Mannoside-Modified Branched Gold Nanoparticles for Photothermal Therapy to MDA-MB-231 Cells
Abstract
:1. Introduction
2. Results
2.1. BAu and Man@BAu Synthesis
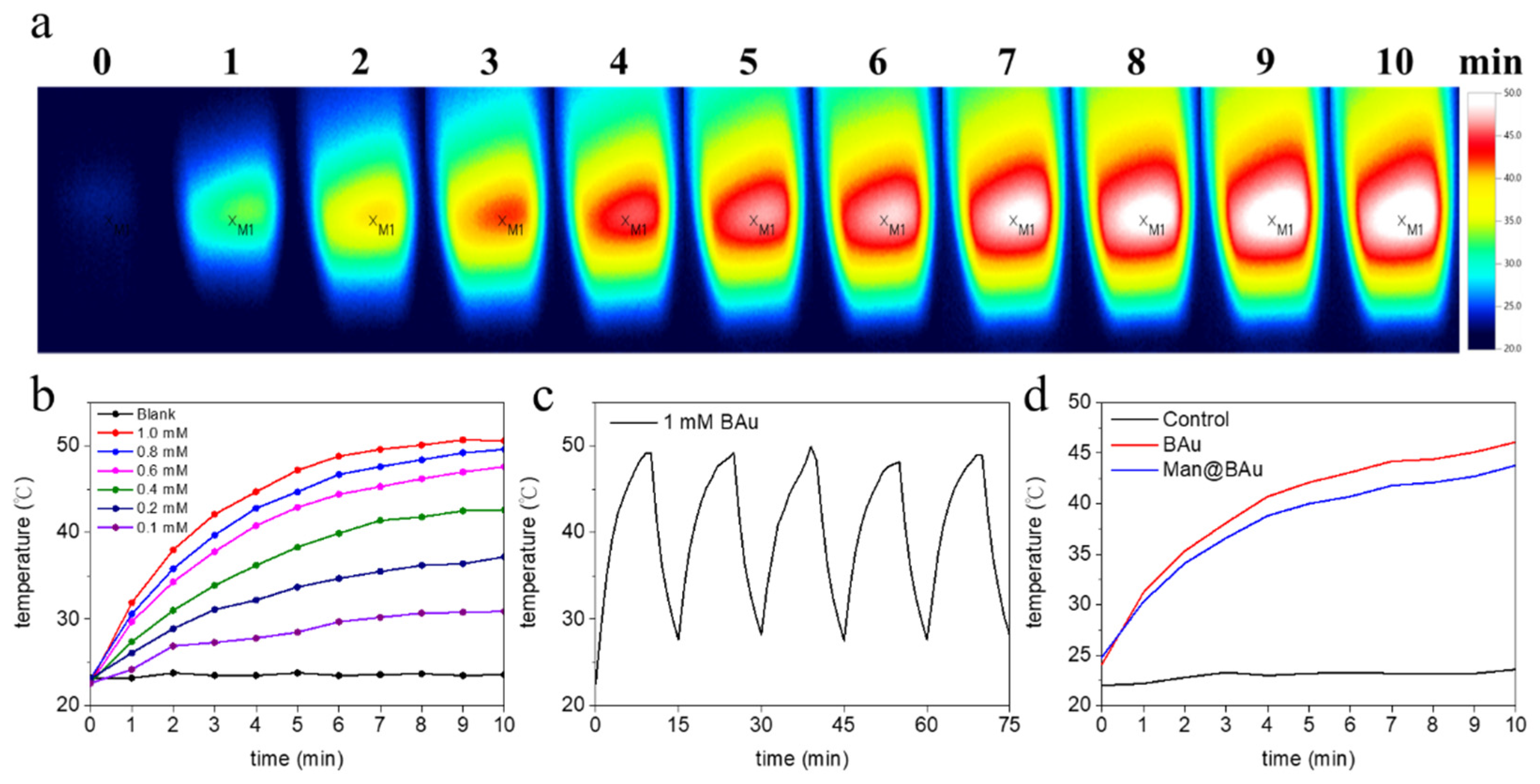
2.2. Photothermal Performance of BAu and Man@BAu
2.3. BAu and Man@BAu Stability
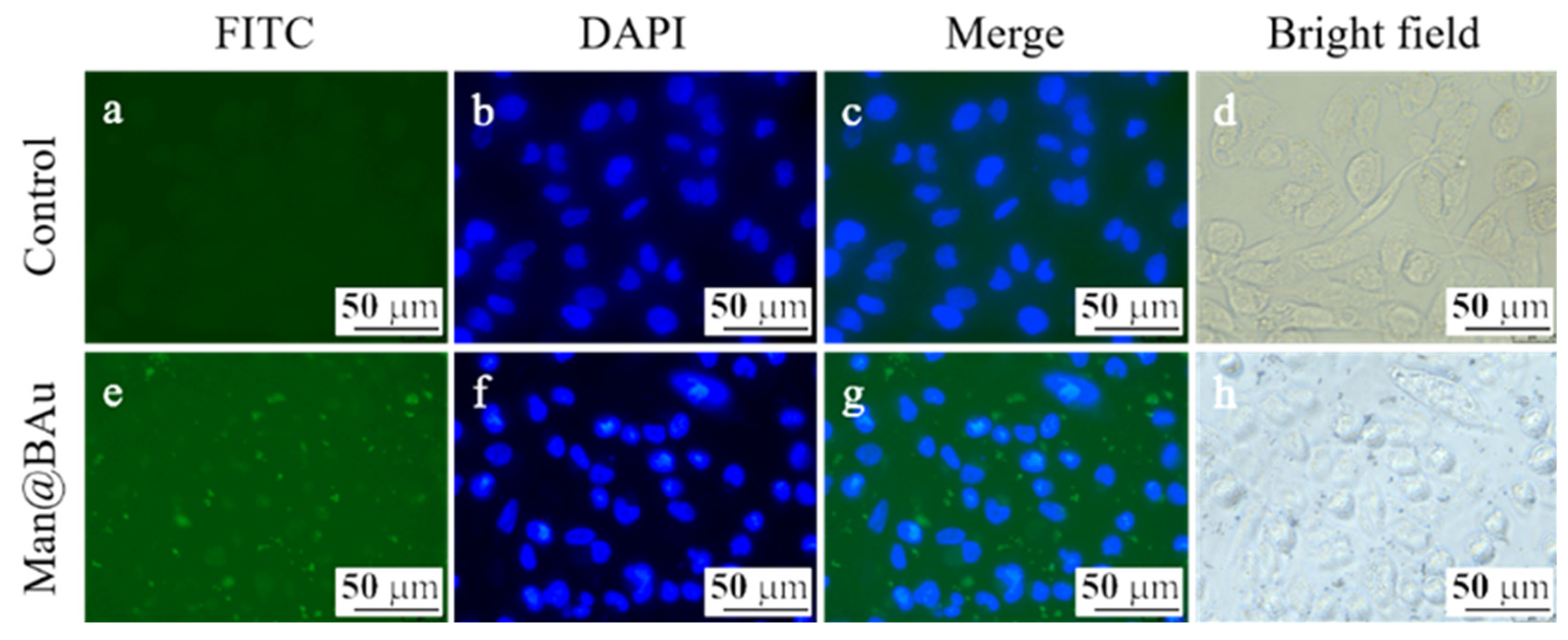
2.4. Fluorescence Microscopy Study and Cytotoxicity Assay
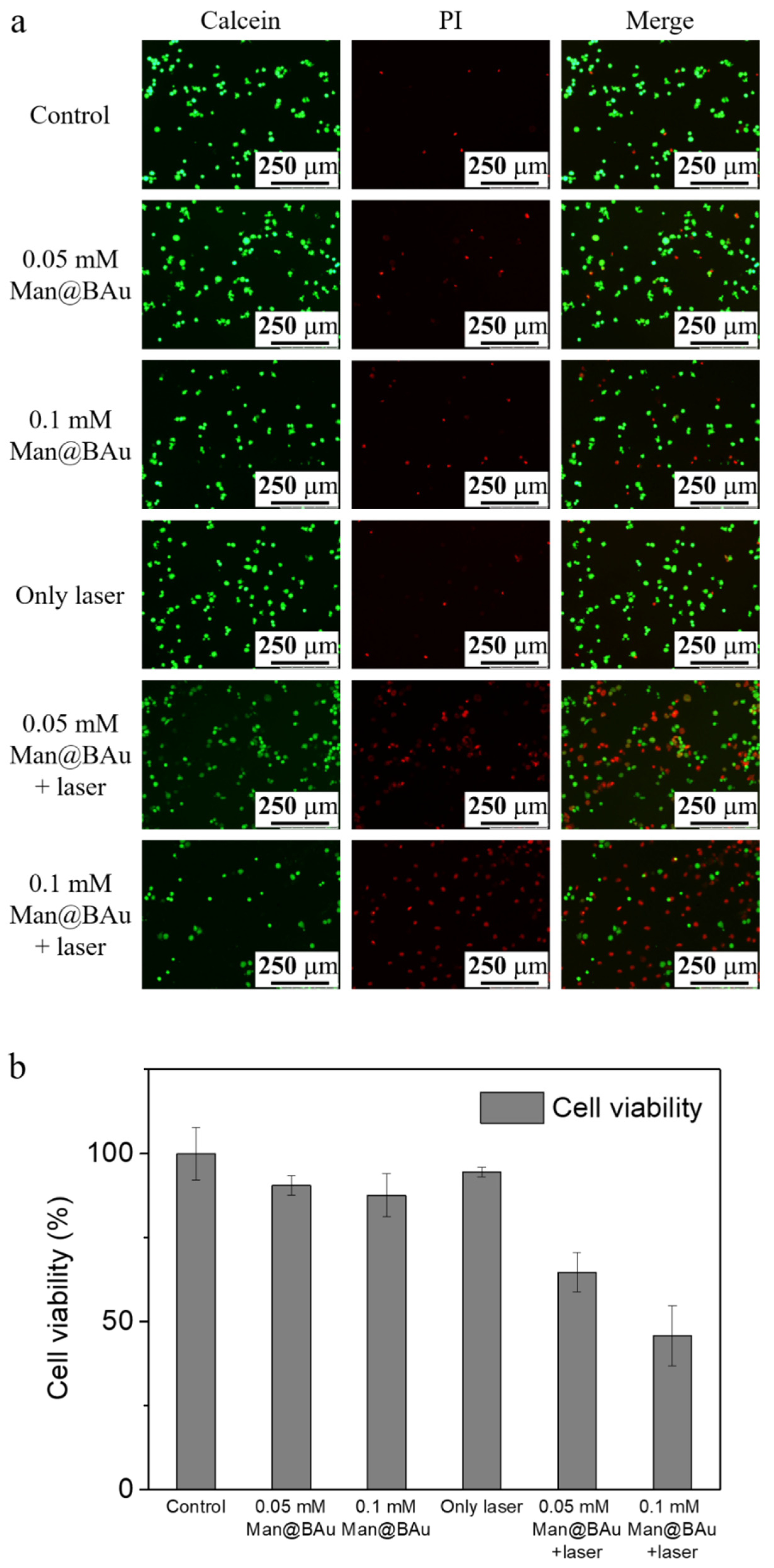
2.5. Laser-Irradiation Experiment and Cell-Killing Effect
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Preparation of Branched Gold Nanoparticles (BAu NPs)
3.3. Preparation of Mannose-Modified Branched Gold Nanoparticles (Man@BAu NPs)
3.4. Preparation of FITC-Labeled Mannoside-Modified Branched Gold Nanoparticles (FITC-Man@BAu NPs)
3.5. Temperature-Elevation Profile Upon 808 nm Laser Irradiation
3.6. Cell-Culture Conditions
3.7. Cellular-Uptake Image Studies
3.8. Photothermal Effect of Man@BAu NPs in MDA-MB-231 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemotherapy. Theranostics 2013, 3, 633–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dam, D.H.M.; Lee, J.H.; Sisco, P.N.; Co, D.T.; Zhang, M.; Wasielewski, M.R.; Odom, T.W. Direct Observation of Nanoparticle–Cancer Cell Nucleus Interactions. ACS Nano 2012, 6, 3318–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhang, M.; Han, Z.; Chen, D.; Zhu, Q.; Gao, W.; Qian, Z.; Gu, Y. A Telomerase-Specific Doxorubicin-Releasing Molecular Beacon for Cancer Theranostics. Angew. Chem. Int. Ed. 2016, 55, 3304–3308. [Google Scholar] [CrossRef]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Hovhannisyan, V.A.; Chao, Y.-C.; Chao, S.-L.; Chiang, S.-J.; Lin, S.-J.; Dong, C.-Y.; Chen, C.-C. Multiple Release Kinetics of Targeted Drug from Gold Nanorod Embedded Polyelectrolyte Conjugates Induced by Near-Infrared Laser Irradiation. J. Am. Chem. Soc. 2010, 132, 14163–14171. