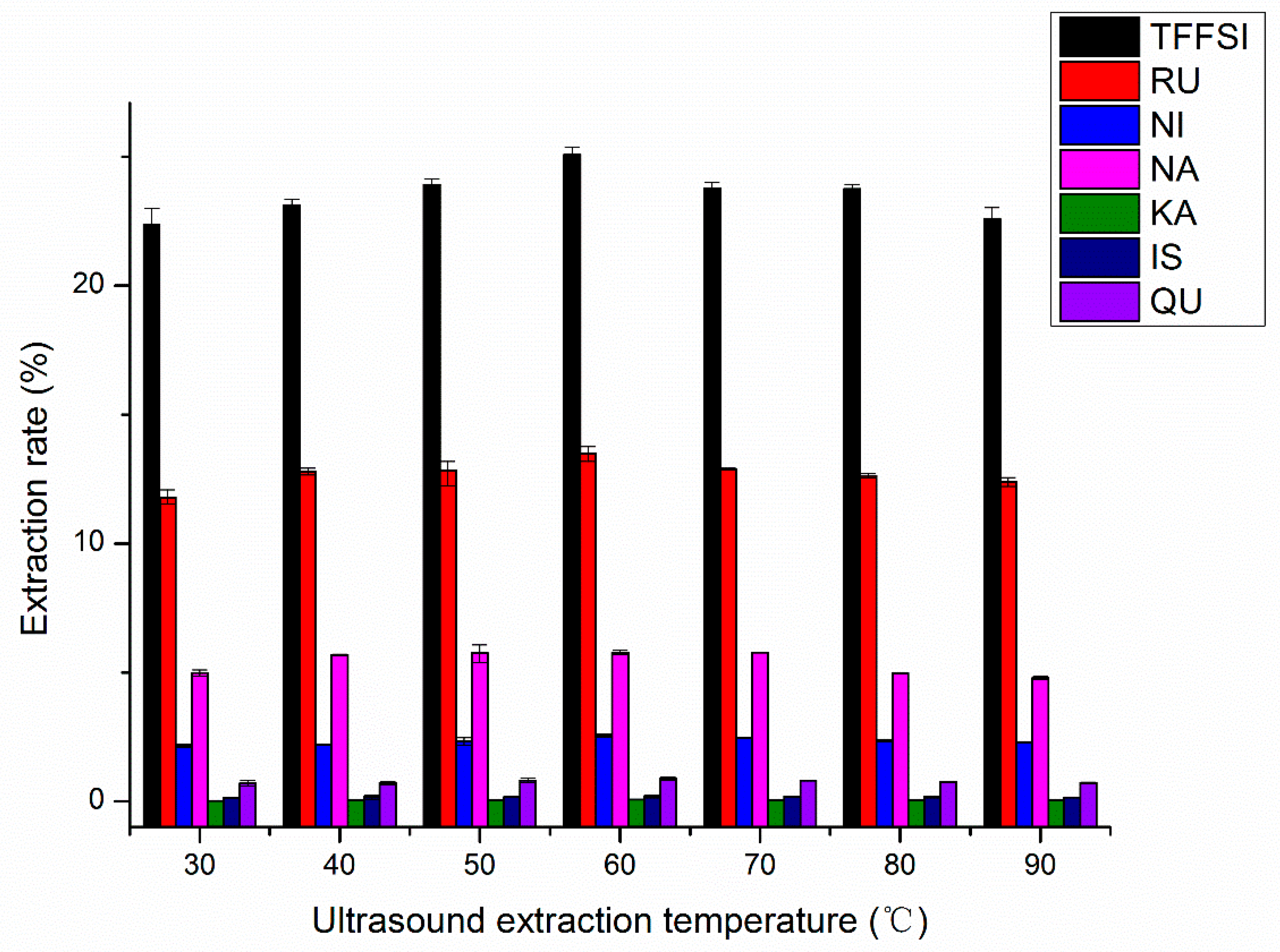
2.1.6. Effects of Ultrasound Extraction Temperature on UAE
2.2. Multi-Response Design and Analysis
2.2.1. Model Fitting
| Y1 = 26.35−0.19A + 0.57B + 0.27C + 0.34D + 0.14AB + 0.53AC−0.20AD + 1.04BC + 0.39BD + 0.53CD−1.11A2−1.81B2−0.50C2−1.45D2 Y2 = 14.52 + 0.11A−4.941E−0.004B + 0.079C + 0.032D−0.45AB−0.16AC + 0.020AD−0.46BC + 0.27BD + 0.27CD−1.03 A2−1.39 B2−0.95 C2−1.02 D2 Y3 = 2.92 + 0.049A + 7.798E−0.003B−0.040C + 0.11D−0.011AB + 0.043AC + 1.688E−003AD + 0.025BC + 0.018BD + 0.079CD−0.19 A2−0.19 B2−0.17 C2−0.35 D2 Y4 = 7.22 + 0.079A−0.044B−0.085C + 0.26D−0.010AB + 0.084AC + 0.054AD + 0.048BC−0.018BD + 0.16CD−0.74 A2−0.73 B2−0.68 C2−1.13 D2 Y5 = 0.10 + 5.705E−0.003A + 0.012B + 6.440E−003C + 0.010D + 9.904E−003AB−4.594E−003AC + 6.230E−003AD + 4.234E−003BC + 0.031BD + 0.012CD−0.050 A2−0.034 B2−0.051 C2−0.03 D2 Y6 = 0.49 + 2.864E−003A + 0.031B−0.010C + 0.022D + 0.016AB + 5.471E−003AC + 0.022AD−0.044BC−1.988E−003BD−0.017CD−0.12 A2−0.14 B2−0.13 C2−0.085 D2 Y7 = 2.85−0.13A + 0.026B + 0.026C−0.046D−1.566E−003AB−0.030AC−7.115E−003AD−0.094BC−0.20BD + 0.45CD−0.86 A2−0.93 B2−0.85 C2−0.66 D2 |
2.2.2. Interpretation of Response Surface Models
2.2.3. Verification of Predictive Models
2.3. Method Validation
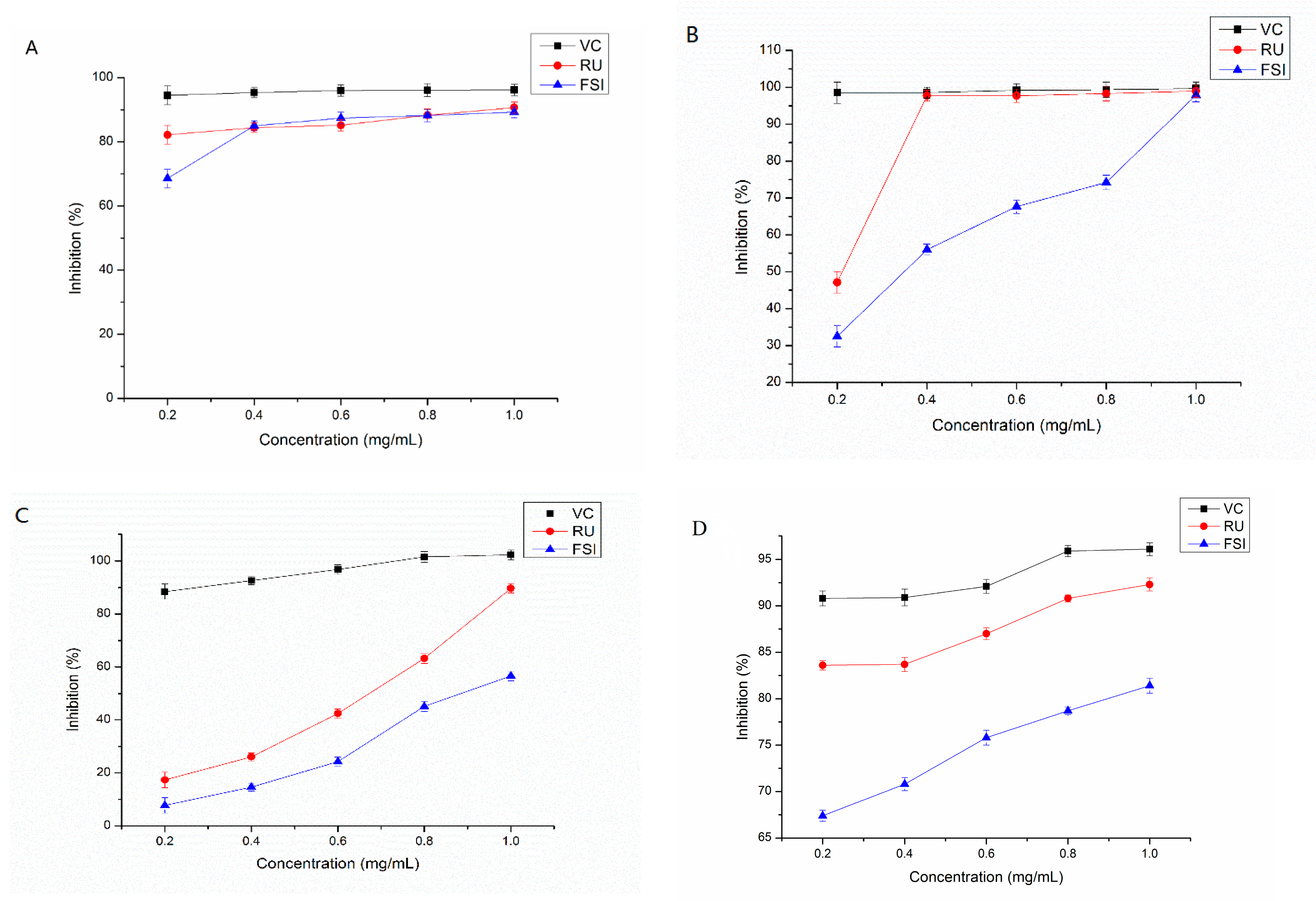
2.4. Assess the Antioxidant Activity
2.4.1. Scavenging Capacity of DPPH Radical
2.4.2. Scavenging Capacity of ABTS•+
2.4.3. Scavenging Capacity of Superoxide Anion (SCSA) Radical
2.4.4. Ferric Reducing/Antioxidant Power
3. Materials and Methods
3.1. Sample of FSI
3.2. Chemicals and Apparatus
3.3. Preparation of Standard Solution
3.4. Determination of TFFSI by UV
3.5. Determination of Six Main Flavonoids by HPLC
3.6. Sample Preparation Based on UAE
3.7. Experimental Design
3.7.1. Univariate Experiments Design
3.7.2. Box–Behnken Design
3.8. Assess the Antioxidant Activity of Hydroalcoholic Extracts from FSI
3.8.1. Sample Preparation
3.8.2. Measurement of Scavenging Capacity on DPPH Radicals
3.8.3. Measurement of Scavenging Activity on ABTS•+ Radicals
3.8.4. Superoxide Anion Scavenging Activity (SASA)
3.8.5. Ferric Reducing/Antioxidant Power
3.9. Statistical Analysis
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Yao, W.; Wang, S.; Chen, Y.; Wang, H. Composition and Antibacterial Activity of Essential Oils of Flos Sophorae Immaturus. Int. J. Food Prop. 2011, 14, 903–913. [Google Scholar] [CrossRef]
