Sensing Precursors of Illegal Drugs—Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry
Abstract
:1. Introduction
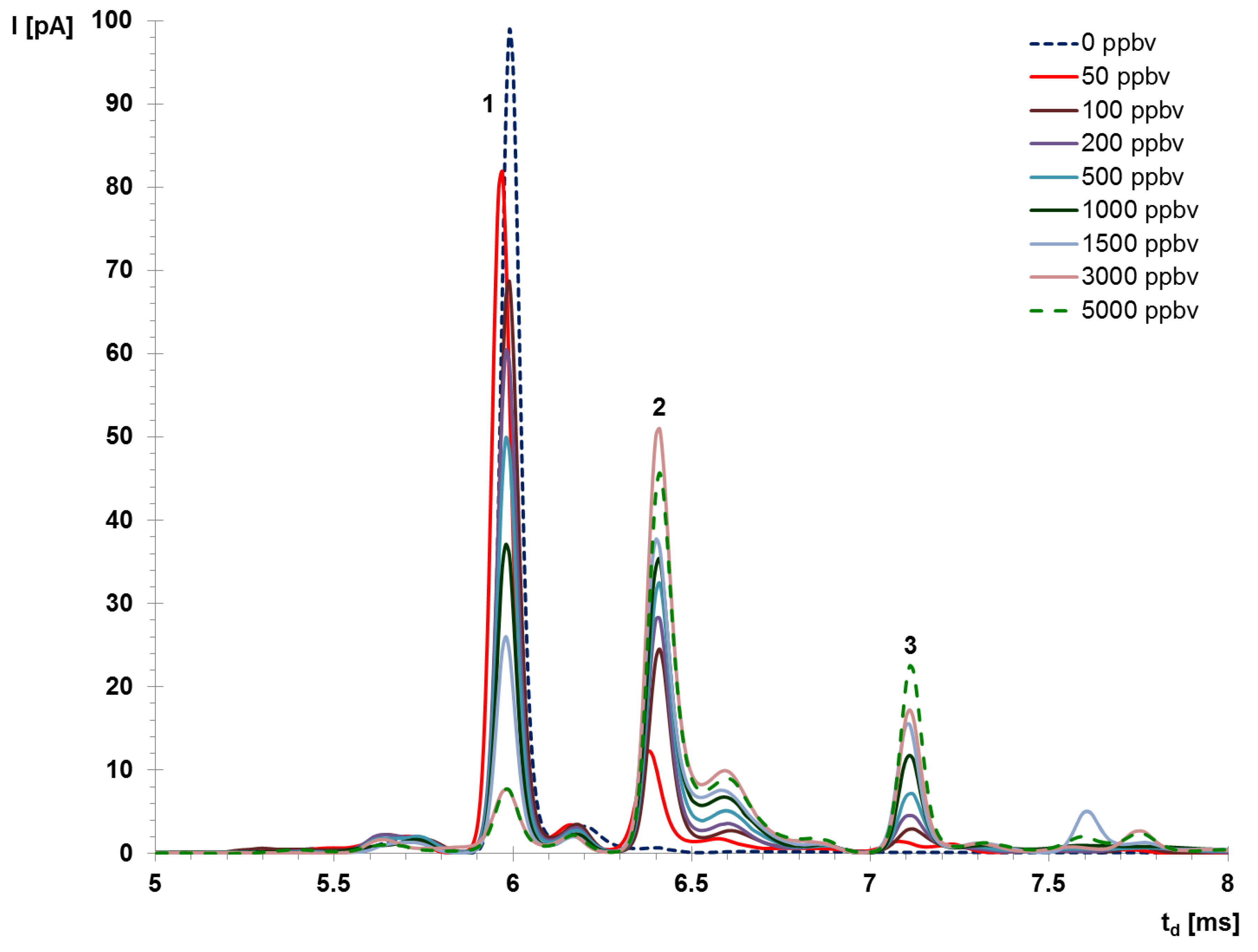
2. Results and Discussion
- 1.
- The saturation threshold of the IMS instrument (indicated by the total disappearance of the reactant ion peak RIP) was not reached. This means that the quantitative data provided by the IMS device is reliable and also that contamination of the IMS cell has been successfully avoided.
- 2.