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chu, S.-H.; Li, C.-H.; Lee, T.R. Surface modification with zwitterionic cysteine betaine for nanoshell-assisted near-infrared plasmonic hyperthermia. Colloids Surf. B 2016, 145, 291–300. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Chen, C.-W.; Syu, W.-J.; Huang, T.-C.; Lee, Y.-C.; Hsiao, J.-K.; Huang, K.-Y.; Yu, H.-P.; Liao, M.-Y.; Lai, P.-S. Encapsulation of Au/Fe3O4 nanoparticles into a polymer nanoarchitecture with combined near infrared-triggered chemo-photothermal therapy based on intracellular secondary protein understanding. J. Mater. Chem. B 2017, 5, 5774–5782. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Stender, C.L.; Lee, M.H.; Nehl, C.L.; Lee, J.; Odom, T.W. Tailoring the Structure of Nanopyramids for Optimal Heat Generation. Nano Lett. 2009, 9, 1555–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xu, Y.; Qin, Y.; Sanderson, W.; Crowley, D.; Turner, C.H.; Bao, Y. Ligand-Directed Formation of Gold Tetrapod Nanostructures. J. Phys. Chem. C 2013, 117, 17143–17150. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotech. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-Targeted Drug Delivery with Mannose-Functionalized Nanoparticles Self-Assembled from Amphiphilic β-Cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morère, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 1475–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.-H.; Lin, H.-C.; Lai, C.-L.; Chen, P.-Y.; Lai, C.-H. Mannosyl Electrochemical Impedance Cytosensor for Label-Free MDA-MB-231 Cancer Cell Detection. Biosens. Bioelectron. 2018, 116, 100–107. [Google Scholar] [CrossRef]