- Lebeau, J.; Neviere, R.; Cotelle, R. Beneficial effects of different flavonoids, on functional recovery after ischemia and reperfusion in isolated rat heart. Bioorg. Med. Chem. Lett. 2001, 11, 23–27. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Ishida, H.; Umino, T.; Tsuji, K.; Kosuge, T. Studies on antihemorrhagic substances in herbs classified as hemostatics in Chinese medicine. VII. On the antihemorrhagic principle in Cirsium japonicum DC. Chem. Pharm. Bull. 1987, 35, 861–864. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Kim, J.M.; Yun-Choi, H.S. Anti-platelet effects of flavonoids and flavonoid-glycosides from Sophora japonica. Arch. Pharmacal Res. 2008, 31, 886–890. [Google Scholar] [CrossRef]
- Koda, T.; Kuroda, Y.; Imai, H. Rutin Supplementation in the Diet has Protective Effects Against Toxicant-Induced Hippocampal Injury by Suppression of Microglial Activation and Pro-Inflammatory Cytokines. Cell. Mol. Neurobiol. 2009, 29, 523–531. [Google Scholar] [CrossRef]
- Lao, C.-J.; Lin, J.-G.; Kuo, J.-S.; Chao, P.-D.L.; Cheng, C.Y.; Tang, N.-Y.; Hsieh, C.-L. Microglia, Apoptosis and Interleukin-1β Expression in the Effect of Sophora Japonica L. on Cerebral Infarct Induced by Ischemia-Reperfusion in Rats. Am. J. Chin. Med. 2005, 33, 425–438. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg Med. Chem. Lett. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- López-Revuelta, A.; Sánchez-Gallego, J.I.; Hernández-Hernández, Á.; Sánchez-Yagüe, J.; Llanillo, M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem. Interactions 2006, 161, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; You, S.; Tao, L.; Xu, F.; Ji, T.; Gu, Z.-Y. Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and d-Galactosamine-Induced Liver Injury in Mice. Int. J. Mol. Sci. 2017, 18, 587. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, H.; Jiang, B.; Li, Z.; Chen, Y.C.; Jiang, B.-H. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, L.; Meng, L.; Sun, H.; Zhang, W.; Xu, Y. Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen-activated protein kinase kinase signaling pathways. Mol. Med. Rep. 2015, 12, 6745–6751. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.-J.; Zheng, X.; Gao, M.-Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2018, 170, 285–294. [Google Scholar] [CrossRef]