- The most sensitive and selective operation mode is the negative ion mode. Apparition of 2 product ion peaks is an additional advantage, since the identification of AA is better by having two peaks to rely on. Minimum concentration measured was 50 ppbv (with both PIP noticed); the estimated minimum concentration level MCL is thought to be ca. 10 ppbv (42 μg m−3), when just the PIP#1 (K0 = 1.90 cm2 V−1 s−1) is to be seen.
- 3.
- In the positive ion mode, one product ion peak was observed, and only at relatively high AA vapor concentrations (>500 ppbv) compared to negative mode. This product ion peak is poorly resolved, being practically almost overlapped with the positive RIP; in order to resolve the RIP and the PIP a deconvolution step is highly recommended. For positive ion mode, the estimated minimum concentration level is ca. 500 ppbv AA.
- 4.
- Saturation occurs at around 3 ppmv in the negative ion mode, which is in accordance with the well-known fact that the dynamic range of an IMS device with a radioactive source ranges typically on two orders of magnitude. In the positive ion mode, saturation is thought to appear at AA vapor concentrations >6 ppmv.
- 5.
- Acetic anhydride produced ion mobility spectra in both positive and negative ion mode, which is definitely an advantage from a qualitative identification by IMS. However, negative operation mode is certainly the most advantageous, as explained previously.
Validation
3. Materials and Methods
- By injecting a volume of 0.04 µL liquid pure AA in the 10 L glass flask, a theoretical concentration C0 (calculated) = 1000 ppbv, while the actual/real value was only 500 ppbv AA (82 ppbv i-Butene). Starting from this initial concentration, standard atmospheres with 200, 100 and 50 ppbv AA were further obtained.
- By injecting a volume of 0.20 µL liquid pure AA in the 10 L glass flask, a theoretical concentration C0 (calculated) = 5,100 ppbv, while the real value (measured using the PID) was only 2400 ppbv AA (395 ppbv i-Butene). From this initial concentration, standard atmospheres with 2000, 1000 and 500 ppbv AA were generated.
- By injecting a volume of 0.50 µL liquid pure AA in the 10 L glass flask, a theoretical concentration C0 (calculated) = 12,750 ppbv, while the real value was 6500 ppbv AA (1065 ppbv i-Butene). From this initial concentration, standard atmospheres with 5000, 4000 and 3000 ppbv AA were produced.
3.1. PID Instrument Used
3.2. IMS Instrument Used
Author Contributions
Funding
Conflicts of Interest
References
- Eiceman, G.A.; Karpas, Z. Ion. Mobility Spectrometry, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bocoş-Binţinţan, V. Modern Techniques in Ultra-Trace Analysis, with Impact in Industrial Hygiene, Environmental Protection and Security Applications. Investigations on Chlorine and Phosgene by Ion Mobility Spectrometry; Cluj University Press: Cluj-Napoca, Romania, 2004. [Google Scholar]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Buszewski, B. Mass spectrometric techniques for the analysis of volatile organic compounds emitted from bacteria. Bioanalysis 2017, 9, 1069–1092. [Google Scholar] [CrossRef] [PubMed]
- Bocos-Bintintan, V.; Brittain, A.H.; Thomas, C.L.P. The response of a membrane inlet ion mobility spectrometer to chlorine and the effect of water contamination of the drying media on ion mobility spectrometric responses to chlorine. Analyst 2001, 126, 1539–1544. [Google Scholar] [CrossRef]
- Bocos-Bintintan, V.; Brittain, A.H.; Thomas, C.L.P. Characterization of the phosgene response of a membrane inlet 63Ni ion mobility spectrometer. Analyst 2002, 127, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Dussy, F.E.; Berchtold, C.; Briellmann, T.A.; Lang, C.; Steiger, R.; Bovens, M. Validation of an ion mobility spectrometry (IMS) method for the detection of heroin and cocaine on incriminated material. Forensic Sci. Int. 2008, 177, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ghira, G.B.; Ratiu, I.A.; Bocos-Bintintan, V. Fast Characterization of Pyridine Using Ion Mobility Spectrometry and Photoionization Detection. Environ. Eng. Manag. J. 2013, 12, 251–256. [Google Scholar]