- Neumann, K.; Conde-González, A.; Owens, M.; Venturato, A.; Zhang, Y.; Geng, J.; Bradley, M. An Approach to the High-Throughput Fabrication of Glycopolymer Microarrays through Thiol–Ene Chemistry. Macromolecules 2017, 50, 6026–6031. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Septiadi, D.; Lai, C.-H.; Chen, P.; Seeberger, P.H.; De Cola, L. Glucose-Modified Silicon Nanoparticles for Cellular Imaging. ChemPlusChem 2017, 82, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Bharate, P.; Lai, C.-H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H.; et al. Multivalency at Interfaces: Supramolecular Carbohydrate-Functionalized Graphene Derivatives for Bacterial Capture, Release, and Disinfection. Nano Lett. 2015, 15, 6051–6057. [Google Scholar] [CrossRef] [Green Version]
- Farr, T.D.; Lai, C.-H.; Grünstein, D.; Orts-Gil, G.; Wang, C.-C.; Boehm-Sturm, P.; Seeberger, P.H.; Harms, C. Imaging Early Endothelial Inflammation Following Stroke by Core Shell Silica Superparamagnetic Glyconanoparticles That Target Selectin. Nano Lett. 2014, 14, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Chang, T.-C.; Chuang, Y.-J.; Tzou, D.-L.; Lin, C.-C. Stepwise Orthogonal Click Chemistry toward Fabrication of Paclitaxel/Galactose Functionalized Fluorescent Nanoparticles for HepG2 Cell Targeting and Delivery. Bioconjugate Chem. 2013, 24, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Lin, C.-Y.; Wu, H.-T.; Chan, H.-S.; Chuang, Y.-J.; Chen, C.-T.; Lin, C.-C. Galactose Encapsulated Multifunctional Nanoparticle for HepG2 Cell Internalization. Adv. Funct. Mater. 2010, 20, 3948–3958. [Google Scholar] [CrossRef]
- Chandra, K.; Culver, K.S.B.; Werner, S.E.; Lee, R.C.; Odom, T.W. Manipulating the Anisotropic Structure of Gold Nanostars using Good’s Buffers. Chem. Mater. 2016, 28, 6763–6769. [Google Scholar] [CrossRef]
- Xi, W.; Haes, A.J. Elucidation of HEPES Affinity to and Structure on Gold Nanostars. J. Am. Chem. Soc. 2019, 141, 4034–4042. [Google Scholar] [CrossRef]