- Liu, J.-L.; Li, L.-Y.; He, G. Optimization of Microwave-Assisted Extraction Conditions for Five Major Bioactive Compounds from Flos Sophorae Immaturus (Cultivars of Sophora japonica L.) Using Response Surface Methodology. Molecules 2016, 21, 296. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Lam, S.C.; Zhao, M.; Liang, Z.; Yu, X.; Yang, D.; Xu, X. Extraction and isolation of flavonoid glycosides from Flos Sophorae Immaturus using ultrasonic-assisted extraction followed by high-speed countercurrent chromatography. J. Sep. Sci. 2014, 37, 957–965. [Google Scholar] [CrossRef]
- Li, F.-J.; Ning, S.-L.; Li, Y.; Yu, Y.-J.; Shen, C.-D.; Duan, G.-L. Optimisation of Infrared-assisted Extraction of Rutin from Crude Flos Sophorae Immaturus Using Response Surface Methodology and HPLC Analysis. Phytochem. Anal. 2011, 23, 292–298. [Google Scholar] [CrossRef]
- Gan, Z.; Chen, Q.; Fu, Y.; Chen, G. Determination of bioactive constituents in Flos Sophorae Immaturus and Cortex Fraxini by capillary electrophoresis in combination with far infrared-assisted solvent extraction. Food Chem. 2012, 130, 1122–1126. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Xie, Z.; Lam, S.C.; Wu, J.; Yang, D.; Xu, X. Chemical fingerprint and simultaneous determination of flavonoids in Flos Sophorae Immaturus by HPLC-DAD and HPLC-DAD-ESI-MS/MS combined with chemometrics analysis. Anal. Methods 2014, 6, 4328–4335. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csoka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Vian, M.A.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Huang, J.; Xie, W. Extraction of rutin from Flos Sophorae Immaturus with an aqueous 1-butyl-3-methylimidazolium chloride solution. Anal. Sci. 2010, 26, 383–386. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Zhang, H.; Chen, G. Carbon nanotube/polystyrene composite electrode for microchip electrophoretic determination of rutin and quercetin in Flos Sophorae Immaturus. Talanta 2007, 73, 932–937. [Google Scholar] [CrossRef]
- Liu, J.-L.; Shunlin, Z.; Fan, Q.-J.; Yuan, J.; Yang, S.-M.; Kong, F.-L. Optimization of High-Pressure Ultrasonic-Assisted Simultaneous Extraction of Six Major Constituents from Ligusticum chuanxiong Rhizome using Response Surface Methodology. Mol. 2014, 19, 1887–1911. [Google Scholar] [CrossRef]