- Moll, V.H.; Bocoş-Binţinţan, V.; Raţiu, I.A.; Ruszkiewicz, D.; Thomas, C.L.P. Control of Dopants/Modifiers in Differential Mobility Spectrometry Using a Piezoelectric Injector. Analyst 2012, 137, 1458–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratiu, I.A.; Bocos-Bintintan, V.; Patrut, A.; Moll, V.H.; Turner, M.; Thomas, C.L.P. Discrimination of bacteria by rapid sensing their metabolic volatiles using an aspiration- type ion mobility spectrometer (a-IMS) and gas chromatography-mass spectrometry GC-MS. Anal. Chim. Acta 2017, 982, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bocos-Bintintan, V.; Thomas, C.L.P.; Ratiu, I.A. Sensors’ array of aspiration ion mobility spectrometer as a tool for bacteria discrimination. Talanta 2020, 206, 120233. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Bocos-Bintintan, V.; Monedeiro, F.; Milanowski, M.; Ligor, T.; Buszewski, B. An Optimistic Vision of Future: Diagnosis of Bacterial Infections by Sensing Their Associated Volatile Organic Compounds. Crit. Rev. Anal. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- International Narcotics Control Board (INCB). Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances. In Report of the International Narcotics Control Board for 2011 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2011/4); International Narcotics Control Board: Vienna, Austria, 2012. [Google Scholar]
- United States Department of State. Bureau for International Narcotics and Law Enforcement Affairs, International Narcotics Control Strategy Report—Volume I. Drug and Chemical Control. 2010. Available online: http://www.state.gov/documents/organization/137411.pdf (accessed on 22 August 2019).
- United States Department of State. Bureau for International Narcotics and Law Enforcement Affairs, International Narcotics Control Strategy Report—Volume I. Drug and Chemical Control. 2012. Available online: http://www.state.gov/documents/organization/187109.pdf (accessed on 22 August 2019).
- European Monitoring Centre for Drugs and Drug Addiction EMCDDA. Statistical bulletin, Table DRD-108, Drug-induced deaths: Detailed qualitative national information on deaths due to specific substances in EU Member. 2012. Available online: http://www.ecdda.europa.eu/stats12#display:/stats12/drdtab108 (accessed on 25 August 2019).
- Romanian Directorate for Investigating Organized Crime and Terrorism, (D.I.I.C.O.T., Nr. 5/CD/2012). 2012. Available online: http://www.diicot.ro/pdf/2012/raport2011.pdf (accessed on 22 August 2019).
- Esseiva, P.; Dujourdy, L.; Anglada, F.; Taroni, F.; Margot, P. A methodology for illicit heroin seizures comparison in a drug intelligence perspective using large databases. Forensic Sci. Int. 2003, 132, 139–152. [Google Scholar] [CrossRef]
- Dujourdy, L.; Barbati, G.; Taroni, F.; Guéniat, O.; Esseiva, P.; Anglada, F.; Margot, P. Evaluation of links in heroin seizures. Forensic Sci. Int. 2003, 131, 171–183. [Google Scholar] [CrossRef]
- Williams, P.L.; James, R.C.; Roberts, S.M. Principles of Toxicology. In Environmental and Industrial Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; p. 203. [Google Scholar]
- Jebrane, M.; Pichavant, F.; Sèbe, G.A. Comparative study on the acetylation of wood by reaction with vinyl acetate and acetic anhydride. Carb. Polym. 2011, 83, 339–345. [Google Scholar] [CrossRef]
- Singh, N.; Chawla, D.; Singh, J. Influence of acetic anhydride on physicochemical, morphological and thermal properties of corn and potato starch. Food Chem. 2004, 86, 601–608. [Google Scholar] [CrossRef]
- de Souza Bergo, P.L.; Correa, J.M.; Nagem, T.J.; Augusti, R.; Nascentes, C.C. Simultaneous Quantification of Amphetamines and Ephedrines in Urine by GC/MS using Analytical-Grade Acetic Anhydride/Pyridine as Derivatizing Reagents: A Suitable Approach to Reduce Costs of Routine Analyses. J. Braz. Chem. Soc. 2009, 20, 348–359. [Google Scholar] [CrossRef]
- Nugroho, D.B.; Rianjanu, A.; Triyana, K.; Kusumaatmaja, A.; Roto, R. Quartz crystal microbalance-coated cellulose acetate nanofibers overlaid with chitosan for detection of acetic anhydride vapor. Results Phys. 2019, 15, 102680. [Google Scholar] [CrossRef]
- Bocos-Bintintan, V.; Smolenschi, A.; Ratiu, I.A. Rapid determination of indoor air contaminants in shoe shops using photoionization detectors. Studia UBB Chemia 2016, LXI, 203. [Google Scholar]
- Bocos-Bintintan, V.; Ratiu, I.A.; Al-Suod, H. Real time monitoring of soil contamination with diesel fuel using photoionization detectors. Arab J. Bas. Appl. Sci. 2019, 26, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Henderson, R.E. Portable Gas Detectors used in Confined Space and Other Industrial Atmospheric Monitoring Programs. 1999. Available online: http://docs.pksafety.com/pk_rae_confspace.pdf (accessed on 30 September 2019).
