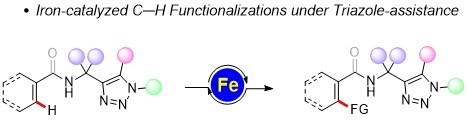
Iron-Catalyzed C–H Functionalizations under Triazole-Assistance
Abstract
Share and Cite
Lanzi, M.; Cera, G. Iron-Catalyzed C–H Functionalizations under Triazole-Assistance. Molecules 2020, 25, 1806. https://doi.org/10.3390/molecules25081806
Lanzi M, Cera G. Iron-Catalyzed C–H Functionalizations under Triazole-Assistance. Molecules. 2020; 25(8):1806. https://doi.org/10.3390/molecules25081806
Chicago/Turabian StyleLanzi, Matteo, and Gianpiero Cera. 2020. "Iron-Catalyzed C–H Functionalizations under Triazole-Assistance" Molecules 25, no. 8: 1806. https://doi.org/10.3390/molecules25081806
APA StyleLanzi, M., & Cera, G. (2020). Iron-Catalyzed C–H Functionalizations under Triazole-Assistance. Molecules, 25(8), 1806. https://doi.org/10.3390/molecules25081806