- Xia, G.H.; Liang, Y.; Han, S. Study on the Extraction Process of Flavonoids in Polygonatum odoratum with Surfactant. Food Res. Dev. 2018, 39, 37–41. [Google Scholar]
- Zhu, X.; Guo, H.Y.; Tan, X.H.; Xiao, J.; Zhang, Y.; Wang, F.; Deng, J.H. Ultrasonic–assisted anionic and cationic surfactant systems extraction of total flavonoids from Ampelopsis grossedentata. J. Food Ind. Sci. Technol. 2016, 37, 213–218. [Google Scholar]
- Luo, K.P.; Li, X.F.; Lin, H.; Luo, J.; Yang, L.; Liu, H.X. Optimization of surfactant assisted ultrasonic extraction for total flavonoids from Turpinia arguta leaves. Chin. Tradit. Pat. Med. 2016, 12, 2585–2589. [Google Scholar]
- Liu, F.F.; Ang, C.Y.W.; Springer, D. Optimization of extraction conditions for active components in Hypericum perforatum using response surface methodology. J. Agric. Food Chem. 2000, 48, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Zhu, L.; Cao, Y.-L.; Jiang, J.-G.; Lin, Q.-S. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower ofCitrus aurantiumL. var.amaraEngl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.-G.; Li, W.-F.; Chen, J.; Wang, D.-Y.; Zhu, L. Optimum extraction Process of polyphenols from the bark ofPhyllanthus emblicaL. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.-S.; Ye, J.-N.; Yan, F.-N. Optimization of extraction technology of polysaccharide from foxtail millet using response surface methodology. Chem. Ind. Chem. Eng. Q. 2014, 20, 579–585. [Google Scholar] [CrossRef]
- Duan, M.H.; Luo, M.; Zhao, C.J.; Wang, W.; Zu, Y.G.; Zhang, D.Y.; Fu, Y.J. Ionic liquid-based negative pressure cavitation-assisted extraction of three mainflavonoids from the pigeonpea roots and its pilot-scale application. Separ. Purif. Technol. 2013, 107, 26–36. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Z.; Tu, Z.-C.; Wang, H.; Zhang, L.; Xie, X.; Liu, G. Influence of in vitro gastrointestinal digestion on the bioavailability and antioxidant activity of polyphenols from Ipomoea batatas leaves. Int. J. Food Sci. Technol. 2017, 52, 1131–1137. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-N.; Woo, H.-C.; Chun, B.-S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between Antioxidant Activities and Phenolic Contents of Radix Angelicae Sinensis (Danggui). Mol. 2009, 14, 5349–5361. [Google Scholar] [CrossRef]