- RAE Systems Inc. Application Note AP-000 RAE Systems PID Training Outline. 2012. Available online: http://www.raesystems.com/sites/default/files/downloads/AP-000_PID_Training_Outline.pdf (accessed on 30 September 2019).
- I.U.T. GmbH Berlin, Description of IUT—Mini IMS Ion Mobility Spectrometer (Technical Note); IUT—Institut für Umwelttechnologien GmbH: Berlin, Germany, 2007; Volmerstraße 9B; p. 12489. [Google Scholar]
- Buszewski, B.; Ratiu, IA.; Milanowski, M.; Pomastowski, P.; Ligor, T. The effect of biosilver nanoparticles on different bacterial strains’ metabolism reflected in their VOCs profiles. J. Breath Res. 2018, 12, 027105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratiu, IA.; Railean Plugaru, V.; Pomastowski, P.; Milanowski, M.; Mametov, R.; Bocos-Bintintan, V.; Buszewski, B. Temporal influence of different antibiotics onto the inhibition of Escherichia coli bacterium grown in different media. Anal. Biochem. 2019, 585, 113407. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Substance Name | Molecular Formula | Properties | Observations |
|---|---|---|---|
| Acetic anhydride (AA) CAS#: 108-24-7 | C4H6O3 (CH3CO)2O | Vapor pressure: 4 mm Hg (20 °C) | Flammable liquid; Acute toxicity, by inhalation; Acute toxicity, by ingestion; Skin corrosion Conversion: 1 ppmv = 4.25 mg m−3 |
| Molecular Weight: 102.09 g/mol | |||
| Melting point: −73 °C | |||
| Boiling point: 138–140 °C | |||
| Relative density: 1.08 g/cm3 | |||
| Vapor density: 3.52 (Air = 1.0) |
| CAA Measured with PID | IMS Data—Positive Ion Mode | IMS Data—Negative Ion Mode | ||
|---|---|---|---|---|
| Drift Time td [ms] | Peak Height h [pA] | Drift Time td [ms] | Peak Height h [pA] | |
| 0 ppbv | POS RIP 6.28 | 60.0 ± 2.5 | NEG RIP 5.98 | 95.0 ± 3.6 |
| 50 ppbv | - | - | PIP#1 6.39 PIP#2 7.10 | 12.3 ± 0.5 1.5 ± 0.1 |
| 100 ppbv | - | - | PIP#1 6.41 PIP#2 7.11 | 24.5 ± 0.8 3.0 ± 0.1 |
| 200 ppbv | - | - | PIP#1 6.41 PIP#2 7.11 | 28.3 ± 1.1 4.6 ± 0.1 |
| 500 ppbv | PIP#1 6.45 | 4.0 ± 0.1 | PIP#1 6.41 PIP#2 7.11 | 32.5 ± 1.3 7.2 ± 0.2 |
| 1000 ppbv | PIP#1 6.45 | 10.0 ± 0.2 | PIP#1 6.41 PIP#2 7.11 | 35.4 ± 1.4 11.8 ± 0.4 |
| 1500 ppbv | - | - | PIP#1 6.41 PIP#2 7.11 | 37.9 ± 1.5 15.5 ± 0.4 |
| 2000 ppbv | PIP#1 6.45 | 21.0 ± 0.5 | - | - |
| 3000 ppbv | PIP#1 6.45 | 27.0 ± 0.7 | PIP#1 6.41 PIP#2 7.11 | 51.0 ± 1.7 17.3 ± 0.6 |
| 5000 ppbv | PIP#1 6.45 | 31.0 ± 1.0 | PIP#1 6.41 PIP#2 7.11 | 45.7 ± 1.8 22.5 ± 0.8 |
| 6500 ppbv | PIP#1 6.45 | 32.0 ± 1.1 | - | - |
| Operation Mode | Ion Drift Time, td [ms] | Reduced Ion Mobility1, K0 [cm2 V−1 s−1] |
|---|---|---|
| POSITIVE: | RIP: 6.28 | 1.94 |
| PIP #1: 6.45 | 1.89 | |
| RIP: 5.98 | 2.04 | |
| NEGATIVE: | PIP #1: 6.41 | 1.90 |
| PIP #2: 7.11 | 1.71 |
| Ion Mode | LOD [ppbv] | LOQ [ppbv] | Linear Range [ppbv] | Equation | R | S [pA/ppbv] |
|---|---|---|---|---|---|---|