- Huang, L.-D.; Adak, A.K.; Yu, C.-C.; Hsiao, W.-C.; Lin, H.-J.; Chen, M.-L.; Lin, C.-C. Fabrication of Highly Stable Glyco-Gold Nanoparticles and Development of a Glyco-Gold Nanoparticle-Based Oriented Immobilized Antibody Microarray for Lectin (GOAL) Assay. Chem. Eur. J. 2015, 21, 3956–3967. [Google Scholar] [CrossRef]
- Lin, C.C.; Yeh, Y.C.; Yang, C.Y.; Chen, C.L.; Chen, G.F.; Chen, C.C.; Wu, Y.C. Selective binding of mannose-encapsulated gold nanoparticles to type 1 pili in Escherichia coli. J. Am. Chem. Soc. 2002, 124, 3508–3509. [Google Scholar] [CrossRef]
- Narayan, S.; Rajagopalan, A.; Reddy, J.S.; Chadha, A. BSA binding to silica capped gold nanostructures: Effect of surface cap and conjugation design on nanostructure–BSA interface. RSC Adv. 2014, 4, 1412–1420. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Liang, T.; Guo, X. Catalytic pyrolysis of mannose as a model compound of hemicellulose over zeolites. Biomass Bioenergy 2013, 57, 106–112. [Google Scholar] [CrossRef]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Goldfeld, D.; Xia, Y. A plasmon-assisted optofluidic (PAOF) system for measuring the photothermal conversion efficiencies of gold nanostructures and controlling an electrical switch. Angew. Chem. Int. Ed. 2013, 52, 4169–4173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Orozco, C.; Urban, C.; Knight, M.W.; Urban, A.S.; Neumann, O.; Bishnoi, S.W.; Mukherjee, S.; Goodman, A.M.; Charron, H.; Mitchell, T.; et al. Au Nanomatryoshkas as Efficient Near-Infrared Photothermal Transducers for Cancer Treatment: Benchmarking against Nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Rong, P.; Lin, J.; Li, W.; Yan, X.; Zhang, M.G.; Nie, L.; Niu, G.; Lu, J.; Wang, W.; et al. Triphase Interface Synthesis of Plasmonic Gold Bellflowers as Near-Infrared Light Mediated Acoustic and Thermal Theranostics. J. Am. Chem. Soc. 2014, 136, 8307–8313. [Google Scholar] [CrossRef]