- Wang, R.; Chang, Y.; Tan, Z.; Li, F. A novel combined process for extracting, separating and recovering flavonoids from flos sophorae immaturus. Sep. Purif. Technol. 2017, 172, 422–432. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity ofPhaseolus vulgarisL. Dry Beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Bruns, R.; Ferreira, H.; Matos, G.; David, J.; Brandão, G.; Da Silva, E.G.P.; Portugal, L.; Dos Reis, P.; Souza, A.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Cheung, L.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Wang, X.; Song, Y.; Fan, X.-H.; Martín, J.F.G. Optimization of Pyrogallol Autoxidation Conditions and Its Application in Evaluation of Superoxide Anion Radical Scavenging Capacity for Four Antioxidants. J. AOAC Int. 2016, 99, 504–511. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Flos Sophorae Immaturus are available from the authors. |






| No. | Flavonoids (Extraction Yield (%)) | Method | Solvent | Ratio mL/g | Time | Temperature (°C) | Literature | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rutin | Nicoti- Florin | Narci- Ssoside | Kaem-Pferol | Isorham-Netin | Querc-Etin | Genistein | |||||||
| 1 | 11.68 | 1.50 | 2.39 | 0.31 | 0.33 | 2.76 | - | Microwave | deep eutectic solvent | 26 | 20 m | 62 | Gen Wang, 2018 [15] |
| 2 | 32.11 | - | - | 0.06 | 0.17 | 6.26 | 0.002 | Microwave | 100% methanol | 50 | 80 s | Unclear | Jin-Liang Liu, 2016 [16] |
| 3 | 20.86 | 0.43 | 2.59 | - | - | - | - | Ultrasound | 82% methanol | 56 | 60 m | Unclear | Zhisheng Xie, 2014 [17] |
| 4 | 13.38 | 1.98 | 0.55 | Ultrasound | 80% methanol | 500:3 | 30 m | 30 | Zhisheng Xie, 2014 [21] | ||||
| 5 | 25.26 | - | - | - | - | - | - | Infrared | 70% methanol | 30 | 4.8 m | Unclear | Fa-jie Li, 2011 [18] |
| 6 | 10.28 | FIASE | Methanol | 25 | 6 m | ZhibinGan, 2011 [19] | |||||||
| 7 | 23.59 | Water bath | 1.72 mol/L 1-butyl-3-methylimidazoliumchloride | 75 | 20 m | 50 | Jianlin Huang, 2010 [25] | ||||||
| 8 | 21.1 | 0.62 | Ultrasound | Methanol | 50 | 30 m | 60 | Jingjing Xu, 2007 [26] | |||||
| Variables | TFFSI | RU | NI | NA | KA | IS | QU | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-value | p-Value | |
| Model | 133.35 | <0.0001 | 48.48 | <0.0001 | 70.29 | <0.0001 | 662.95 | <0.0001 | 112.16 | <0.0001 | 79.43 | <0.0001 | 690.27 | <0.0001 |
| A | 17.38 | 0.0009 | 4.33 | 0.0564 | 22.94 | 0.0003 | 52.04 | <0.0001 | 15.35 | 0.0015 | 0.41 | 0.5305 | 160.99 | <0.0001 |