| Negative | 1.1 | 3.7 | 3.7–100 | Y = 0.275X + 0.0167 | 1.000 | 0.28 |
| Positive | 190.0 | 325.0 | 325–2000 | Y = 0.0113X – 1.5 | 0.999 | 0.01 |
| Parameter | Specifications |
|---|---|
| Type of the ion mobility cell | Classic, with stacked rings design (conducting rings alternated with insulating rings). |
| Ionization source | Radioactive—using the β isotope 3H (tritium), with an activity of 300 MBq; tritium was embedded within a stainless steel disc. |
| IMS cell temperature | ca. 50 °C |
| Operating pressure | atmospheric pressure (ca. 990 mbar) |
| IMS cell drift length | ld = 55 mm |
| IMS cell internal diameter | 20 mm |
| Shutter grid opening time | 60 μs |
| Electric field intensity | E = 400 V cm−1 |
| Resolution of the IMS cell | 50 |
| Drift gas flow | Purified, dry air at 400 cm3 min−1; recirculated in a closed-loop pneumatic circuit that contains a filter with 10A molecular sieve. |
| Sample gas flow | 50 cm3 min−1; provided by a pump operated sequentially |
| Sampling frequency | Every 15 s (automatic mode) |
| Inlet gas flow | 200 cm3 min−1; Inlet system uses a sequentially pulsed valve. |
| Drift gas flow | 400 cm3 min−1; purified air moving inside an internal loop gasflow |
| Size of the instrument | 26.5 × 22 × 14 cm; weight: 3.8 kg |
| Speed of response | ca. 1 sec after sampling (real time response) |
| Power supply | Internal rechargeable Li-Ion battery at 19 V d.c. (autonomous mode; min. operation time–ca. 8 h.) or mains (220 V/50 Hz). |
| Power consumption | 6 W |
| Minimal detectable concentrations MDC | Between 1 ppbv and 100 ppbv, depending on the proton affinities of the target analyte (in the positive mode) or on the electron affinities (in the negative mode). |
| Software | IMS Control Program, ver. 2.209 (IUT GmbH), with spectra deconvolution integrated capability. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocos-Bintintan, V.; Ghira, G.-B.; Anton, M.; Martiniuc, A.-V.; Ratiu, I.-A. Sensing Precursors of Illegal Drugs—Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry. Molecules 2020, 25, 1852. https://doi.org/10.3390/molecules25081852
Bocos-Bintintan V, Ghira G-B, Anton M, Martiniuc A-V, Ratiu I-A. Sensing Precursors of Illegal Drugs—Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry. Molecules. 2020; 25(8):1852. https://doi.org/10.3390/molecules25081852
Chicago/Turabian StyleBocos-Bintintan, Victor, George-Bogdan Ghira, Mircea Anton, Aurel-Vasile Martiniuc, and Ileana-Andreea Ratiu. 2020. "Sensing Precursors of Illegal Drugs—Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry" Molecules 25, no. 8: 1852. https://doi.org/10.3390/molecules25081852
APA StyleBocos-Bintintan, V., Ghira, G. -B., Anton, M., Martiniuc, A. -V., & Ratiu, I. -A. (2020). Sensing Precursors of Illegal Drugs—Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry. Molecules, 25(8), 1852. https://doi.org/10.3390/molecules25081852