- Santos, G.M.; Zhao, F.; Zeng, J.; Shih, W.-C. Characterization of nanoporous gold disks for photothermal light harvesting and light-gated molecular release. Nanoscale 2014, 6, 5718–5724. [Google Scholar] [CrossRef]
- Xi, W.; Phan, H.T.; Haes, A.J. How to accurately predict solution-phase gold nanostar stability. Anal. Bioanal. Chem. 2018, 410, 6113–6123. [Google Scholar] [CrossRef]
- Ivanov, M.G.; Ivanov, D.M. Chapter 14—Nanodiamond Nanoparticles as Additives to Lubricants. In Ultananocrystalline Diamond, 2nd ed.; Shenderova, O.A., Gruen, D.M., Eds.; William Andrew Publishing: Oxford, UK, 2012; pp. 457–492. [Google Scholar]
Sample Availability: Samples of the compounds 1, BAu and Man@BAu are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-C.; Hsu, K.-F.; Lai, C.-L.; Wu, T.-C.; Chen, H.-F.; Lai, C.-H. Mannoside-Modified Branched Gold Nanoparticles for Photothermal Therapy to MDA-MB-231 Cells. Molecules 2020, 25, 1853. https://doi.org/10.3390/molecules25081853
Lin H-C, Hsu K-F, Lai C-L, Wu T-C, Chen H-F, Lai C-H. Mannoside-Modified Branched Gold Nanoparticles for Photothermal Therapy to MDA-MB-231 Cells. Molecules. 2020; 25(8):1853. https://doi.org/10.3390/molecules25081853
Chicago/Turabian StyleLin, Han-Chen, Keng-Fang Hsu, Chiao-Ling Lai, Tzu-Chien Wu, Hui-Fen Chen, and Chian-Hui Lai. 2020. "Mannoside-Modified Branched Gold Nanoparticles for Photothermal Therapy to MDA-MB-231 Cells" Molecules 25, no. 8: 1853. https://doi.org/10.3390/molecules25081853
APA StyleLin, H.-C., Hsu, K.-F., Lai, C.-L., Wu, T.-C., Chen, H.-F., & Lai, C.-H. (2020). Mannoside-Modified Branched Gold Nanoparticles for Photothermal Therapy to MDA-MB-231 Cells. Molecules, 25(8), 1853. https://doi.org/10.3390/molecules25081853