| B | 156.13 | <0.0001 | 0.00008 | 0.9928 | 0.59 | 0.4569 | 16.22 | 0.0012 | 64.38 | <0.0001 | 49.18 | <0.0001 | 6.09 | 0.0271 |
| C | 34.55 | <0.0001 | 2.16 | 0.1636 | 15.29 | 0.0016 | 60.81 | <0.0001 | 19.56 | 0.0006 | 5.26 | 0.0378 | 6.15 | 0.0265 |
| D | 54.73 | <0.0001 | 0.36 | 0.5600 | 106.87 | <0.0001 | 568.71 | <0.0001 | 51.42 | <0.0001 | 24.77 | 0.0002 | 18.99 | 0.0007 |
| AB | 3.24 | 0.0936 | 23.43 | 0.0003 | 0.41 | 0.5317 | 0.29 | 0.6006 | 15.42 | 0.0015 | 4.30 | 0.0570 | 0.0074 | 0.9328 |
| AC | 45.36 | <0.0001 | 2.89 | 0.1111 | 5.82 | 0.0302 | 19.49 | 0.0006 | 3.32 | 0.0899 | 0.50 | 0.4898 | 2.75 | 0.1198 |
| AD | 6.30 | 0.0250 | 0.045 | 0.8352 | 0.00915 | 0.9252 | 8.22 | 0.0124 | 6.10 | 0.0270 | 8.30 | 0.0121 | 0.15 | 0.7024 |
| BC | 176.26 | <0.0001 | 24.28 | 0.0002 | 2.04 | 0.1751 | 6.51 | 0.0231 | 2.82 | 0.1153 | 32.78 | <0.0001 | 26.70 | 0.0001 |
| BD | 25.02 | 0.0002 | 8.46 | 0.0114 | 0.98 | 0.3379 | 0.91 | 0.3557 | 152.88 | <0.0001 | 0.066 | 0.8003 | 121.18 | <0.0001 |
| CD | 44.90 | <0.0001 | 8.54 | 0.0111 | 20.25 | 0.0005 | 74.23 | <0.0001 | 21.86 | 0.0004 | 5.09 | 0.0406 | 613.28 | <0.0001 |
| A2 | 323.61 | <0.0001 | 200.73 | <0.0001 | 191.76 | <0.0001 | 2462.20 | <0.0001 | 648.50 | <0.0001 | 373.58 | <0.0001 | 3626.45 | <0.0001 |
| B2 | 866.73 | <0.0001 | 361.20 | <0.0001 | 184.16 | <0.0001 | 2429.41 | <0.0001 | 288.60 | <0.0001 | 502.16 | <0.0001 | 4196.71 | <0.0001 |
| C2 | 66.60 | <0.0001 | 170.37 | <0.0001 | 152.27 | <0.0001 | 2085.05 | <0.0001 | 654.16 | <0.0001 | 428.39 | <0.0001 | 3500.95 | <0.0001 |
| D2 | 555.98 | <0.0001 | 194.72 | <0.0001 | 637.69 | <0.0001 | 5760.41 | <0.0001 | 232.85 | <0.0001 | 197.93 | <0.0001 | 2151.17 | <0.0001 |
| Lack of fit | 0.68 | 0.7197 | 0.57 | 0.7879 | 0.19 | 0.9859 | 0.38 | 0.9041 | 0.41 | 0.8828 | 0.44 | 0.8691 | 0.89 | 0.6025 |
| R2 | 0.9926 | 0.9798 | 0.9860 | 0.9985 | 0.9912 | 0.9876 | 0.9986 | |||||||
| Adjusted R2 | 0.9851 | 0.9596 | 0.9719 | 0.9970 | 0.9823 | 0.9751 | 0.9971 | |||||||
| Compound | Calibration Curve | R2 | Linearity Range/(mg/mL) | LOD/(mg/mL) c | LOQ/(mg/mL) d |
|---|---|---|---|---|---|
| The total flavonoids of Flos Sophorae Immaturus a | Y = 9.93278X + 0.01922 | 0.99796 | 0.004–0.100 | 0.0030 | 0.0040 |
| Rutin b | Y = 9759.82662X−1.23991 | 0.99994 | 0.003125–1.000 | 0.0020 | 0.0030 |
| Nicotiflorin b | Y = 2299.7295X + 19.66392 | 0.99162 | 0.006–2.000 | 0.0050 | 0.0055 |
| Narcissoside b | Y = 2206.89713X + 67.81067 | 0.9973 | 0.004147–1.6 | 0.0030 | 0.0041 |
| Kaempferol b | Y = 21654.77389X + 139.37839 | 0.99905 | 0.004–1.000 | 0.0030 | 0.0038 |
| Isorhamnetin b | Y = 10605.6954X−30.38794 | 0.99682 | 0.00816–0.1700 | 0.0070 | 0.0080 |
| Quercetin b | Y = 12930.14145X + 159.16393 | 0.99631 | 0.0045–1.2000 | 0.0040 | 0.0042 |
| Analyte | Intra-Day RSD | Inter-Day RSD | RSD | AmountAdded(mg) | Recovery(%) | RSD(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UVv a (%) | Pa b (%) | Rt c (%) | UVv (%) | Pa (%) | Rt (%) | UVv (%) | Pa (%) | Rt (%) | ||||
| The total flavonoids of Flos Sophorae Immaturus | 2.33 | - | - | 2.46 | - | - | 3.21 | - | - | 0.020 | 98.93 | 2.51 |
| 0.040 | 98.90 | 2.68 | ||||||||||
| Rutin | - | 0.40 | 0.16 | - | 0.89 | 0.22 | - | 0.19 | 0.20 | 0.020 | 99.30 | 2.65 |
| 0.040 | 98.70 | 2.14 | ||||||||||
| Nicotiflorin | - | 0.50 | 0.70 | - | 0.67 | 0.10 | - | 0.44 | 0.04 | 0.020 | 99.41 | 2.31 |
| 0.040 | 98.77 | 2.47 | ||||||||||
| Narcissoside | - | 0.13 | 0.05 | - | 0.24 | 0.10 | - | 0.04 | 0.04 | 0.020 | 97.98 | 2.41 |
| 0.040 | 99.12 | 2.84 | ||||||||||
| Kaempferol | - | 0.31 | 0.20 | - | 0.47 | 0.21 | - | 0.21 | 0.30 | 0.020 | 99.21 | 2.36 |
| 0.040 | 97.87 | 3.10 | ||||||||||
| Isorhamnetin | - | 0.32 | 0.11 | - | 0.41 | 0.09 | - | 0.18 | 0.10 | 0.020 | 98.68 | 2.98 |
| 0.040 | 99.21 | 3.16 | ||||||||||
| Quercetin | - | 0.89 | 0.55 | - | 0.95 | 0.62 | - | 0.91 | 0.60 | 0.020 | 97.94 | 2.98 |
| 0.040 | 97.68 | 2.78 | ||||||||||
| Runs | Factors | Extraction Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A (E a/%) | B (L b/mL/g) | C (t c/min) | D (T d/°C) | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | |
| 1 | 0(70) | 1(20) | 0(30) | −1(55) | 23.05 | 11.80 | 2.25 | 5.06 | 0.0095 | 0.2846 | 0.2846 |
| 2 | 0 | −1(10) | −1(25) | 0(60) | 24.31 | 11.51 | 2.61 | 5.96 | 0.0060 | 0.1655 | 0.9456 |
| 3 | 1(75) | −1 | 0 | 0 | 22.62 | 12.53 | 2.59 | 5.89 | 0.0054 | 0.1865 | 0.9224 |
| 4 | 0 | −1 | 1(35) | 0 | 22.54 | 12.89 | 2.48 | 5.70 | 0.0074 | 0.2325 | 1.1910 |
| 5 | 1 | 0(15) | 0 | 1(65) | 23.67 | 12.50 | 2.54 | 5.76 | 0.0394 | 0.3166 | 1.1283 |
| 6 | 0 | 0 | −1 | −1 | 24.28 | 12.75 | 2.41 | 5.42 | 0.0124 | 0.2426 | 1.8381 |
| 7 | 0 | 0 | 0 | 0 | 26.67 | 14.91 | 2.85 | 7.25 | 0.1022 | 0.4938 | 2.8469 |
| 8 | 0 | 0 | 0 | 0 | 26.27 | 14.42 | 2.90 | 7.21 | 0.1042 | 0.4880 | 2.8669 |
| 9 | 0 | −1 | 0 | 1 | 22.34 | 11.98 | 2.49 | 5.72 | 0.0049 | 0.2464 | 1.3541 |
| 10 | 1 | 0 | 1 | 0 | 25.49 | 12.67 | 2.60 | 5.87 | 0.0054 | 0.2497 | 0.99661 |
| 11 | 0 | 1 | −1 | 0 | 23.36 | 12.38 | 2.59 | 5.81 | 0.0170 | 0.2967 | 1.1399 |
| 12 | 0 | 0 | 0 | 0 | 26.27 | 14.42 | 2.89 | 7.15 | 0.0922 | 0.4898 | 2.8969 |
| 13 | 1 | 1 | 0 | 0 | 23.98 | 11.77 | 2.57 | 5.76 | 0.0477 | 0.2886 | 0.9891 |
| 14 | 0 | 0 | 0 | 0 | 26.27 | 14.42 | 2.95 | 7.28 | 0.1102 | 0.5038 | 2.7947 |
| 15 | 0 | 1 | 1 | 0 | 25.75 | 11.92 | 2.56 | 5.74 | 0.0354 | 0.1871 | 1.0083 |
| 16 | 0 | 0 | 1 | 1 | 25.65 | 12.83 | 2.52 | 5.73 | 0.0531 | 0.2814 | 1.8018 |
| 17 | −1(65) | 0 | 1 | 0 | 24.75 | 12.57 | 2.45 | 5.59 | 0.0064 | 0.2151 | 1.3006 |
| 18 | −1 | −1 | 0 | 0 | 23.25 | 11.46 | 2.46 | 5.71 | 0.0076 | 0.2178 | 1.1868 |
| 19 | 0 | −1 | 0 | −1 | 22.65 | 12.43 | 2.28 | 5.12 | 0.0438 | 0.2062 | 1.0557 |
| 20 | 0 | 0 | −1 | 1 | 24.07 | 12.36 | 2.44 | 5.58 | 0.0102 | 0.3369 | 0.8409 |
| 21 | 1 | 0 | −1 | 0 | 23.68 | 12.90 | 2.59 | 5.87 | 0.0051 | 0.2580 | 1.0143 |
| 22 | −1 | 0 | 0 | 1 | 24.52 | 12.37 | 2.41 | 5.45 | 0.0185 | 0.2792 | 1.4327 |
| 23 | 0 | 0 | 1 | −1 | 23.76 | 12.14 | 2.17 | 4.91 | 0.0081 | 0.2567 | 0.9920 |
| 24 | −1 | 1 | 0 | 0 | 24.05 | 12.50 | 2.49 | 5.62 | 0.0103 | 0.2559 | 1.2598 |
| 25 | −1 | 0 | 0 | −1 | 23.43 | 12.46 | 2.22 | 5.05 | 0.0132 | 0.2864 | 1.4970 |
| 26 | 0 | 0 | 0 | 0 | 26.27 | 14.42 | 2.99 | 7.19 | 0.0992 | 0.4514 | 2.8688 |
| 27 | −1 | 0 | −1 | 0 | 25.06 | 12.17 | 2.61 | 5.93 | 0.0011 | 0.2453 | 1.1978 |
| 28 | 1 | 0 | 0 | −1 | 23.36 | 12.51 | 2.34 | 5.14 | 0.0092 | 0.2349 | 1.2211 |
| 29 | 0 | 1 | 0 | 1 | 24.30 | 12.43 | 2.53 | 5.59 | 0.0953 | 0.3169 | 1.0328 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. https://doi.org/10.3390/molecules25081767
Fan S, Yang G, Zhang J, Li J, Bai B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules. 2020; 25(8):1767. https://doi.org/10.3390/molecules25081767
Chicago/Turabian StyleFan, Sanhong, Gege Yang, Jinhua Zhang, Jiani Li, and Baoqing Bai. 2020. "Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity" Molecules 25, no. 8: 1767. https://doi.org/10.3390/molecules25081767
APA StyleFan, S., Yang, G., Zhang, J., Li, J., & Bai, B. (2020). Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules, 25(8), 1767. https://doi.org/10.3390/molecules25081767






